ABSTRACT
Colorectal cancer (CRC) is one of the leading malignancies that causes death worldwide. Cancer vaccines and oncolytic immunotherapy bring new hope for patients with advanced CRC. The capability of vaccinia virus (VV) in carrying foreign genes as antigens or immunostimulatory factors has been demonstrated in animal models. VV of Wyeth, Western Reserve, Lister, Tian Tan, and Copenhagen strains have been engineered for the induction of antitumor response in multiple cancers. This paper summarized the preclinical and clinical application and development of VV serving as cancer vaccines and oncolytic vectors in CRC treatment. Additionally, the remaining challenges and future direction are also discussed.
Introduction
Colorectal cancer (CRC) is the third common cancer and second common cause of cancer death in the world. There are more than 1.9 million new cases and 935,000 deaths caused by CRC in 2020.Citation1 Incidence rates of CRC are the highest in European regions, Australia/New Zealand, and Northern America and low in most regions of Africa and in South Central Asia.Citation1 A lot of risk factors including family history,Citation2 colitis,Citation3 smoking,Citation4 alcohol drinking,Citation5 obesity, and diabetesCitation6 have been identified for CRC. The 5-year relative survival is reported over 65% in Northern America and European countries, while it is much lower (less than 50%) in developing countries.Citation7 However, the 5-year survival for metastatic CRC is only 12.5%. Endoscopic resection, surgery, radiotherapy, and systemic therapy have been employed for the treatment of CRC. Antibodies of vascular endothelial growth factor, such as bevacizumab and aflibercept, and epidermal growth factor receptor, such as cetuximab and panitumumab, have been developed for treating metastatic CRC.Citation8 Nevertheless, novel agents are still in need as cancer relapse soon after chemotherapy.
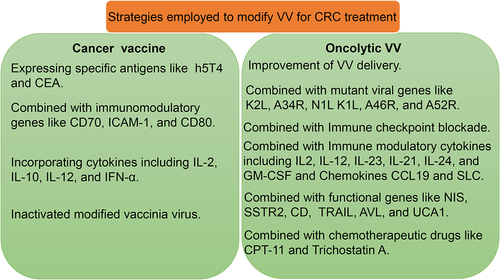
Vaccinia virus (VV) is one extensively studied member of poxvirus family and belongs to the orthopoxvirus genus of the Chordopoxvirinae subfamily. The genomic DNA of VV is double-stranded and encodes more than 200 genes, which enable virus infection, replication, and immune evasion in the host.Citation9 VV replicates from the expression of viral gene, dissolution of virion core, replication of genome and then virion assembly. Mature virus (MV) exists in the host cytoplasm and enveloped virus (EV) spreads outside. The size of MV is about 270 × 350 nm. The large genome (about 190 kb) of VV potentiates the insertion of multiple immunostimulatory transgenes and the ability to tune tumor microenvironment for tumor immunotherapy.Citation10 Furthermore, VV can broadly infect many laboratory animals and is suitable for preclinical investigation of VV in laboratory animal models.Citation11 Several strains of VVs have been employed for preclinical and clinical research in battling human cancers. These are strains named Wyeth, Western Reserve, Lister, Tian Tan, and Copenhagen strains.Citation12 Here, we will review the application and development of VV in the treatment of CRC based on both preclinical and clinical aspects. Various strategies have been employed to modify VV for CRC treatment (). In addition, the remaining challenges and future direction also will be discussed in this study.
Preclinical investigation of oncolytic VV in CRC
VV as a cancer vaccine
At present, strategies commonly employed to develop CRC vaccine include dendritic cell (DC), peptide, tumor cell, and viral vaccines, which have been detailly summarized by Berry J et al.Citation13 Multiple viruses can be engineered to deliver tumor antigens to activate immune response, such as mammalian poxviruses (VV and modified virus Ankara), avian poxviruses, adenovirus, alphavirus, measles virus, herpes simplex virus, and vesicular stomatitis virus.Citation14 What is noteworthy, the capability of VV platform for delivering tumor antigens and costimulatory molecules to enhance immunogenicity has been extensively demonstrated (). Studies have validated the antitumor effect of VV expressing human 5T4 gene or carcinoembryonic antigen (CEA) in CRC. Human 5T4 gene, also called trophoblast glycoprotein, locates at 6q14.1 and expresses a 72 kDa, heavily N-glycosylated protein. 5T4 is highly expressed on human trophoblast cells and most tumors but rarely on normal tissues.Citation24,Citation25 In CRC, immunization with VV (TroVax) expressing h5T4 exerted antitumor effect in a CD4+-dependent manner.Citation26 Indeed, VV expressing human and mouse 5T4 triggered protective immunity for mice implanted with colon or melanoma tumor cells.Citation27 CEA subgroup contains seven members which express predominantly on cell membrane.Citation28 CEA has been identified as an important marker for CRC and other malignancies for more than 50 years.Citation29 The immunization of VV expressing human CEA through mucosa induced mucosal and systemic CEA-specific antibody titers and CD4+ and CD8+ T cell responses. The immune response was associated with tumor regression in the mouse models implanted with CEA-expressing colon cancer cells.Citation15 During the development of vaccine therapy, researchers have noted that tumor-mediated immune suppression remains a major challenge. Thus, the development of critical strategies is needed to combine other forms of immunotherapy.
Table 1. Recombinant VVs in cancer vaccine for colon cancer.
Immunomodulatory genes have been implicated in the improvement of VV immunogenicity. The T cell costimulatory molecule CD70 is a type II transmembrane glycoprotein and composed of 193 amino acids. CD70 acts as a receptor of CD27 and the interaction between them leads to the activation of TNF receptor-associated factors (TRAFs) in lymphocytes. CD70-CD27 axis induces proliferation and cytokine production of T lymphocytes, which are critical for tumor immunity.Citation30 Infection of VV encoding human CD70 (rV-CD70) in CD70-negative murine colon tumor cells was able to provoke a polyclonal response of murine T cells via CD27.Citation16 The combination of rV-CD70 and virus expressing carcinoembryonic antigen (rV-CEA) could constrain tumor growth of colon cancers.Citation16 Intercellular adhesion molecule-1 (ICAM-1) is a glycoprotein expressed on cell membrane of lymphocytes, endothelial cells, and epithelial cells at a low level. ICAM-1 is implicated in leukocyte migration along the vessel wall and across the endothelial layer. As well, the expression of ICAM-1 on lymphocytes facilitates antigen presentation by the augment of cell–cell interaction. The delivering of ICAM-1 on ICAM-1-negative murine colon carcinomas by VV prevented tumor formation in mouse models, with elevation of cytokines production and cytotoxic T lymphocytes activity.Citation17 CD70 and ICAM-1 can interact with different receptors on T lymphocytes and facilitate T cell activation. The combination of these costimulatory molecules may improve the efficacy of VV vaccine in the clinical setting.
