ABSTRACT
Previous clinical studies had not shown expected results in advanced pancreatic cancer (APC) with single-agent checkpoint inhibitors. Until the present day, little is known about their performance in real-world settings. So, in this study, we investigate the ICIs’ efficacy and safety in Chinese APC patients. Patients with APC who received ICIs between November 2018 to June 2021 were enrolled in this retrospective study. The efficacy end points included overall survival (OS), progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR) and adverse events (AEs). This study included 104 patients and the median OS (mOS) and median PFS (mPFS) were 9.1 and 5.4 months, respectively. In the subgroup analyses, the mOS was longer for patients receiving combined radiotherapy than for those that didn’t (13.8 vs 7.0 months, p < .001), whereas the mPFS was also longer, and the ORR and DCR were higher. Specifically, the mOS was longer for patients who had received a combination of chemotherapy than for those combined with targeted therapy (11.6 vs 5.6 months, p = .002), with the mPFS being also longer. ICIs as a first-line treatment could resulted to better survival. The mOS was longer for patients with a high TMB compared to those with low (19.3 vs 7.2 months, p = .004), whereas AEs were considered to be tolerable. The combination therapy of ICIs was proved to be safe and effective for treating APC, especially the combination of chemotherapy and radiotherapy, which would benefit from additional prospective studies.
Introduction
Pancreatic cancer is a highly fatal disease with a 5-year survival rate of no more than 10% and is said to become the third leading cause of cancer death in the United States in 2030.Citation1,Citation2 Since the early symptoms of the disease are not evident, most patients have already developed distant metastases at the moment of diagnosis, and can only be treated with palliative chemotherapy.Citation3,Citation4 Even though there have been signs of progresses in medical research from gemcitabine single-agent chemotherapy and two-drug combination (gemcitabine plus nab-paclitaxel/capecitabine) to FOLFIRINOX (fluorouracil plus leucovorin plus irinotecan plus oxaliplatin), and the survival period of advanced pancreatic cancer (APC) patients has been albeit prolonged, the 5-year survival rate has not improved significantlyCitation5–7 And a low surgical resection rate, a high postoperative recurrence rate, a short survival time, and a poor prognosis are still problems in the treatment of APC that need to be tackled.
Immune checkpoint inhibitors (ICIs) that target the interaction of programmed death 1 (PD-1) with its ligands, programmed death ligands 1 (PD-L1) and programmed death ligands 2 (PD-L2), have achieved gratifying results in several solid tumors.Citation8–10 However, only patients with a mismatch repair deficiency or a microsatellite instability-high (dMMR/MSI-H) respond to ICIs, with the 5-year survival rate of postoperative patients and advanced patients being able to reach 100% and 25%Citation11 respectively. Partially advanced patients maintain complete response (CR) for 3 years, but dMMR/MSI-H is only present in 0.5%-1% pancreatic cancer patients.Citation11–13
The efficacy of single-agent ICIs in the treatment of pancreatic cancer is unresponsive due to the low cancer immunogenicity and the particularity of the tumor microenvironment.Citation14–16 Bearing in mind the objective response rate (ORR) of the dual immune checkpoint inhibitors (PD-1+T-lymphocyte associated protein (CTLA-4)) is only 3.1%, with limited efficacy and increased toxicity.Citation17,Citation18 Early studies have also shown that the PD-L1 expression is related to a poor prognosis of pancreatic cancer, specifically with the KEYNOTE-028 study showing that patients with a high PD-L1 expression had no objective response in pancreatic cancer.Citation19,Citation20 How to overcome the immune resistance and the search for biomarkers to identify patients, who have a higher likelihood of response to immunotherapy in pancreatic cancer, are, therefore, problems faced by clinical practices worldwide.
In its turn, chemotherapy can promote the release of tumor antigens, restore or enhance the expression of tumor antigens, which make it easier to be recognized by the immune system while also, increasing the sensitivity of tumor cells to immunotherapy.Citation21–23 The COMBAT trial showed that median overall survival (mOS), ORR, and disease control rate (DCR) were all significantly better for patients who had received pembrolizumab plus chemotherapy than for those who had received chemotherapy only in the NAPOLI-1 trial (mOS: 7.5 months vs 6.1 months, ORR: 32% vs 17%, DCR: 77% vs 52%) for second-line treatment of advanced pancreatic cancer.Citation22 Other Phase Ib/II studies of ICIs, plus chemotherapy, have also been shown to have a considerable efficacy.Citation24–26
On the other hand, preclinical studies have shown that radiotherapy can induce dendritic cell migration, T-cell activation and proliferation, and increase tumor-infiltrating lymphocytes, which eventually leads to tumor mutations producing new antigens.Citation27,Citation28 Combined with ICIs, radiotherapy is safe and tolerable in phase II, single-arm clinical studies of small cell lung cancer, triple-negative breast cancer and liver cancer.Citation29–31 The efficacy and safety of radiotherapy combined with ICIs in pancreatic cancer need further exploration and research. Despite a phase II study is ongoing,Citation32,Citation33 phase Ib studies showed the combination of ICIs and tumor neoantigen vaccines can overcome immune suppression in the tumor microenvironment, induce immune memory, and lead to long-term resistance to cancer, with disease free survivals (DFS) being able to reach 15.4 months in the postoperative adjuvant treatment.
