ABSTRACT
Immunization is cost-effective preventive strategy for child morbidity and mortality. PubMed, Google Scholar, Scopus, Science Direct, and online institutional repository homes were searched. Data were extracted by Microsoft excel. Begg’s rank test, and Egger’s regression test was done. A pooled prevalence, Sub-group analysis, sensitivity analysis and meta-regression were conducted. A total of 12 articles were included in this study. The pooled prevalence of vaccination dropout was 26.06% (95% CI: 11.59, 30.53), I2 =91.2%. In sub-group analysis, Nigeria had the highest prevalence of immunization dropouts (33.59%). It was 18.01% and 29.25%, respectively, for published and unpublished research. Community-based studies and institutional-based studies also yield a prevalence of dropout 39.04% and 13.73% respectively. Dropout rate was 22.66% for sample sizes under 500 and 18.01% for sample sizes beyond 500. In Sub-Saharan Africa, the prevalence of vaccination dropout was high. Community education about vaccinations importance should be prioritized.
Introduction
Immunization is the practice of preventing an infectious pathogen from attacking the body by administering vaccines.Citation1,Citation2 It is among the most economically advantageous public health strategies for reducing child morbidity and mortality.Citation3 Between 2010 and 2018, the measles vaccine avoided nearly 23 million fatalities.Citation4 More than half of child and young child mortality are caused by diseases that may easily be prevented by taking simple, cheap measures, like receiving vaccines.Citation5 Vaccine-preventable diseases continue to pose a public health danger in South-East Asia and sub-Saharan Africa because of insufficient and poor vaccination coverage.Citation6
Pneumonia (12%), diarrhea (8%), injuries (6%), congenital anomalies(4%), malaria (5%) and measles (2%) are the leading causes of death in children under the age of five.Citation7 Vaccine-preventable diseases cost the lives of 8.8 million children under the age of five worldwide.Citation8 Vaccination is the most effective way to prevent infectious childhood diseases like measles, pertussis, diphtheria, tetanus, TB, meningitis, and tuberculosis in children.Citation9 The percentages of mortality brought on by pneumonia and diarrhea that can be avoided by vaccination are 59% and 29%, respectively.Citation10
A significant number of children are not fully vaccinated despite a huge decline in the occurrence of vaccine-preventable mortality, which causes a major regional and global variation in vaccination coverage.Citation11,Citation12 For instance, out of more over 17 million cases of measles in the world in 2017, there were 83,439 fatalities.Citation13,Citation14 In 2018, a full three doses of the diphtheria-tetanus-pertussis (DTP) vaccine were given to 116.3 million children worldwide (86%).Citation15,Citation16
In 2019, 19.7 million children worldwide did not receive the third dose of the diphtheria, tetanus, and pertussis (DTP3) vaccination during the first year of life, which is a crucial sign of the effectiveness of immunization programs.Citation17–19 Of all the children who did not finish the three-dose DTP series, 6.2 million (31%) started it but never finished it.Citation20 Every year, 2–3 million children become ill and are vulnerable to diseases that can be prevented by vaccination, resulting in mortality, and about 22.4% of children fail to receive the DPT3 vaccine.Citation21 One in five infants around the world does not receive the three essential doses of the diphtheria, tetanus, and pertussis vaccine.Citation22
Every year, 4.4 million children in sub-Saharan Africa die from communicable diseases that could be prevented by vaccination.Citation23,Citation24 It is associated to inadequate vaccination Coverage, challenges and setup was not fully equipped in sub-Saharan Africa countries.Citation25
The WHO’s global approach known as the Immunization Agenda 2030 states that by the year 2030, every child should have received all recommended vaccinations, irrespective of their geographical region, age, socioeconomic status, or gender-related constraints.Citation26 The Expanded Program on Immunization (EPI) was established by the WHO in 1974 to control diseases that may be prevented by immunization.Citation27 After the launch of the EPI program, the number of fatalities among children under five remained at 5.3 million in 2018.Citation28
Despite significant progress, the expanded immunization program still faces many obstacles. These are categorized as follows: policy, standards, and guidelines, human resources, management of vaccine, cold chain, and logistics, service delivery, communication and community partnerships; and sustainable financing.Citation29
A review of the literature revealed various factors that were related to immunization dropout. Long travel distances to medical facilities, living in a rural region, and having a mother without a regular work were reported to be risk factors for immunization dropout in Nepal.Citation30 Children in other parts of Ghana were more likely to drop out of school if they lacked immunization records than those who did.Citation31 Last but not least, a randomized controlled experiment conducted in urban Pakistan found that receiving both a redesigned card and center-based education was expected to significantly enhance DPT3 completion compared to the control group.Citation32
There are no data at the regional level, despite the fact that numerous primary researches have confirmed the percentage of dropout rate in Sub-Saharan Africa. Therefore, the goal of this systematic review and meta-analysis study was to identify the prevalence of dropout of vaccination among children in Sub-Saharan Africa. Clinicians and other stakeholders will be able to address gaps in immunization coverage by prioritizing and customizing immunization campaigns and operational plans based on the study’s findings, which will provide them with the fundamental knowledge they need to provide every child with a life-saving vaccination.
Methods
Reporting
This systematic review and meta-analysis study was conducted to determine vaccination dropout among children in Sub-Saharan Africa using the standard PRISMA checklist guideline.Citation33 (Supplementary file 1).