Evidence has shown that the combination of cytokines and VV vectors could synergistically suppress colon cancer growth. Cytokines including IL-2, IL-10, IL-12, and IFN-α play essential roles in modulating antitumor response triggered by VV vectors. In one previous study, colon cancer cells were infected with VV encoding the murine T cell co-stimulatory gene B7.1 (CD 80) (NYVAC-B7.1) and the murine interleukin-2 gene (NYVAC-IL-2).Citation18 Immunization of these cancer cells helped to contain tumor development in mouse model.Citation18 Although the levels of anti-vaccinia antibody titer and natural killer activity were not influenced by IL-10 administration, IL-10 did enhance vaccinia-specific cytotoxic T-lymphocyte activity.Citation19 IL-12 could prolong survival of tumor-bearing mice when treated with VV expressing B7-1 and β-galactosidase (β-gal).Citation20 And CD4+ and CD8+ T cells were required for a superior antitumor effect.Citation20 Another pleiotropic cytokine IFN-α plays an essential role in innate and adaptive immune response. The combination of IFN-α and CEA containing VV suppressed tumor growth, improved survival, and seduced CEA-specific CTL response in model mice.Citation21 The inhibition of VV by IFN-α could be avoided by a distant site of vaccination.Citation21 The cooperation between cytokines and VV enhanced antitumor response in vivo, which might represent a potential strategy for colon cancer therapy.
The cGAS/STING pathway plays a central role for the induction of antitumor immunity, as STING- or Batf3-deficiency resulted in a less effective response.Citation31 Heat inactivated modified VV Ankara (iMVA) induced higher type I IFN production than MVA depending on cGAS/STING pathway in conventional dendritic cells.Citation22 Treatment with heat-iMVA via intratumoral injection stimulated antitumor immune response in murine melanoma and colon cancer models.Citation22 The combination of heat-iMVA and immune checkpoint blockade provided synergistic antitumor therapeutic effects.Citation22 These findings provided novel insights for employing innate immune response to treat colon cancer.
Additionally, the influence of vaccination methods of VV vaccines on antitumor activity has been studied. As expected, intratumoral vaccination induced superior antitumor effect compared with systemic manner.Citation23 Compared with single systemic or intratumoral injection, the sequential regime by systemic vaccination and then intratumoral vaccination led to a further elevation, with the boost of long-term immunological memory.Citation23 Nonetheless, the exploration on how to improve the efficacy of systemic vaccination of VV will be on the way, as colon cancer and many other solid cancers are not clinically suitable for intratumoral vaccination.
Oncolytic VV
Since the employment of VV as oncolytic virus from 1999, researchers have endeavored to modified various oncolytic VV for preclinical and clinical research.Citation32 Till now, several strategies have been put forward to further enhance antitumor effects of oncolytic VV in CRC ().
Table 2. Recombinant VV in oncolytic research for colon cancer.
Refinement of VV delivery in vivo
The delivery and tumor localization of VV may be an essential factor that contributes to its oncolytic activity. Ferguson MS et al.Citation33 found that macrophage was one barrier for VV systemic delivery. Transient inhibition of phosphoinositide 3-kinase δ suppressed the attachment of VV to macrophage and increased tumor localization of VV as well as antitumor efficacy by promoting T cell infiltration and immune response. Moreover, tumor vasculature also plays a role in VV delivery and distribution. The controlling of colorectal peritoneal carcinomatosis by vvDD-SR-RFP was correlated with tumor vasculature formation.Citation34 Other researchers chose to directly modify VV to facilitate virus delivery. For instance, Badrinath N et al.Citation35 coated a cancer-favoring VV with a Poly lactide-co-glycolic acid (PLGA) nanofiber (CVV-PLGA). The superior antitumor activity of CVV-PLGA over CVV without PLGA was demonstrated in murine CRC models. These results suggested that inhibition of premature clearance of VV supported therapeutic efficacy enhancement in tumor-bearing models. Nonetheless, the clinical feasibility of these modified VV in human needs to be further studied.
Employment of mutant viral gene
K2 L gene of VV encodes a protein named serine protease inhibitor (SPI-3).Citation65 SPI-3 suppresses cell–cell fusion via the conjugation with the A56 polypeptide and inhibition of viral entry-fusion complex. The recombinant VV, FUVAC obtained a nonsense mutation in K2 L in addition to the deletion of VV growth factor (VGF) and O1 L (MDRVV). The mutation enhanced viral replication ability and cytotoxic effect in cancer cells. FUVAC showed antitumor effect in a CD8+ T-cell-dependent manner and also inhibited infiltration of tumor-associated immune suppressive cells.Citation36 These results suggested that cell fusion is involved in oncolytic and antitumor effect of VV. A34 R is an EV glycoprotein of VV and involved in cell-to-cell transmission.Citation66 Researchers have noticed that mutation of A34 R promoted viral spread and replication in mouse model bearing MC38 colon cancer.Citation38 Their results stressed on the improvement of viral spread for a higher efficacy in tumor cell elimination. N1 L is a conserved VV gene that encodes a protein of 14 kDa and is critical in the virus life-cycle.Citation67 N1 L protein could suppress the expression of TNF-α, IL-1β, IFN-α, IFN-β, and IL-10.Citation68 K1 L acts as an antagonist of type I IFN and inhibits antiviral effectors.Citation69 K1 L also inhibited NF-κB activation by preventing IκBα degradation.Citation70 K3 L can constrain the growth-inhibitory effects of PKR by preventing autophosphorylation of PKR and phosphorylation by eIF2a.Citation71 Toll-like-interleukin-1 resistance (TIR) domain in A46 R facilitates its interaction with myeloid differentiation factor 88 (MyD88), MyD88 adaptor-like, TIR domain-containing adaptor inducing IFN-beta (TRIF), and the TRIF-related adaptor molecule and inactivation of mitogen-activated protein kinases and NF-κB.Citation72 A52 R also contains TIR domain and potently blocks NF-κB activation induced by IL-1 and TLR4.Citation73 Ho TY et al.Citation37 tested a panel of VV with a deletion of immunomodulatory genes like N1 L, K1 L, K3 L, A46 R, or A52 R in treating colon and ovarian cancer. The mutation of K1 L, A46 R, and A52 R potentiated the ability of VV in prolonging survival and immunomodulation in animal models.Citation37 The deletion of some immunomodulatory genes in VV might be a promising way for the enhancement of VV potency in cancer therapy.
Combination with immune checkpoint blockade
T-lymphocyte-associated antigen 4 (CTLA4) binds with ligands on antigen-presenting cells (APCs) to inhibit T-cell responses and plays roles in immunosuppression. VV encodes a M2 protein that binds with CD80 and CD86 and blocks their interaction with soluble CD28 and CTLA4. The expression of M2 protein resulted in the inhibition of the host immune response.Citation74 Notably, anti-CTLA4 antibody was found to hamper VV replication in murine tumor models. An optimized combination of VV and A TLA4 antibody promoted systemic and tumor-specific immune response.Citation39 In the tumor microenvironment, the programmed cell death protein 1 (PD1or PDCD1)–PD1 ligand 1 (PDL1) receptor–ligand pair is expressed by cancer cells to evade immune attack. VV infection upregulated PD-L1 expression in colon and ovarian cancer cells and tumor tissues. Combination of PD-L1 blockade and VV exerted antitumor effect in synergy and relied on CD4 and CD8 T cells and IFN-γ.Citation40 The combination of cancer favoring VV (CVV) and anti-PD-1 antibody prolonged survival in murine models. The antitumor effect was linked with increased CD8+ PD-1+ T-cell infiltration in the tumor.Citation41 These results revealed that blockade of immune checkpoint could be harnessed for the improvement of therapeutic effects of VV.