ICIs combined with chemotherapy and radiotherapy have the potential to convert pancreatic cancer from a “cold” tumor to a “hot” one, and this study was designed to equate that possibility, specifically to evaluate the effects and toxicity of ICIs treatment of APC in a real-world setting.
Methods
Patients
We retrospectively reviewed patients with APC who had received a combination therapy of ICIs between November 2018 to June 2021 at the Comprehensive Cancer Centre of Drum Tower Hospital, Clinical Cancer Institute of Nanjing University. All patient information was extracted from the electronic medical record system by the following inclusion criteria: a) diagnosis of APC by histology or pathology; b) no other tumors and no autoimmune diseases; c) have not previously received any targeting T-cell co-stimulation and ICIs therapy; d) received at least 2 cycles of ICIs treatment and possess a complete and objective efficacy evaluation; e) have complete medical and follow-up information. All patients completed laboratory serum testing, computed tomography (CT) and magnetic resonance imaging (MRI) baseline assessment. Bearing in mind this study was in retrospect, there was no need for the patients’ informed consent.
Treatment
All patients received anti-PD-1 (pembrolizumab, camrelizumab, toripalimab, sintilimab, tislelizumab) treatment, and combination therapy, which includes chemotherapy (nab-paclitaxel, gemcitabine, irinotecan, cisplatin, pemetrexed, oxaliplatin, S-1), targeted therapy (anlotinib, apatinib, palbociclib, olaparib, nimotuzuma) and neoantigen vaccine therapy, with or without radiotherapy (3 Gy × 10f/5 Gy × 10f/8 Gy × 3f/30 Gy × 10f). According to the recommended standard treatment dose and cycles, chemotherapeutic or targeted drugs were administered while PD-1 inhibitors were administered with the first chemotherapy in each cycle. The treatment with a PD-1 inhibitor was followed by a concurrent initiation of radiotherapy until the prescribed dose was completed, which meant the patient showed intolerable toxicity or disease progression (PD) or voluntary withdraws. After 6 or 8 cycles of treatment, the duration of each cycle could be extended up to one treatment a month, and the follow-up time is up to 31 August 2021. The curative effect was evaluated by CT or MRI every 2 cycles during the treatment period, and every 2 months during the follow-up period.
Data collection and assessment
Patient history, pathology, and treatment-related information were separately collected both by an oncologist and a pharmacist, whereas imaging evaluation was conducted separately by two doctors, from which any objection was determined by the imaging director.
The primary study endpoints were OS and PFS, while the secondary study endpoints included ORR, DCR, and safety (treatment-related adverse events). Based on the Response Evaluation Criteria in Solid Tumors (version 1.1), the response was divided into CR, partial response (PR), stable disease (SD) and PD. The ORR consisted of any patient with both CR and PR, and the DCR contains all CR, PR, SD. In its turn, OS was the time of death or the follow-up deadline from the start of treatment, whereas PFS was defined as being the time of death, from the start of treatment to the progression of the disease or any other related cause. On the other hand, safety evaluates adverse reaction events and Immune-related adverse events (irAEs) per the Common Terminology Criteria for Adverse Events version 5.0, and the relationship with immunotherapy was determined by the physician and clinical pharmacists.
Statistical analysis
Patients who had received at least two immunotherapy sessions and underwent imaging assessments formed the efficacy and safety analysis, whereas variables in the baseline features were compared using Pearson’s χ2 test or Fisher’s exact test, and continuous variables were expressed as median and range and categorical variables as a percentage. Having performed two-group comparisons using the two-tailed log-rank-test, OS and PFS were assessed by using the Kaplan-Meier survival curve method while the A-cox proportional-risk model was used to determine the relationship between patient characteristics and survival outcome. Factor analysis was, however, performed first, and it was included in the multivariate analysis to look for factors that independently influenced prognosis, in case there was a comparison p < .05 between both groups. Results were expressed as a hazard ratio (HR) of 95% confidence intervals (CIs) and were considered statistically significant if p-value <.05. All statistical analyses were performed using SPSS statistical software (version 22.0, IBM Corporation, USA), Stata Corp LLC (MP16.0, College Station, USA) and an R-programming environment (version 4.0.2).
Results
Patient and clinical characteristics
The study consisted of 104 APC who had received ICIs combination therapy (, ) and from which the median age was 63 years of age (range: 30–80), with 62 and 42 patients in male and female, respectively. The ECOG scores were mostly 0 and 1, with only three patients (2.9%) of 2. Moreover, there were 48 cases of distant metastasis, 30 cases of which were locally advanced (28.8%), and 26 of them were postoperative recurrence (25.0%). There were 69 cases of ductal adenocarcinoma (66.3%), 3 cases of adenosquamous carcinoma (2.9%), and 32 cases of an unknown tumor type (30.8%). Twenty-four patients were tested for PD-L1 protein expression, including 8 (7.7%) positive with a PD-L1 combined positive score (CPS) of more than 1 and 16 (15.4%) with a negative score of less than 1. Next-generation sequencing (NGS) using the targeted gene panel used tumor samples (FFPE sections of resected tumor or biopsy) in 51 patients, and according to the tumor mutation burden (TMB) status was divided into 2 groups with the cutoff value of TMB being 10 mutations/Mb. Seven patients (6.7%) showed a TMB-H of above 10 mutations/Mb and 44 (42.3%) patients a TMB-L of below 10 mutations/Mb. Patients with MSI-H and microsatellite stability (MSS) were, respectively, 1 patient and 50 patients Whereas the number of patients treated with Pembrolizumab, Camrelizumab, Toripalimab, Sintilimab and Tislelizumab was 7 (6.7%), 11 (10.6%), 19 (18.3%), 43 (41.3%) and 24 (23.1%), also respectively.