Combination with immune modulatory cytokines
The deletion of thymidine kinase (TK) and VGF genes has been reported to enhance tumor selectivity and diminished toxicity. For instance, the treatment of cancer-favoring oncolytic vaccinia virus (CVV) with a disruption of TK gene overcame chemoresistance in stem cell-like CRC cells.Citation42 CVV synergistically repressed tumor growth in tumor-bearing mouse models.Citation42 Further, researchers have found that insertion of exogenous genes into TK gene can enhance VV therapeutic effects in CRC. These exogenous genes, especially immune modulatory cytokines and chemokines, could synergistically improve oncolytic effect of VV.
vvDD-mIL2 is an OV with the deletion of TK and vaccinia growth factor and insertion of murine IL2.Citation43 The combination of vvDD-mIL2 with TLR9 ligand increased CD8+ T cell/regulatory T cell (Treg) ratio and enhanced CD11c+ cells infiltration in the tumor microenvironment.Citation43 Depletion of macrophage and blockade of PD-1 further reduced the tumor burden.Citation43 Ge Y et al.Citation44 remolded the genome of vvDD to express membrane-bound IL-12 (vvDD-IL-12-FG, and vvDD-IL-12-RG) in target tumor cells. Tethered IL-12 could be maintained in the TME without adverse effects on lung, kidney, and liver.Citation44 The treatment induced infiltration of activated CD4+ and CD8+ T cells and reduced infiltration of Tregs, granulocytic myeloid-derived suppressor cells, and exhausted CD8+T cells.Citation44 vvDD-IL-12-FG treatment elicited its antitumor effect depending on IFN-γ and CD8+ T cells.Citation44 IL-23 is a cytokine belonging to IL-12 family and formed by the pairing of IL-12p40 and IL-23p19 or IL-23A subunits. IL-23 expressing-VV curtained tumor growth in multiple tumor models depending on CD8+ and CD4+ T cells and IFN-γ. Notably, both secreted and membrane-bound IL-23 functioned as useful antitumor cytokine when delivering with VV. However, IL-23A, a potent tumor promoter, could not be delivered to the tumor tissues due to tristetraprolin expression.Citation45 VVLΔTKΔN1 L-mIL-21 was modified by the deletion TK and N1 L gene and insertion of mouse IL-21. The modification further enhanced virus safety and antitumor immune response. VVLΔTKΔN1 L-mIL-21 controlled tumor growth in murine colon cancer tumor models through CD8+ T cells and stimulating memory T cell formation.Citation46 IL-24, a broad-spectrum tumor suppressor, has been inserted into the TK locus of Tian Tan VV (VG9-IL-24).Citation47 VG9-IL-24 induced apoptosis of CRC cells by stimulating PKR and MAPK signaling activation and compressing STAT3 phosphorylation.Citation47 VG9-IL-24 also induced tumor-specific immune response in murine models.Citation47 Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a multifunctional cytokine regulating immune response including the hematopoiesis and the development of immature or mature myeloid cells.Citation75 GM-CSF play a role in the differentiation, maturation, and migration of DCs, thus potentially strengthening antitumor response. Recombinant VV mJX-594 (JX) encoded murine GM-CSF driven by p7.5 promoter.Citation48 JX treatment reduced tumor angiogenesis and enhanced the infiltration of CD11c+ DCs and CD8+ T cells into tumor nodules.Citation48 JX cooperated with anti-PD-1 antibody to improve immune response and eliminated peritoneal metastases of colon cancer.Citation48 The regimens employed in these studies took advantage of cytokines that could modulate tumor immune microenvironment and thus stimulate immune-mediated responses in tumors.
Chemokine CCL19 is abundantly expressed in lymphoid organs and modulates the activation of immune cells like lymphocytes and DCs through its receptor CCR7.Citation76 CCL19 treatment contributed to the infiltration of CD4+ and CD8+ T cells and DCs into the tumor tissues and exerted antitumor effect.Citation77 Likewise, recombinant mouse CCL19 suppressed tumor growth and improved overall survival (OS) of mice with CRC implantation by increasing IFN-γ and IL-12 expression.Citation78 vvCCL19 expressing the chemokine CCL19 elevated the infiltration of T cell and dendritic cell into murine colon cancer tissues. The combination of vvCCL19 and cytokine-induced killer cells expressing CCR7, the receptor for CCL19, could enhance antitumor activity.Citation49 Secondary lymphoid chemokine (SLC) could help the co-localization of dendritic cell and naive T cells and facilitate immune response. Local injection of VV containing SLC improved the infiltration of CD4 T cells and suppressed tumor growth.Citation50 Colon cancer cells infected with VV expressing CD40 L obtained highly expression of CD40 L and stimulated IL-12 secretion from DC.Citation51 The infected cancer cells also incurred proliferation of B cells and IFN-γ production by T cells and NK/NKT cells.Citation51
Combination with other functional genes
The sodium/iodide symporter (NIS or SLC5A5) primarily functions as a membrane protein for the uptake of iodide into thyroid follicular cells. NIS gene has been employed for the radiotreatment and imaging of nonthyroidal tumors.Citation79 In a study, NIS gene was incorporated into VV, which could infect, replicate in, and kill colon cancer cells. Interestingly, functional NIS enabled imaging of tumor cells by the uptake of radioisotope.Citation52 The recombinant VV GLV-1h151 was modified by deletion of thymidine kinase gene. GLV-1h151 could selectively infect, replicate in, and kill colorectal and other tumor cells in vitro and in vivo.Citation53 Eveno C et al.Citation54 established orthotopic colorectal peritoneal carcinomatosis xenograft models and intraperitoneally treated using GLV-1h153. The expression of NIS in tumor tissues facilitated monitoring by computed tomography to confirm the effect of GLV-1h153.Citation54 Another functional gene, human somatostatin receptor type 2 (SSTR2), can also be applied for tumor imaging. SSTR2 binds with pentetreotide, a synthetic peptide used for receptor imaging after being radiolabeled with indium-111.Citation55 SSTR2 has been inserted into oncolytic VV genome and is feasible for colon cancer imaging in a mouse model.Citation55
Cytosine deaminase (CD) is expressed by Escherichia coli and yeast and can deaminate 5-fluorocytosine to 5-fluorouracil for antitumor purpose. Researchers have taken advantage of the property of CD gene to generate recombinant VV with new application. For instance, VG9-CD was modified with TK deletion and yeast CD insertion.Citation56 The combination of VG9-CD and 5-FC improved antitumor activity of VG9-CD.Citation56 However, their results showed that VG9-TK- without CD expression showed superior effects in prolonging survival, for CD may further increased cytotoxicity.Citation56 Notably, when VVCD was given at high MOI (higher than 0.1), it could alone kill most tumor cells,Citation57 whereas when it was given at low MOIs, only the effect of 5-FC was seen.Citation57 Moreover, fusion suicide gene FCU1 was designed by the combination of the yeast CD gene and uracil phosphoribosyltransferase gene.Citation58 The incorporation of FCU1 into TK-deleted VV (VV-FCU1) enhanced tumor regression ability in metastatic colon cancer.Citation58
A membrane-bound tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) was inserted into VV genome (vvTRAIL). vvTRAIL infection enhanced TRAIL expression and cytotoxic potency. The combination of vvTRAIL and oxaliplatin exhibited synergistic or additive antitumor activity and prolonged the survival of the tumor-bearing mice.Citation59 In another study, both angiopoietin 1 (Ang1) and TRAIL were inserted into the VGF and TK region of VV genome.Citation60 The novel VV induced cancer cell apoptosis and provoked antitumor immunity in murine models.Citation60 These results suggested that TRAIL signaling activation might be useful during VV therapy.
Aphrocallistes vastus lectin (AVL) is a C-type lectin isolated from Aphrocallistes vastus.Citation80 OncoVV-AVL expressing AVL was constructed and elicited antitumor activity in mice bearing colon and liver cancers.Citation61 AVL enhanced virus replication in cancer cells by activating ERK pathway.Citation61 Long noncoding RNAs (lncRNAs) serve as competing endogenous RNAs (ceRNAs) and modulate expression of downstream genes by competing for shared miRNAs. UCA1 is a lncRNA expressed highly in multiple cancers and involved in carcinogenesis.Citation81 In human CRC cells, UCA1 was demonstrated to enhanced OVV cell-to-cell spread via activating Cdc42 expression, a process mediated by miR-18a and miR-182. The results validated the potential value of lncRNAs in VV therapy.Citation62
Combination with chemotherapeutic drugs
Irinotecan (also called CPT-11), an inhibitor targeting DNA topoisomerase I, and its derivatives have been largely used in regimen, like FOLFIRI and FOLFIRINOX, to treat solid cancers like CRC. Oncolytic vvDD synergized with CPT-11 in decreasing CRC cell viabilities and improving survival in tumor-bearing models. Although SN-38, the active metabolite of CPT-11, restricted virus replication, the combination therapy raised apoptotic levels and immune cell infiltration in tumors.Citation63 Trichostatin A (TSA), a histone deacetylase inhibitor, promoted VV replication and spread and enhanced antitumor activity. The combination of TSA and VV could prolong survival of murine colon cancer models.Citation64 These results highlighted the effect of the combination of chemotherapeutic drugs and VV in tumor therapy.
Clinical trials of oncolytic VV in CRC
The evaluation of VV in CRC therapy has been carried out by multiple groups ( and ). The poxviral-based vaccine BN-CV301 contains recombinant vaccinia Ankara (MVA-BN-CV301) and recombinant fowlpox. Transgenes including MUC1, CEA, B7.1, ICAM-1, and LFA-3 were incorporated into the recombinant viruses.Citation82 Gatti-Mays M et al.Citation82 conducted a phase I, dose-escalation trial to evaluate the safety and efficacy of BN-CV301 in patients with CRC and other malignancies. The vaccine stimulated MUC1- and CEA-specific T cells in patients and prolonged stable disease (SD) in several patients, especially in KRAS-mutant gastrointestinal tumors. The median progression-free survival (PFS) was 15 weeks (range: 6 to ongoing at 82 weeks). However, most patients (9/12) eventually had disease progression, in spite of one patient with the unconfirmed partial response (PR). In addition, BN-CV301 in combination with anti-PD-L1 antibody prolonged SD in patients with KRAS-mutant CRC. CRC is characterized by high level of 5T4 expression, which has been an attractive target for cancer immunotherapy. The injection of MVA-5T4 increased anti-5T4 responses in patients with mCRC, while cyclophosphamide exposure led to depletion of regulatory T cells.Citation87 Both cyclophosphamide and MVA-5T4 treatment improved PFS compared with no treatment.Citation87 However, the combination treatment by cyclophosphamide and MVA-5T4 did not received further improvement.Citation87 Cyclophosphamide depleted regulatory T cells in 24 of 27 patients with MVA-5T4 treatment and independently prolonged PFS (5.0 vs 2.5 months; hazard ratio [HR] = 0.48; P = .09). MVA-5T4 doubled baseline anti-5T4 responses in 16 of 35 patients and significantly prolonged PFS (5.6 vs 2.4 months; HR = 0.21; P < .001) and OS (20.0 vs 10.3 months; HR = 0.32; P = .008).Citation87 Vaccination of another 5T4 expressing VV-TroVax in mCRC patients obviously induced 5T4-specific and MVA-specific antibody responses.Citation88 5T4-specific but not MVA-specific antibody positively correlated with time to progression.Citation88 Five out of 17 patients showed periods of disease stabilization ranging from 3 to 18 months.Citation88 Co-administration of Trovax with 5-fluorouracil, folinic acid, and oxaliplatin or with leukovorin and irinotecan in patients with mCRC was safe and well tolerated. The regime was able to induce 5T4-specific immune responses and bring clinical benefit in these patients.Citation89,Citation90
Table 3. VVs employed in clinical trials for colon cancer.
Table 4. Clinical trials for colon cancers and other solid tumors using VVs (https://clinicaltrials.gov/).
CRC patients were administered with a regime comprising GM-CSF, IFN-α, and Recombinant (r) vaccinia-based or recombinant avipox-based vectors (rV-CEA(6D)-TRICOM and rF-CEA(6D)-TRICOM).Citation83 With the treatment, eight patients (24%) had SD for more than 3 months. The median PFS was 1.8 months, and the median OS was 6.3 months. Although the vaccine regimen produced no clinical responses, the addition of IFN-α significantly prolonged OS in patients (6.40 vs 3.94 months).Citation83 Pexa-Vec is an oncolytic VV expressing GM-CSF and β-galactosidase with TK inactivation.Citation84 Intravenous treatment of Pexa-Vec into patients with treatment-refractory CRC was well tolerated and associated with antitumor activity.Citation84 After the treatment, 10 patients (67%) achieved SD.Citation84 PFS and OS were 61 days and 10.3 months, respectively.Citation84 Intratumoral dose escalation of VGF and TK deletion VV (vvDD) treatment proceeded without dose-limiting toxicities in patients with CRC or other cancers.Citation85 What is noteworthy, patients would experience fever, malaise, and/or pain during the expected peak in the VV replication and the corresponding immune response.Citation85 In patients with mCRC and other cancers, intravenous injection trial of vvDD did not cause dose-limiting toxicities and treatment-related severe adverse events during the therapy session.Citation86 Elevation of anti-VV antibody and Th1 cytokines (IL-2, IFN-gamma, and TNF-alpha) in the sera was detected in these patients.Citation86 The median survival of these patients was 4.8 months (range 2.6–23.9 months).Citation86 These studies validated the safety and efficacy of vvDD in patients with CRC and other solid cancers.
Discussion – challenges and future directions
Since the approval of talimogene laherparepvec (T-VEC, an engineered herpes simplex virus-1 expressing GM-CSF) for treating melanoma by the US FDA, researchers have ignited their passion to evaluate efficient oncolytic viruses in cancer therapy. VV represents promising vectors that may be employed for the improvement of cancer therapy condition. In the present study, we reviewed the preclinical and clinical progression and development of VV in CRC therapy. VV has considerable versatility, because it can be modified to enhance tumor selectivity, and its ability to spread within tumors, or combined with immunostimulatory molecules to activate antitumor immunity.Citation91 Collective evidence suggests that VV represents a potential and promising treatment choice for CRC. However, challenges would be faced before sophisticated strategies are established to apply these vectors to cancer therapy.