Table 1. Patient characteristics.
In total, 74 (71.2%) patients discontinued treatment, and the main reason for this interruption consisted of disease progression in 70 (67.3%) patients, with the remaining four patients (3.8%) suspending treatment for economic reasons or adverse reactions, and 55 patients (52.9%) had ultimately died. The median number of ICIs was 5 doses (range, 2–22) and the median follow-up period was 30 weeks (range, 10–87 weeks).
Efficacy
Overall population
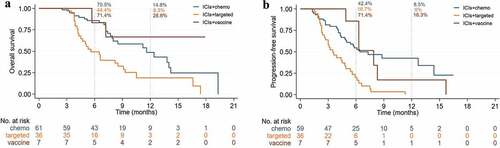
Overall, 2 patients with PFS were lost to follow-up. The mOS was 9.1 months (95%CI, 6.7–11.4, ) and the mPFS was 5.4 months (95%CI, 4.5–6.3, ). The 12-month OS rate was 13.5%, 6-month and 12-month PFS rate were 34.6% and 5.8%. The ORR and DCR were 31.7% and 72.1% (), including 4 patients with CR, 29 patients with PR, 42 patients with SD, and 29 patients with PD. The duration of reaching CR was 10, 26, 10 and 25 weeks, respectively, and while the median reaching times of PR were about 9 weeks (range, 3–36 weeks) (), the maintenance time of SD for each patient was shown in . ICIs median treatment dose of CR, PR, SD, and PD was 12.5 (range, 7–22), 6 (range, 2–16), 5 (range, 2–18) and 3 (range, 2–3), respectively. At the end of the follow-up, 34 patients were able to continue the ICIs treatment while 2 patients were in CR, with their respective PFS being 16.5 months and 15.3 months, and 9 patients remained in PR. There were 7 patients (6.7%) with an OS of more than 15 months, of which 3 were TMB-H, and the longest OS could reach 19.3 months. In its turn, another patient with MSI-H was still in CR at the end of follow-up (follow-up time was 5.5 months).
Figure 2. Kaplan-Meier curves of survival outcomes of patients in the overall population. (A) Overall survival. (B) Progression-free survival.
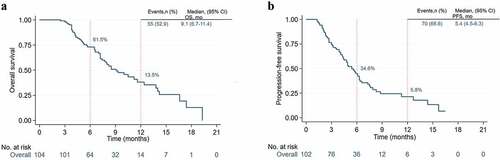
Figure 3. Swimmer’s plot illustrating times of response and disease progression and durations of response and survival for patients. (a) Patients of complete response and partial response. (b) Patients with stable disease.
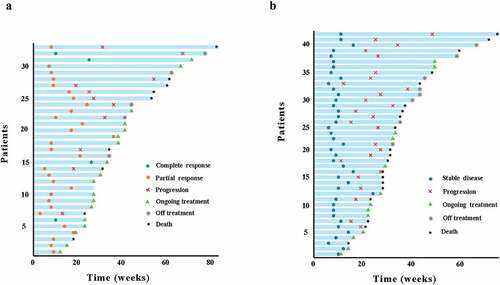
Table 2. Activity of immunotherapy in patients with APC in overall population.
The multivariate analysis concluded disease stage and TMB were independent predictors for OS (supplementary Table S1)., and that postsurgical recurrent or distant metastasis had a shorter OS (HR 2.33, 95% CI: 1.32–4.11, p = .003) compared with locally advanced patients. Moreover, TMB was independently associated with OS (HR 1.84, 95% CI: 1.14–.96, p = .013), whereas the degree of tumor differentiation and bone metastasis, regardless of radiotherapy, were independently associated with PFS (supplementary Table S1). It was also shown the higher the degree of tumor differentiation, the better the prognosis of PFS (HR 0.78, 95% CI: 0.61–1.00, p = .048). However, bone metastasis (HR 3.44, 95% CI: 1.03–11.53, p = .045) and the lack of radiotherapy (HR 1.88, 95% CI: 1.03–3.42, p = .039) consisted of poor prognosis factors of PFS. Age, gender, ECOG, tumor location, tumor type, the number of metastatic sites and PD-L1 expression had no significant impact on OS and PFS.
Subgroup analysis
Combined radiotherapy
Combination therapy of ICIs with radiotherapy showed an mOS of 13.8 months against the 7.0 months of therapy without radiotherapy (HR 2.79, 95% CI: 1.55–5.04, p < .001) (), while the mPFS was 8.3 months vs 3.9 months (HR 3.21, 95% CI: 1.91–5.40, p < .001) (), 12-month OS rate (21.3% vs 7.0%), 6-month PFS rate (53.2% vs 19.3%) and 12-month (12.8% vs 0%), ORR (53.2% vs 14.0%), and DCR (91.5% vs 56.1%). Among the group with or without radiotherapy (CR: 4 vs 0) and (PR: 21 vs 8) (). It is worth mentioning that patients with radiotherapy had higher ORR than those without radiotherapy in local advanced cases (63.6% vs 37.5%). It was pity that the advantage of ORR did not improve the mOS in the subgroup. In postsurgical recurrent or distant metastasis cases, the mOS of ICIs with or without radiotherapy was 12.4 months vs 6.5 months (HR 2.78, 95% CI: 1.37–5.59, p = .003) (), the mPFS was 6.3 months vs 3.6 months (HR 2.88, 95% CI: 1.58–5.25, p < .001) ().