There are more than 200 genes encoded by VV genome. However, most of them are functionally ambiguous. Full understanding of these functional genes would help to further develop more efficient oncolytic VV for CRC therapy.
Systemic delivery and location of VV into solid tumors has been viewed as a critical factor that significantly influences its antitumor effect. Polymeric materials have been developed to reduce neutralizing anti-VV antibody and promote tumor tropism.Citation92 These results may suggest an important direction for VV development.
Although the safety of VV has been validated in many human clinical trials, attentions need be paid to the biosafety and risk of the therapies based on VV. Because VV can replicate in a wide range of human cells, VV may also affect normal and untargeted cells during the therapy.
Author contributions
All authors contributed to the study conception and design. Material preparation was performed by Qiaoyun Ling, Bichun Zheng, Xudong Chen, Shaoshun Ye, and Quan Cheng. The first draft of the manuscript was written by Qiaoyun Ling and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Disclosure statement
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: gLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:1–13. doi:10.3322/caac.21660.
- Kastrinos F, Samadder NJ, Burt RW. Use of family history and genetic testing to determine risk of colorectal cancer. Gastroenterology. 2020;158:389–403. doi:10.1053/j.gastro.2019.11.029.
- Perera AP, Sajnani K, Dickinson J, Eri R, Korner H. NLRP3 inflammasome in colitis and colitis-associated colorectal cancer. Mamm Genome. 2018;29:817–30. doi:10.1007/s00335-018-9783-2.
- Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer. 2009;124:2406–15. doi:10.1002/ijc.24191.
- Vieira AR, Abar L, Chan DSM, Vingeliene S, Polemiti E, Stevens C, Greenwood D, Norat T. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR continuous update project. Ann Oncol. 2017;28:1788–802. doi:10.1093/annonc/mdx171.
- Soltani G, Poursheikhani A, Yassi M, Hayatbakhsh A, Kerachian M, Kerachian MA. Obesity, diabetes and the risk of colorectal adenoma and cancer. BMC Endocr Disord. 2019;19:113. doi:10.1186/s12902-019-0444-6.
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–502. doi:10.1016/S0140-6736(13)61649-9.
- Piawah S, Venook AP. Targeted therapy for colorectal cancer metastases: a review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer. 2019;125:4139–47. doi:10.1002/cncr.32163.
- Van Vliet K, Mohamed MR, Zhang L, Villa NY, Werden SJ, Liu J, McFadden G. Poxvirus proteomics and virus-host protein interactions. Microbiol Mol Biol Rev. 2009;73:730–49. doi:10.1128/MMBR.00026-09.
- Guse K, Cerullo V, Hemminki A. Oncolytic vaccinia virus for the treatment of cancer. Expert Opin Biol Ther. 2011;11:595–608. doi:10.1517/14712598.2011.558838.
- Smith GL, Benfield CTO, Maluquer de Motes C, Mazzon M, Ember SWJ, Ferguson BJ, Sumner RP. Vaccinia virus immune evasion: mechanisms, virulence and immunogenicity. J Gen Virol. 2013;94:2367–92. doi:10.1099/vir.0.055921-0.
- Guo ZS, Lu B, Guo Z, Giehl E, Feist M, Dai E, Liu W, Storkus WJ, He Y, Liu Z, et al. Vaccinia virus-mediated cancer immunotherapy: cancer vaccines and oncolytics. J Immunother Cancer. 2019;7:6. doi:10.1186/s40425-018-0495-7.
- Berry J, Vreeland T, Trappey A, Hale D, Peace K, Tyler J, Walker A, Brown R, Herbert G, Yi F, et al. Cancer vaccines in colon and rectal cancer over the last decade: lessons learned and future directions. Expert Rev Clin Immunol. 2017;13:235–45. doi:10.1080/1744666X.2016.1226132.
- Larocca C, Schlom J. Viral vector-based therapeutic cancer vaccines. Cancer J. 2011;17:359–71. doi:10.1097/PPO.0b013e3182325e63.
- Kim-Schulze S, Kim HS, Wainstein A, Kim DW, Yang WC, Moroziewicz D, Mong PY, Bereta M, Taback B, Wang Q, et al. Intrarectal vaccination with recombinant vaccinia virus expressing carcinoembronic antigen induces mucosal and systemic immunity and prevents progression of colorectal cancer. J Immunol. 2008;181:8112–19. doi:10.4049/jimmunol.181.11.8112.
- Lorenz MG, Kantor JA, Schlom J, Hodge JW. Anti-tumor immunity elicited by a recombinant vaccinia virus expressing CD70 (CD27L). Hum Gene Ther. 1999;10:1095–103. doi:10.1089/10430349950018094.
- Uzendoski K, Kantor JA, Abrams SI, Schlom J, Hodge JW. Construction and characterization of a recombinant vaccinia virus expressing murine intercellular adhesion molecule-1: induction and potentiation of antitumor responses. Hum Gene Ther. 1997;8:851–60. doi:10.1089/hum.1997.8.7-851.
- Sivanandham M, Shaw P, Bernik SF, Paoletti E, Wallack MK. Colon cancer cell vaccine prepared with replication-deficient vaccinia viruses encoding B7.1 and interleukin-2 induce antitumor response in syngeneic mice. Cancer Immunol Immunother. 1998;46:261–67. doi:10.1007/s002620050486.
- Kaufman HL, Rao JB, Irvine KR, Bronte V, Rosenberg SA, Restifo NP. Interleukin-10 enhances the therapeutic effectiveness of a recombinant poxvirus-based vaccine in an experimental murine tumor model. J Immunother (1991). 1999;22:489–96. doi:10.1097/00002371-199911000-00003.
- Rao JB, Chamberlain RS, Bronte V, Carroll MW, Irvine KR, Moss B, Rosenberg SA, Restifo NP. IL-12 is an effective adjuvant to recombinant vaccinia virus-based tumor vaccines: enhancement by simultaneous B7-1 expression. J Immunol. 1996;156:3357–65.
- Hance KW, Rogers CJ, Zaharoff DA, Canter D, Schlom J, Greiner JW. The antitumor and immunoadjuvant effects of IFN-alpha in combination with recombinant poxvirus vaccines. Clin Cancer Res. 2009;15:2387–96. doi:10.1158/1078-0432.CCR-08-1752.
- Dai P, Wang W, Yang N, Serna-Tamayo C, Ricca JM, Zamarin D, Shuman S, Merghoub T, Wolchok JD, Deng L. Intratumoral delivery of inactivated modified vaccinia virus Ankara (iMVA) induces systemic antitumor immunity via STING and Batf3-dependent dendritic cells. Sci Immunol. 2017;2. doi:10.1126/sciimmunol.aal1713.
- Kudo-Saito C, Schlom J, Hodge JW. Intratumoral vaccination and diversified subcutaneous/intratumoral vaccination with recombinant poxviruses encoding a tumor antigen and multiple costimulatory molecules. Clin Cancer Res. 2004;10:1090–99. doi:10.1158/1078-0432.CCR-03-0145.
- Tykodi SS, Thompson JA. Development of modified vaccinia Ankara-5T4 as specific immunotherapy for advanced human cancer. Expert Opin Biol Ther. 2008;8:1947–53. doi:10.1517/14712590802567298.