Figure 4. Kaplan-Meier curves of ICIs plus chemo/target/vaccine with or without radiotherapy in the overall population. (a) Overall survival. (b) Progression-free survival.
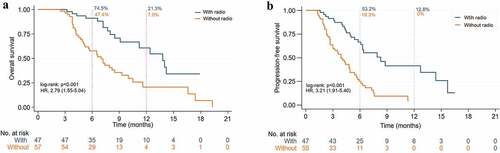
Figure 5. Kaplan-Meier curves of ICIs plus chemo/target/vaccine with or without radiotherapy in recurrent and metastasis pancreatic cancer patients. (A) Overall survival. (B) Progression-free survival.
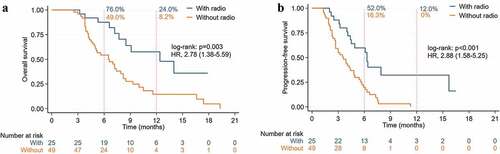
Table 3. Activity of immunotherapy in patients with APC in subgroup analysis.
Combine different drugs
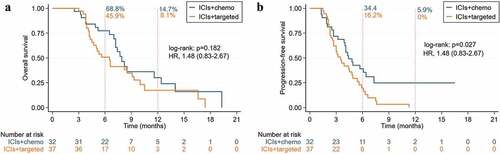
The mOS of ICIs combined chemotherapy compared to targeted therapy was 11.6 months vs 5.6 months (HR 2.35, 95% CI: 1.35–4.07, p = .002) (), the mPFS was 6.4 months vs 3.5 months (HR 3.03, 95% CI: 1.82–5.05, p < .001) (), ORR (45.9% vs 4.6%), DCR (95.1% vs 63.9%), 12-month OS rate (14.8% vs 8.6%) and PFS rate (8.5% vs 0%) (). In local advanced group, the mOS and mPFS were no statistical significance between combination chemotherapy or targeted therapy. In the recurrent or metastasis group, the mOS of ICIs combined chemotherapy compared to targeted therapy was 7.9 months vs 6.5 months (HR 1.48, 95% CI: 0.83–2.67, p = .182) (), the mPFS was 4.3 months vs 3.5 months (HR 1.48, 95% CI: 0.83–2.67, p = .027) (). Combination chemotherapy if compared to the neoantigen vaccine (mOS not research), neither combination chemotherapy (HR 0.69, 95% CI: 0.34–1.43, p = .280) nor mPFS (HR 1.03, 95% CI: 0.66–1.61, p = .897) were considered to be significantly different. ORR and DCR of neoantigen vaccine were 28.6% and 85.7%, respectively, whereas the 12-month OS rate and PFS rate was 28.6% and 16.3%, also respectively.
Different treatment lines
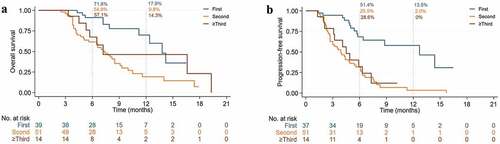
First-line treated patients’ mOS compared to second-line in 13.8 months vs 7.4 months (HR 3.46, 95% CI: 1.71–6.99, p < .001) (), whereas mPFS was 12.7 months vs 3.9 months (HR 4.33, 95% CI: 2.34–7.99, p < .001) (), the 12-month OS rate (17.9% vs 9.8%) and PFS rate (13.5% vs 2.0%). Compared to second-line, multi-line of mOS was 7.2 months (HR 1.42, 95% CI: 0.85–2.36, p = .275) and PFS was 4.5 months, which also proved not to be significantly different(HR 1.93, 95% CI: 1.28–2.92, p = .618), and the 12-month OS rate and PFS rate of multi-line was 14.3% and 0%, respectively.
PD-L1 expression and TMB
As shown in , the expression of the PD-L1 mOS was divided into a positive and a negative group, on which the former comprised 7.2 months against the 9.1 months of the latter. Likewise, shows the mPFS was also not significantly different (4.8 months vs 6.6 months). As per , the mOS was significantly higher in the TMB-H group than in the TMB-L (19.3 months vs 7.2 months) whereas proves the mPFS was not significantly different (7.3 months vs 4.3 months). The similar result was shown on the recurrent and metastasis patients (). While the tumor response of the PD-L1 expression and the TMB is shown in , the ORR of the PD-L1 positive group and TMB-H groups was 42.9% and 62.5%, respectively, and the DCR of all groups reached 100%.
Figure 9. Kaplan-Meier curves of ICIs with PD-L1 expression positive and negative in the overall population. (a) Overall survival. (b) Progression-free survival.
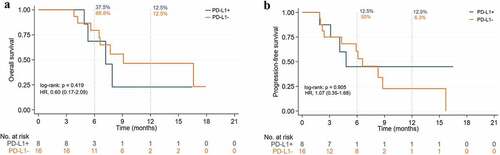
Figure 10. Kaplan-Meier curves of ICIs with TMB-H and TMB-L in the overall population. (a) Overall survival. (b) Progression-free survival.
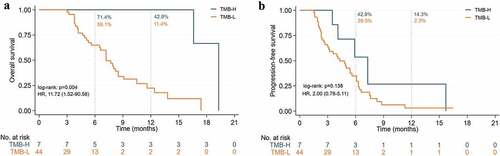
Figure 11. Kaplan-Meier curves of ICIs with TMB-H and TMB-L in recurrent and metastasis pancreatic cancer patients. (a) Overall survival. (b) Progression-free survival.
Table 4. Activity of immunotherapy in patients with APC in PD-L1 protein expression and TMB.
Safety
Of the 104 patients in the overall population, three patients (2.9%) discontinued ICIs therapy due to irAE, which included one patient with hepatitis, Rash and Herpes, respectively. One patient occurred treatment-related death due to Immune-related Jaundice. In total, 23 (22.1%) patients had irAE, among hyperthyroidism (4.8%), hypothyroidism (3.9%), herpes (4.8%), and rash (4.8%) were the highest incidence in all grades (). Eight patients had grade 3 irAE, including myocarditis (2.9%), hepatitis (1.9%) and herpes (1.9%). No patients had grade 4 irAE. There were 80 patients (76.9%) with hematologic toxicity, among 24 patients (22.0%) with grade 3–4 toxicity, the most commonly toxicity was myelosuppression and elevated transaminase, such as white blood cell count decreased (36.5%), neutropenia (36.5%), anemia (29.8%) and thrombocytopenia (42.3%), as well as increased alanine aminotransferase (31.7%) and increased aspartate aminotransferase (33.7%). The highest incidence in grade 3–4 hematologic toxicity was neutropenia (5.7%) and thrombocytopenia (5.8%), and no grade 5 toxicity occurred. Most of the blood system toxicity was be reversed by symptomatic treatment or stopped tumor treatment. Overall, combination therapy with ICIs is safely tolerated for APC.
Table 5. Treatment-related adverse events occurring in ≥1% of patients.
Discussion
While APC had low survival rates and showed a poor prognosis due to a low rate of early diagnosis, a high postsurgical recurrent rate, and limited treatment options, it becomes urgently necessary to explore better treatment methods. ICIs with a broad antitumor activity did achieve a promising efficacy in multiple solid tumors, but patients with pancreatic cancer failed to respond to ICIs, which is different from other tumors due to a low mutational burden and a low neoantigen-expressing.Citation10,Citation24,Citation34 Not only the effect on patients who had received single ICIs (PD-1/PDL-1/CTLA-4) or double ICIs (PDL-1 + CTLA-4) was limited, but the ORR also reached only 3.1% and, no patient reached CR.Citation16–18–Citation35 While chemotherapy was able to promote the release of tumor antigens and shows synergy with immunotherapy in other tumors,Citation36 tremelimumab with gemcitabine, or Pembrolizumab with gemcitabine/nab-paclitaxel of ORR were 10.5% and 18%, respectively, showing similar efficacy to exclusive administration of gemcitabine or nab-paclitaxel.Citation24,Citation26,Citation37 How to overcome the immunotherapy resistance of the pancreatic tumor immune microenvironment has, therefore, become the primary problem in the current treatment of pancreatic cancer. Pre-clinical studies were able to show that CXC chemokine receptor 4 (CXCR4) blockade promotes T cell tumor infiltration, and a phase II clinical trial of a CXCR4 antagonist BL-8040 (motixafortide) with pembrolizumab and chemotherapy showed an ORR of 32%, a DCR of 77% and a median duration of 7.8 months in pancreatic ductal adenocarcinoma.Citation22 This particular research has brought new hope for the importance of immunotherapy in pancreatic cancer, whereas radiotherapy is also capable of altering the composition of the tumor immune microenvironment, inducing low-mutation tumors to produce new antigenic targets, and producing a synergistic effect with immunotherapy.Citation27 Combined with immunotherapy, radiotherapy has achieved significant efficacy in other solid tumors, but needs to be verified by prospective studies focused specifically on pancreatic cancer.Citation30 Moreover, there was no standard treatment option after second-line progression, and there were limited treatment options for APC. However, most patients will experience, in real-life treatment, multiple progress as well as they will also need multi-line treatment.
In this real-world study, we retrospectively analyzed the efficacy and safety of the ICIs combination therapy in 104 patients with APC, to the best of our abilities, and our cohort was the largest to demonstrate ICIs efficacy in this relatively small area. While all patients received combined immunotherapy with more than two drugs, no patients were administered alone, with the combination therapy among them including traditional chemotherapy, targeted therapy, and neoantigen vaccines. The results were encouraging, especially with ICIs combined with radiotherapy, in which the patient’s survival time and disease response rate were better, which shows a surprising efficacy related to the possibility that small-dose radiotherapy may be a sensitizer for immunotherapy in pancreatic cancer, being able to convert “cold” tumors into “hot” tumors and subsequently bringing new hope for the struggles found in pancreatic cancer’s immunotherapy. In the subgroup analysis, the ICIs’ mOS, which was combined with targeting drugs (most antiangiogenic drugs) was only half that of conventional cytotoxic drugs, so pancreatic cancer may not be suitable for antiangiogenic drugs due to a poor blood supply. The vaccine has a long-lasting anti-tumor effect, as well as compared with the longest survival period of FOLFIRINOX, the mOS of the first-line treatment patients was prolonged by nearly 3 months (13.8 months vs 11.1 months), whereas the mPFS was doubled (12.7 months vs 6.4 months).Citation7 The ORR and DCR of the first-line treatment were 54.9% and 94.9%, respectively, which are encouraging compared to the current MPACT of 23% and 48%, also respectively, or FOLFIRINOX, which was 31.6% and 71.2%.Citation7,Citation37 Some patients (n = 17) with locally advanced treatment were planned to undergo surgical transformation, and four patients had a successful surgical resection at the end of the follow-up.