- Stern PL, Harrop R. 5T4 oncofoetal antigen: an attractive target for immune intervention in cancer. Cancer Immunol Immunother. 2017;66:415–26. doi:10.1007/s00262-016-1917-3.
- Harrop R, Ryan MG, Myers KA, Redchenko I, Kingsman SM, Carroll MW. Active treatment of murine tumors with a highly attenuated vaccinia virus expressing the tumor associated antigen 5T4 (TroVax) is CD4+ T cell dependent and antibody mediated. Cancer Immunol Immunother. 2006;55:1081–90. doi:10.1007/s00262-005-0096-4.
- Mulryan K, Ryan MG, Myers KA, Shaw D, Wang W, Kingsman SM, Stern PL, Carroll MW. Attenuated recombinant vaccinia virus expressing oncofetal antigen (tumor-associated antigen) 5T4 induces active therapy of established tumors. Mol Cancer Ther. 2002;1:1129–37.
- Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi:10.1006/scbi.1998.0119.
- Beauchemin N, Arabzadeh A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013;32:643–71. doi:10.1007/s10555-013-9444-6.
- Jacobs J, Deschoolmeester V, Zwaenepoel K, Rolfo C, Silence K, Rottey S, Lardon F, Smits E, Pauwels P. CD70: an emerging target in cancer immunotherapy. Pharmacol Ther. 2015;155:1–10. doi:10.1016/j.pharmthera.2015.07.007.
- Zheng J, Mo J, Zhu T, Zhuo W, Yi Y, Hu S, Yin J, Zhang W, Zhou H, Liu Z. Comprehensive elaboration of the cGAS-STING signaling axis in cancer development and immunotherapy. Mol Cancer. 2020;19:133. doi:10.1186/s12943-020-01250-1.
- Timiryasova TM, Li J, Chen B, Chong D, Langridge WH, Gridley DS, Fodor I. Antitumor effect of vaccinia virus in glioma model. Oncol Res. 1999;11:133–44.
- Ferguson MS, Chard Dunmall LS, Gangeswaran R, Marelli G, Tysome JR, Burns E, Whitehead MA, Aksoy E, Alusi G, Hiley C, et al. Transient inhibition of PI3Kδ enhances the therapeutic effect of intravenous delivery of oncolytic vaccinia virus. Mol Ther. 2020;28:1263–75. doi:10.1016/j.ymthe.2020.02.017.
- Ottolino-Perry K, Tang N, Head R, Ng C, Arulanandam R, Angarita FA, Acuna SA, Chen Y, Bell J, DaCosta RS, et al. Tumor vascularization is critical for oncolytic vaccinia virus treatment of peritoneal carcinomatosis. Int J Cancer. 2014;134:717–30. doi:10.1002/ijc.28395.
- Badrinath N, Jeong YI, Woo HY, Bang SY, Kim C, Heo J, Kang DH, Yoo SY. Local delivery of a cancer-favoring oncolytic vaccinia virus via poly (lactic-co-glycolic acid) nanofiber for theranostic purposes. Int J Pharm. 2018;552:437–42. doi:10.1016/j.ijpharm.2018.10.020.
- Nakatake M, Kuwano N, Kaitsurumaru E, Kurosaki H, Nakamura T. Fusogenic oncolytic vaccinia virus enhances systemic antitumor immune response by modulating the tumor microenvironment. Mol Ther. 2021;29:1782–93. doi:10.1016/j.ymthe.2020.12.024.
- Ho TY, Mealiea D, Okamoto L, Stojdl DF, McCart JA. Deletion of immunomodulatory genes as a novel approach to oncolytic vaccinia virus development. Mol Ther Oncolytics. 2021;22:85–97. doi:10.1016/j.omto.2021.05.007.
- Thirunavukarasu P, Sathaiah M, Gorry MC, O’Malley ME, Ravindranathan R, Austin F, Thorne SH, Guo ZS, Bartlett DL. A rationally designed A34R mutant oncolytic poxvirus: improved efficacy in peritoneal carcinomatosis. Mol Ther. 2013;21:1024–33. doi:10.1038/mt.2013.27.
- Rojas JJ, Sampath P, Hou W, Thorne SH. Defining effective combinations of immune checkpoint blockade and oncolytic virotherapy. Clin Cancer Res. 2015;21:5543–51. doi:10.1158/1078-0432.CCR-14-2009.
- Liu Z, Ravindranathan R, Kalinski P, Guo ZS, Bartlett DL. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat Commun. 2017;8:14754. doi:10.1038/ncomms14754.
- Yoo SY, Badrinath N, Jeong SN, Woo HY, Heo J. Overcoming tumor resistance to oncolyticvaccinia virus with anti-PD-1-based combination therapy by inducing antitumor immunity in the tumor microenvironment. Vaccines (Basel). 2020;8:321. doi:10.3390/vaccines8020321.
- Yoo SY, Bang SY, Jeong SN, Kang DH, Heo J. A cancer-favoring oncolytic vaccinia virus shows enhanced suppression of stem-cell like colon cancer. Oncotarget. 2016;7:16479–89. doi:10.18632/oncotarget.7660.
- Liu W, Dai E, Liu Z, Ma C, Guo ZS, Bartlett DL. In Situ therapeutic cancer vaccination with an oncolytic virus expressing membrane-tethered IL-2. Mol Ther Oncolytics. 2020;17:350–60. doi:10.1016/j.omto.2020.04.006.
- Ge Y, Wang H, Ren J, Liu W, Chen L, Chen H, Ye J, Dai E, Ma C, Ju S, et al. Oncolytic vaccinia virus delivering tethered IL-12 enhances antitumor effects with improved safety. J Immunother Cancer. 2020;8:e000710. doi:10.1136/jitc-2020-000710.
- Chen L, Chen H, Ye J, Ge Y, Wang H, Dai E, Ren J, Liu W, Ma C, Ju S, et al. Intratumoral expression of interleukin 23 variants using oncolytic vaccinia virus elicit potent antitumor effects on multiple tumor models via tumor microenvironment modulation. Theranostics. 2021;11:6668–81. doi:10.7150/thno.56494.
- Wang N, Wang J, Zhang Z, Cao H, Yan W, Chu Y, Chard Dunmall LS, Wang Y. A novel vaccinia virus enhances anti-tumor efficacy and promotes a long-term anti-tumor response in a murine model of colorectal cancer. Mol Ther Oncolytics. 2021;20:71–81. doi:10.1016/j.omto.2020.11.002.
- Deng L, Yang X, Fan J, Ding Y, Peng Y, Xu D, Huang B, Hu Z. IL-24-armed oncolytic vaccinia virus exerts potent antitumor effects via multiple pathways in colorectal cancer. Oncol Res. 2021;28:579–90. doi:10.3727/096504020X15942028641011.
- Lee YS, Lee WS, Kim CW, Lee SJ, Yang H, Kong SJ, Ning J, Yang K-M, Kang B, Kim WR, et al. Oncolytic vaccinia virus reinvigorates peritoneal immunity and cooperates with immune checkpoint inhibitor to suppress peritoneal carcinomatosis in colon cancer. J Immunother Cancer. 2020;8:e000857. doi:10.1136/jitc-2020-000857.
- Li J, O’Malley M, Sampath P, Kalinski P, Bartlett DL, Thorne SH. Expression of CCL19 from oncolytic vaccinia enhances immunotherapeutic potential while maintaining oncolytic activity. Neoplasia. 2012;14:1115–21. doi:10.1593/neo.121272.