Compared with multi-line patients, first-line patients have better body tolerance and low tumor burden, and immunotherapy patients will benefit more. Compared with historical mOS, the second-line ICIs treatment’s mOS was 7.2 months and the only currently approved second-line treatment for metastatic pancreatic ductal adenocarcinoma mOS was 6.1 months (NOPALI-1 regimen).Citation38 In this study, a PFS of 3.9 months and DCR of 58.8% were also better, compared to mPFS of 3.1 months and DCR of 52%, but our ORR 13.5% was lower than the 17% of NOPALI-1, as most second-line patients used anti-angiogenic drugs, whose curative effect was not very satisfactory. The 7.2 months of mOS with multiline treatment was a good choice for patients with multiline progression in clinical treatment, and in general treatment of patients with APC, chemotherapy and radiotherapy can synergize the efficacy of immunotherapy, with patients with combined drugs benefitting significantly.
TMB is a potential biomarker associated with response to treatment with ICIs, but there are significant differences between cancer types, and until now there has been no clear consensus on the threshold of TMB in specific cancer types. From initial Foundation Medicine defines TMB-H as more than 20 mut/Mb,Citation39 later 16 to 12, until recently the FDA approved pembrolizumab for the treatment of unresectable or metastatic TMB-H solid tumors with TMB ≥10 mut/Mb.Citation40 The evidence for TMB is still weak for various reasons, and the evidence for TMB in pancreatic cancer is even less sparse. In pan-tumor species studies with TMB ≥10 mut/Mb,Citation41,Citation42 APC patients with TMB-H respond better to ICIs than TMB-L patients, but more studies should further confirm this conclusion because the proportion of APC patients is too low. In our study TMB was an independent prognostic factor, patients with TMB-H had an OS significantly better than patients with TMB-L. However, since this study is retrospective, more prospective studies are needed to verify whether TMB is a potential biomarker of ICIs in APC patients.
PD-L1 expression in some tumor types was also considered to be able to predict the efficacy of immunotherapy, whose value in pancreatic cancer remains to be further verified. This study indicated that patients with a positive PD-L1 expression have a worse survival rate, which was a factor of poor prognosis which may also be due to limitations regarding the sample size and difference from the PD-L1 detection reagent and method, which makes the research results different from other cancers. Likewise, while TMB was an independent prognostic factor, patients with TMB-H had an OS significantly better than patients with TMB-L. MSI-H pancreatic cancer patients benefit from immunotherapy, specifically, a patient with MSI-H (2.0%) in this study was still in CR at the end of the follow-up (the follow-up time was comprised of 24 weeks). From this point of view, TMB and MSI-H can be used as biomarkers for the benefit of immunotherapy for APC. The association between the efficacy of pancreatic cancer immunotherapy, PD-L1 expression and TMB also needs to be demonstrated by more clinical studies, to better guide the level of customization in the the clinical medication and realize the personalized precise treatment of pancreatic cancer patients.
This study brings new light on the importance of immunotherapy for patients with APC, and besides the efficacy being more beneficial than the existing regimen, its safety is also completely acceptable to anyone, as the occurrence of all adverse events was less than 10%, which is significantly reduced compared to the previous CTLA-4 combined or not with PD-L1. The regretful death of one patient was related to immune cholangitis.
Significance and limitations
To the best of our abilities, this is the largest study to date to explore the efficacy and the safety of ICIs in advanced pancreatic cancer (APC) patients, especially ICIs that were combined with radiotherapy, and the role of PD-L1 and TMB in the treatment of advanced pancreatic cancer. Additionally, our study not only shows that chemotherapy in patients with advanced pancreatic cancer is superior to patients on targeted therapy, but it also concluded the earlier the use of ICIs, the greater the benefit to the patient. However, this study has several limitations. While on one hand, this study was a retrospective study, which gives rise to a selection bias. On the other hand, it only includes Chinese patients, which makes additional research needed to verify whether our results are equally applicable to other racial groups. Finally, The radiotherapy dose was varied and limited, and more prospective studies need to be conducted to verify the efficacy, and the most appropriate radiotherapy dose and radiotherapy time.
Conclusion
Our real-world study was able to demonstrate that the combination treatment of ICIs is a safe and effective way to treat patients with APC. ICIs combined with dual chemotherapeutics with radiotherapy could significantly prolong the patients’ survival rate while also increase the tumor response rate. Both first-line and second-line treatments of ICIs performed better than the renowned studies MPACT and NOPALI-1. While, antiangiogenic drugs benefited less in patients with pancreatic cancer and were not recommended, traditional cytotoxic drugs were still the most effective therapeutic drugs. Patients with TMB-H and MSI-H had a higher likelihood of response to immunotherapy, whereas patients with PD-L1 may be related to a poor prognosis, which beholds a guiding significance in the clinical treatment of patients with APC while providing direction for APC’s future immunotherapy.
Authors contributions
Xiaoling Gong, Yahui Zhu and Qianning Zhang contributed equally to this work. Xiaoling Gong, Yahui Zhu, Qianning Zhang, Xin Qiu, Changchang Lu, Fan Tong, Qiaoli Wang, Weiwei Kong, Haihui Zhou, Brorui Liu, Yujie Zhou and Juan Du conceived, wrote and edited the manuscript. All authors read and approved the final version of the manuscript.
Consent for publication
All patient (or that person’s parent or legal guardian) had given informed consent to collect their clinical data for the research.
Supplemental Material
Download PDF (150.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2143154.
Additional information
Funding
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):1–12. doi:10.3322/caac.21654.
- Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395(10242):2008–20. doi:10.1016/s0140-6736(20)30974-0.
- Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7(4):e1000267. doi:10.1371/journal.pmed.1000267. PMID:PMC2857873.
- Shrikhande SV, Kleeff J, Reiser C, Weitz J, Hinz U, Esposito I, Schmidt J, Friess H, Buchler MW. Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2007;14(1):118–27. doi:10.1245/s10434-006-9131-8.
- Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, Carter R, Tebbutt NC, Dervenis C, Smith D, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308(2):147–56. doi:10.1001/jama.2012.7352.
- Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, Scheithauer W, Siena S, Tabernero J, Teixeira L, et al. Nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107(2):dju413. doi:10.1093/jnci/dju413.
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul J-L, Gourgou-Bourgade S, de la Fouchardière C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25. doi:10.1056/NEJMoa1011923.
- Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17(6):717–26. doi:10.1016/s1470-2045(16)00175-3.
- Heery CR, O’Sullivan-Coyne G, Madan RA, Cordes L, Rajan A, Rauckhorst M, Lamping E, Oyelakin I, Marté JL, Lepone LM, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN solid tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017;18(5):587–98. doi:10.1016/s1470-2045(17)30239-5.
- Herzberg B, Campo MJ, Gainor JF. Immune checkpoint inhibitors in non-small cell lung cancer. Oncologist. 2017;22(1):81–88. doi:10.1634/theoncologist.2016-0189. PMID:PMC5313266.
- Cloyd JM, Katz MHG, Wang H, Cuddy A, You YN. Clinical and genetic implications of DNA mismatch repair deficiency in patients with pancreatic ductal adenocarcinoma. JAMA Surg. 2017;152(11):1086–88. doi:10.1001/jamasurg.2017.2631. PMID:PMC5831418.
- Grant RC, Denroche R, Jang GH, Nowak KM, Zhang A, Borgida A, Holter S, Topham JT, Wilson J, Dodd A, et al. Clinical and genomic characterisation of mismatch repair deficient pancreatic adenocarcinoma. Gut. 2021;70(10):1894–903. doi:10.1136/gutjnl-2020-320730.
- Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science (New York, N Y). 2017;357(6349):409–13. doi:10.1126/science.aan6733.
- Zhou W, Zhou Y, Chen X, Ning T, Chen H, Guo Q, Zhang Y, Liu P, Zhang Y, Li C, et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021;268:120546. doi:10.1016/j.biomaterials.2020.120546.
- Patnaik A, Kang SP, Rasco D, Papadopoulos KP, Elassaiss-Schaap J, Beeram M, Drengler R, Chen C, Smith L, Espino G, et al. Phase I study of pembrolizumab (MK-3475; anti–PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res. 2015;21(19):4286–93. doi:10.1158/1078-0432.CCR-14-2607.
- Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi:10.1056/NEJMoa1200694.
- O’Reilly EM, Oh DY, Dhani N, Renouf DJ, Lee MA, Sun W, Fisher G, Hezel A, Chang SC, Vlahovic G, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5(10):1431–38. doi:10.1001/jamaoncol.2019.1588. PMID:PMC6647002.
- Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I, et al. Phase 2 trial of single agent ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33(8):828–33. doi:10.1097/CJI.0b013e3181eec14c.
- Ott PA, Bang Y-J, Piha-Paul SA, Razak ARA, Bennouna J, Soria J-C, Rugo HS, Cohen RB, O’Neil BH, Mehnert JM, et al. T-cell–Inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol. 2018;37(4):318–27. doi:10.1200/JCO.2018.78.2276.
- Gong J, Hendifar A, Tuli R, Chuang J, Cho M, Chung V, Li D, Salgia R. Combination systemic therapies with immune checkpoint inhibitors in pancreatic cancer: overcoming resistance to single-agent checkpoint blockade. Clin Transl Med. 2018;7(1):32. doi:10.1186/s40169-018-0210-9. PMID:PMC6174117.
- Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8(3):151–60. doi:10.1038/nrclinonc.2010.223.
- Bockorny B, Semenisty V, Macarulla T, Borazanci E, Wolpin BM, Stemmer SM, Golan T, Geva R, Borad MJ, Pedersen KS, et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat Med. 2020;26(6):878–85. doi:10.1038/s41591-020-0880-x.
- Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39(1):74–88. doi:10.1016/j.immuni.2013.06.014.