- Flanagan K, Glover RT, Horig H, Yang W, Kaufman HL. Local delivery of recombinant vaccinia virus expressing secondary lymphoid chemokine (SLC) results in a CD4 T-cell dependent antitumor response. Vaccine. 2004;22:2894–903. doi:10.1016/j.vaccine.2003.12.021.
- Bereta M, Bereta J, Park J, Medina F, Kwak H, Kaufman HL. Immune properties of recombinant vaccinia virus encoding CD154 (CD40L) are determined by expression of virally encoded CD40L and the presence of CD40L protein in viral particles. Cancer Gene Ther. 2004;11:808–18. doi:10.1038/sj.cgt.7700762.
- Warner SG, Kim SI, Chaurasiya S, O’Leary MP, Lu J, Sivanandam V, Woo Y, Chen NG, Fong Y. A novel chimeric poxvirus encoding hNIS is tumor-tropic, imageable, and synergistic with radioiodine to sustain colon cancer regression. Mol Ther Oncolytics. 2019;13:82–92. doi:10.1016/j.omto.2019.04.001.
- Haddad D, Chen N, Zhang Q, Chen CH, Yu YA, Gonzalez L, Aguilar J, Li P, Wong J, Szalay AA, et al. A novel genetically modified oncolytic vaccinia virus in experimental models is effective against a wide range of human cancers. Ann Surg Oncol. 2012;19(Suppl 3):S665–74. doi:10.1245/s10434-011-2198-x.
- Eveno C, Mojica K, Ady JW, Thorek DL, Longo V, Belin LJ, Gholami S, Johnsen C, Zanzonico P, Chen N, et al. Gene therapy using therapeutic and diagnostic recombinant oncolytic vaccinia virus GLV-1h153 for management of colorectal peritoneal carcinomatosis. Surgery. 2015;157:331–37. doi:10.1016/j.surg.2014.09.008.
- McCart JA, Mehta N, Scollard D, Reilly RM, Carrasquillo JA, Tang N, Deng H, Miller M, Xu H, Libutti SK, et al. Oncolytic vaccinia virus expressing the human somatostatin receptor SSTR2: molecular imaging after systemic delivery using 111in-pentetreotide. Mol Ther. 2004;10:553–61. doi:10.1016/j.ymthe.2004.06.158.
- Ding Y, Fan J, Deng L, Huang B, Zhou B. Antitumor efficacy of cytosine deaminase-armed vaccinia virus plus 5-fluorocytosine in colorectal cancers. Cancer Cell Int. 2020;20:243. doi:10.1186/s12935-020-01340-6.
- McCart JA, Puhlmann M, Lee J, Hu Y, Libutti SK, Alexander HR, Bartlett DL. Complex interactions between the replicating oncolytic effect and the enzyme/prodrug effect of vaccinia-mediated tumor regression. Gene Ther. 2000;7:1217–23. doi:10.1038/sj.gt.3301237.
- Foloppe J, Kintz J, Futin N, Findeli A, Cordier P, Schlesinger Y, Hoffmann C, Tosch C, Balloul J-M, Erbs P. Targeted delivery of a suicide gene to human colorectal tumors by a conditionally replicating vaccinia virus. Gene Ther. 2008;15:1361–71. doi:10.1038/gt.2008.82.
- Ziauddin MF, Guo ZS, O’Malley ME, Austin F, Popovic PJ, Kavanagh MA, Li J, Sathaiah M, Thirunavukarasu P, Fang B, et al. TRAIL gene-armed oncolytic poxvirus and oxaliplatin can work synergistically against colorectal cancer. Gene Ther. 2010;17:550–59. doi:10.1038/gt.2010.5.
- Jeong SN, Yoo SY. Novel oncolytic virus armed with cancer suicide gene and normal vasculogenic gene for improved anti-tumor activity. Cancers (Basel). 2020;12:1070. doi:10.3390/cancers12051070.
- Wu T, Xiang Y, Liu T, Wang X, Ren X, Ye T, Li G. Oncolytic vaccinia virus expressing aphrocallistes vastus lectin as a cancer therapeutic agent. Mar Drugs. 2019;17:363. doi:10.3390/md17060363.
- Horita K, Kurosaki H, Nakatake M, Ito M, Kono H, Nakamura T. Long noncoding RNA UCA1 enhances sensitivity to oncolytic vaccinia virus by sponging miR-18a/mir-182 and modulating the Cdc42/filopodia axis in colorectal cancer. Biochem Biophys Res Commun. 2019;516:831–38. doi:10.1016/j.bbrc.2019.06.125.
- Ottolino-Perry K, Acuna SA, Angarita FA, Sellers C, Zerhouni S, Tang N, McCart JA. Oncolytic vaccinia virus synergizes with irinotecan in colorectal cancer. Mol Oncol. 2015;9:1539–52. doi:10.1016/j.molonc.2015.04.009.
- MacTavish H, Diallo JS, Huang B, Stanford M, Le Boeuf F, De Silva N, Cox J, Simmons JG, Guimond T, Falls T, et al. Enhancement of vaccinia virus based oncolysis with histone deacetylase inhibitors. PLoS One. 2010;5:e14462. doi:10.1371/journal.pone.0014462.
- Zhou J, Sun XY, Fernando GJ, Frazer IH. The vaccinia virus K2L gene encodes a serine protease inhibitor which inhibits cell-cell fusion. Virology. 1992;189:678–86. doi:10.1016/0042-6822(92)90591-C.
- Wolffe EJ, Katz E, Weisberg A, Moss B. The A34R glycoprotein gene is required for induction of specialized actin-containing microvilli and efficient cell-to-cell transmission of vaccinia virus. J Virol. 1997;71:3904–15. doi:10.1128/jvi.71.5.3904-3915.1997.
- Bartlett N, Symons JA, Tscharke DC, Smith GL. The vaccinia virus N1L protein is an intracellular homodimer that promotes virulence. J Gen Virol. 2002;83:1965–76. doi:10.1099/0022-1317-83-8-1965.
- Zhang Z, Abrahams MR, Hunt LA, Suttles J, Marshall W, Lahiri DK, Kotwal GJ. The vaccinia virus N1L protein influences cytokine secretion in vitro after infection. Ann N Y Acad Sci. 2005;1056:69–86. doi:10.1196/annals.1352.005.
- Meng X, Jiang C, Arsenio J, Dick K, Cao J, Xiang Y. Vaccinia virus K1L and C7L inhibit antiviral activities induced by type I interferons. J Virol. 2009;83:10627–36. doi:10.1128/JVI.01260-09.
- Shisler JL, Jin XL. The vaccinia virus K1L gene product inhibits host NF-kappaB activation by preventing IkappaBalpha degradation. J Virol. 2004;78:3553–60. doi:10.1128/JVI.78.7.3553-3560.2004.
- Kawagishi-Kobayashi M, Silverman JB, Ung TL, Dever TE. Regulation of the protein kinase PKR by the vaccinia virus pseudosubstrate inhibitor K3L is dependent on residues conserved between the K3L protein and the PKR substrate eIf2alpha. Mol Cell Biol. 1997;17:4146–58. doi:10.1128/MCB.17.7.4146.
- Stack J, Haga IR, Schroder M, Bartlett NW, Maloney G, Reading PC, Fitzgerald KA, Smith GL, Bowie AG. Vaccinia virus protein A46R targets multiple Toll-like–interleukin-1 receptor adaptors and contributes to virulence. J Exp Med. 2005;201:1007–18. doi:10.1084/jem.20041442.
- Bowie A, Kiss-Toth E, Symons JA, Smith GL, Dower SK, O’Neill LA. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc Natl Acad Sci USA. 2000;97:10162–67. doi:10.1073/pnas.160027697.
- Kleinpeter P, Remy-Ziller C, Winter E, Gantzer M, Nourtier V, Kempf J, Hortelano J, Schmitt D, Schultz H, Geist M, et al. By binding CD80 and CD86, the vaccinia virus M2 protein blocks their interactions with both CD28 and CTLA4 and potentiates CD80 binding to PD-L1. J Virol. 2019;93. doi:10.1128/JVI.00207-19.
- Yan WL, Shen KY, Tien CY, Chen YA, Liu SJ. Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy. 2017;9:347–60. doi:10.2217/imt-2016-0141.
- Gowhari Shabgah A, Al-Obaidi ZMJ, Sulaiman Rahman H, Kamal Abdelbasset W, Suksatan W, Bokov DO, Thangavelu L, Turki Jalil A, Jadidi-Niaragh F, Mohammadi H, et al. Does CCL19 act as a double-edged sword in cancer development? Clin Exp Immunol. 2022;207:164–75. doi:10.1093/cei/uxab039.
- Hillinger S, Yang SC, Zhu L, Huang M, Duckett R, Atianzar K, Batra RK, Strieter RM, Dubinett SM, Sharma S. EBV-induced molecule 1 ligand chemokine (ELC/CCL19) promotes IFN-γ-dependent antitumor responses in a lung cancer model. J Immunol. 2003;171:6457–65. doi:10.4049/jimmunol.171.12.6457.
- Lu J, Ma J, Cai W, Wangpu X, Feng H, Zhao J, Guan S, Zong Y, Lu A. CC motif chemokine ligand 19 suppressed colorectal cancer in vivo accompanied by an increase in IL-12 and IFN-γ. Biomed Pharmacother. 2015;69:374–79. doi:10.1016/j.biopha.2014.12.032.
- Darrouzet E, Lindenthal S, Marcellin D, Pellequer JL, Pourcher T. The sodium/iodide symporter: state of the art of its molecular characterization. Biochim Biophys Acta. 2014;1838:244–53. doi:10.1016/j.bbamem.2013.08.013.
- Gundacker D, Leys SP, Schroder HC, Muller IM, Muller WE. Isolation and cloning of a C-type lectin from the hexactinellid sponge Aphrocallistes vastus: a putative aggregation factor. Glycobiology. 2001;11:21–29. doi:10.1093/glycob/11.1.21.
- Xuan W, Yu H, Zhang X, Song D. Crosstalk between the lncRNA UCA1 and microRnas in cancer. FEBS Lett. 2019;593:1901–14. doi:10.1002/1873-3468.13470.
- Gatti-Mays ME, Strauss J, Donahue RN, Palena C, Del Rivero J, Redman JM, Madan RA, Marté JL, Cordes LM, Lamping E, et al. A phase I dose-escalation trial of BN-CV301, a recombinant poxviral vaccine targeting MUC1 and CEA with costimulatory molecules. Clin Cancer Res. 2019;25:4933–44. doi:10.1158/1078-0432.CCR-19-0183.
- Duggan MC, Jochems C, Donahue RN, Richards J, Karpa V, Foust E, Paul B, Brooks T, Tridandapani S, Olencki T, et al. A phase I study of recombinant (r) vaccinia-CEA(6D)-TRICOM and rFowlpox-CEA(6D)-TRICOM vaccines with GM-CSF and IFN-α-2b in patients with CEA-expressing carcinomas. Cancer Immunol Immunother. 2016;65:1353–64. doi:10.1007/s00262-016-1893-7.
- Park SH, Breitbach CJ, Lee J, Park JO, Lim HY, Kang WK, Moon A, Mun J-H, Sommermann EM, Maruri Avidal L, et al. Phase 1b trial of biweekly intravenous Pexa-Vec (JX-594), an oncolytic and immunotherapeutic vaccinia virus in colorectal cancer. Mol Ther. 2015;23:1532–40. doi:10.1038/mt.2015.109.
- Zeh HJ, Downs-Canner S, McCart JA, Guo ZS, Rao UN, Ramalingam L, Thorne SH, Jones HL, Kalinski P, Wieckowski E, et al. First-in-man study of western reserve strain oncolytic vaccinia virus: safety, systemic spread, and antitumor activity. Mol Ther. 2015;23:202–14. doi:10.1038/mt.2014.194.
- Downs-Canner S, Guo ZS, Ravindranathan R, Breitbach CJ, O’Malley ME, Jones HL, Moon A, McCart JA, Shuai Y, Zeh HJ, et al. Phase 1 study of intravenous oncolytic poxvirus (vvDD) in patients with advanced solid cancers. Mol Ther. 2016;24:1492–501. doi:10.1038/mt.2016.101.
- Scurr M, Pembroke T, Bloom A, Roberts D, Thomson A, Smart K, Bridgeman H, Adams R, Brewster A, Jones R, et al. Effect of modified vaccinia Ankara–5T4 and low-dose cyclophosphamide on antitumor immunity in metastatic colorectal cancer. JAMA Oncol. 2017;3:e172579. doi:10.1001/jamaoncol.2017.2579.
- Harrop R, Connolly N, Redchenko I, Valle J, Saunders M, Ryan MG, Myers KA, Drury N, Kingsman SM, Hawkins RE, et al. Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial. Clin Cancer Res. 2006;12:3416–24. doi:10.1158/1078-0432.CCR-05-2732.
- Harrop R, Drury N, Shingler W, Chikoti P, Redchenko I, Carroll MW, Kingsman SM, Naylor S, Melcher A, Nicholls J, et al. Vaccination of colorectal cancer patients with modified vaccinia ankara encoding the tumor antigen 5T4 (TroVax) given alongside chemotherapy induces potent immune responses. Clin Cancer Res. 2007;13:4487–94. doi:10.1158/1078-0432.CCR-07-0704.
- Harrop R, Drury N, Shingler W, Chikoti P, Redchenko I, Carroll MW, Kingsman SM, Naylor S, Griffiths R, Steven N, et al. Vaccination of colorectal cancer patients with TroVax given alongside chemotherapy (5-fluorouracil, leukovorin and irinotecan) is safe and induces potent immune responses. Cancer Immunol Immunother. 2008;57:977–86. doi:10.1007/s00262-007-0428-7.
- Morse MA. Virus-based therapies for colon cancer. Expert Opin Biol Ther. 2005;5:1627–33. doi:10.1517/14712598.5.12.1627.
- Hill C, Grundy M, Bau L, Wallington S, Balkaran J, Ramos V, Fisher K, Seymour L, Coussios C, Carlisle R. Polymer stealthing and mucin-1 retargeting for enhanced pharmacokinetics of an oncolytic vaccinia virus. Mol Ther Oncolytics. 2021;21:47–61. doi:10.1016/j.omto.2021.03.011.