- Weiss GJ, Waypa J, Blaydorn L, Coats J, McGahey K, Sangal A, Niu J, Lynch CA, Farley JH, Khemka V. A phase Ib study of pembrolizumab plus chemotherapy in patients with advanced cancer (PembroPlus). Br J Cancer. 2017;117(1):33–40. doi:10.1038/bjc.2017.145. PMID:PMC5520208.
- Weiss GJ, Blaydorn L, Beck J, Bornemann-Kolatzki K, Urnovitz H, Schutz E, Khemka V. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. N Engl J Med. 2018;36(1):96–102. doi:10.1007/s10637-017-0525-1.
- Kamath SD, Kalyan A, Kircher S, Nimeiri H, Fought AJ, Benson A 3rd, Mulcahy M. Ipilimumab and gemcitabine for advanced pancreatic cancer: a phase Ib study. Oncologist. 2020;25(5):e808–15. doi:10.1634/theoncologist.2019-0473. PMID:PMC7216436.
- Lussier DM, Alspach E, Ward JP, Miceli AP, Runci D, White JM, Mpoy C, Arthur CD, Kohlmiller HN, Jacks T, et al. Radiation-induced neoantigens broaden the immunotherapeutic window of cancers with low mutational loads. Proc Natl Acad Sci U S A. 2021;118(24). doi: 10.1073/pnas.2102611118. PMID:PMC8214694.
- Dyer BA, Feng CH, Eskander R, Sharabi AB, Mell LK, McHale M, Mayadev JS. Current status of clinical trials for cervical and uterine cancer using immunotherapy combined with radiation. Int J Radiat Oncol Biol Phys. 2021;109(2):396–412. doi:10.1016/j.ijrobp.2020.09.016.
- Pakkala S, Higgins K, Chen Z, Sica G, Steuer C, Zhang C, Zhang G, Wang S, Hossain MS, Nazha B, et al. Durvalumab and tremelimumab with or without stereotactic body radiation therapy in relapsed small cell lung cancer: a randomized phase II study. J Immunother Cancer. 2020;8(2):e001302. doi:10.1136/jitc-2020-001302. PMID:PMC7754662.
- Ho AY, Barker CA, Arnold BB, Powell SN, Hu ZI, Gucalp A, Lebron-Zapata L, Wen HY, Kallman C, D’Agnolo A, et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer. 2020;126(4):850–60. doi:10.1002/cncr.32599.
- Lee YH, Tai D, Yip C, Choo SP, Chew V. Combinational Immunotherapy for hepatocellular carcinoma: radiotherapy, immune checkpoint blockade and beyond. Front Immunol. 2020;11:568759. doi:10.3389/fimmu.2020.568759. PMID:PMC7561368.
- Chung V, Kos FJ, Hardwick N, Yuan Y, Chao J, Li D, Waisman J, Li M, Zurcher K, Frankel P, et al. Evaluation of safety and efficacy of p53mva vaccine combined with pembrolizumab in patients with advanced solid cancers. Clin Transl Oncol. 2019;21(3):363–72. doi:10.1007/s12094-018-1932-2.
- Bassani-Sternberg M, Digklia A, Huber F, Wagner D, Sempoux C, Stevenson BJ, Thierry AC, Michaux J, Pak H, Racle J, et al. A phase Ib study of the combination of personalized autologous dendritic cell vaccine, aspirin, and standard of care adjuvant chemotherapy followed by nivolumab for resected pancreatic adenocarcinoma-a proof of antigen discovery feasibility in three patients. Front Immunol. 2019;10:1832. doi:10.3389/fimmu.2019.01832. PMID:PMC6694698.
- Knudsen ES, Vail P, Balaji U, Ngo H, Botros IW, Makarov V, Riaz N, Balachandran V, Leach S, Thompson DM, et al. Stratification of pancreatic ductal adenocarcinoma: combinatorial genetic, stromal, and immunologic markers. Clin Cancer Res. 2017;23(15):4429–40. doi:10.1158/1078-0432.CCR-17-0162. PMID:PMC5951386.
- Ko AH. Progress in the treatment of metastatic pancreatic cancer and the search for next opportunities. J Clin Oncol. 2015;33(16):1779–86. doi:10.1200/JCO.2014.59.7625.
- Reck M, Rodríguez–abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non–Small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37(7):537–46. doi:10.1200/JCO.18.00149.
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703. doi:10.1056/NEJMoa1304369. PMID:Pmc4631139.
- Wang-Gillam A, C-P L, Bodoky G, Dean A, Shan Y-S, Jameson G, Macarulla T, Lee K-H, Cunningham D, Blanc JF, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387(10018):545–57. doi:10.1016/s0140-6736(15)00986-1.
- Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. doi:10.1186/s13073-017-0424-2. PMID:PMC5395719.
- Food U, Administration D. D FDA approves pembrolizumab for adults and children with TMB-H solid tumors. US US Food and Drug Administration; 2020.
- Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin JA, Miller WH, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–65. doi:10.1016/s1470-2045(20)30445-9.
- Valero C, Lee M, Hoen D, Zehir A, Berger MF, Seshan VE, Chan TA, Morris LGT. Response rates to anti-PD-1 immunotherapy in microsatellite-stable solid tumors with 10 or more mutations per megabase. JAMA Oncol. 2021;7(5):739–43. doi:10.1001/jamaoncol.2020.7684. PMID:PMC7893543.