Search strategy
International online databases (Pub Med, Science Direct, Scopus, and Google Scholar) were used to search articles on the prevalence of dropout of vaccination in Sub-Saharan Africa. We also retrieved gray literature from Bangladesh University (Daffodil International University) and United States of America University (Walden University) and University of Ghana online research institutional repository. The search string was established using “AND” and “OR” Boolean operators. The following core search terms and phrases with Boolean operators were used to search related articles: ((((Dropout) OR (“Dropout” OR “failure to complete”)) AND Immunization) OR (“Immunization” OR “Vaccine” OR “Vaccination” OR “Immunisation”)) AND Children) OR (“Child” OR “Childhood” OR “Baby”) AND Sub-Saharan Africa. Search terms were based on PICO principles to retrieve relevant articles through the databases mentioned above. The searching period was from May 1/2022, to June 10/2022.
Outcome measurement
The outcome variable of the current study was immunization dropout and it indicates that one has received the first recommended dose of vaccine and missed the next recommended dose. It was used for pentavalent vaccines (Diphtheria, Pertussis, Tetanus, Hepatitis B, and Hib) for the shots that require successive doses and was determined by asking yes/no questions (1–5 doses). Citation34
Inclusion and exclusion criteria
Only English language papers, including published and unpublished studies with full text available for research, and studies that took place in Africa were included in this meta-analysis. This systematic review and meta-analysis excluded research that used duplicated sources, qualitative studies, studies from developed nations, and articles without full text.
Quality assessment
Two authors (NAG and KDT) independently appraised the standard of the studies using the Joanna Briggs Institute (JBI) standardized quality appraisal checklist.Citation35 The disagreement raised during the quality assessment was resolved through a discussion led by the third author (GAA). Finally, the argument was solved and reached with an agreement. The critical analysis checklist has eight parameters with yes, no, unclear, and not applicable options. The parameters involve the following questions:
Where were the criteria for inclusion in the sample clearly defined?
Were the study subjects and, therefore, the setting described in detail?
Was the exposure measured result validly and reliably?
Were the main objective and standard criteria used to measure the event?
Were confounding factors identified?
Were strategies to affect confounding factors stated?
Were the results measured indeed and dependably? And (8) Was the statistical analysis suitable?. Studies were considered low risk when they scored 50% and above on the quality assessment indicators, as reported in a supplementary file (Supplementary file2).
Risk of bias assessment
Two authors (NAG and GAA) independently assessed included studies for risk of bias through the bias assessment tool developed by Hoy et al,Citation36 consisting of ten items that assess four domains of bias and internal and external validity. Any disagreement raised during the risk of bias assessment was resolved through a discussion led by the third author (KDT). Finally, the argument was solved and reached with an agreement. The first four items (items 1–4) evaluate the presence of selection bias, non-response bias, and external validity. The other six items (items 5–10) assess the presence of measuring the bias, analysis-related bias, and internal validity. Therefore, studies that received ‘yes’ for eight or more of the ten questions were classified as ‘low risk of bias.’ If studies that received ‘yes’ for six to seven of the ten questions were classified as ’moderate risk’ whereas studies that received ‘yes’ for five or fewer of the ten questions were classified as ‘high risk’ as reported in a supplementary file (Supplementary file 3).
Data extraction
Microsoft Excel spreadsheet (2016) and STATA version 11 software were utilized for data extraction and analysis. Two authors (NAG and BB) independently extracted all relevant data using a standardized Joanna Briggs Institute data extraction format. The disagreement raised during data extraction was resolved through a discussion led by the third author (GAA). Finally, the argument was solved and reached with an agreement. The data automation tool was not used due to this study’s absence of the paper form (manual data). The name of the first author, year of publication, study country, study setting, study design, the prevalence of dropout of immunization, sample size, and quality of each paper was extracted.
Data analysis
After extracting all relevant findings in a micro-soft excel spreadsheet, the data were exported to STATA software version 14 for analysis. The pooled prevalence of dropout of vaccination was computed using a 95% confidence interval. Publication bias was checked by funnel plot and more objectively through Begg and Egger’s regression tests, with P < .05 indicating potential publication bias. The presence of between-study heterogeneity was checked by using the Cochrane Q statistic. This heterogeneity between studies was quantified using I2, in which a value of 0, 25, 50, and 75% represented no, low, medium, and high heterogeneity, respectively. A forest plot was used to visually assess the presence of heterogeneity, which presented at a high-level random-effect model was used for analysis to estimate the pooled estimate of dropout of vaccination. Subgroup analysis was done by sample size, country, publication, and study setting. Sensitivity analysis was executed to see the effect of a single study on the overall prevalence of the meta-analysis estimate. The findings of the study were presented in the form of text, tables, and figures
Results
Search findings and study characteristics
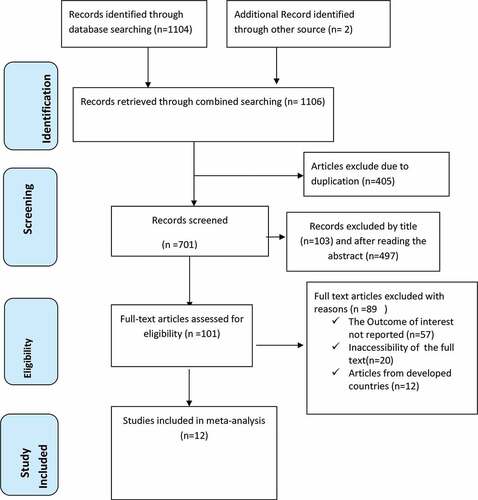
One thousand one hundred six articles were retrieved using a search strategy about vaccination dropout in Sub-Saharan Africa through online search engines such as; PubMed, Scopus, Google Scholar, Science direct, and online research repository home. After removing duplicated studies, we obtained 701 studies selected for screening full title and abstracts. Of these, 600 studies were excluded due to title and abstracts and the remaining 101 articles were assessed for full text articles. After reviewing the full text, 89 articles were then eliminated because they contained qualitative studies, lacked full titles and abstracts, and reported findings from developed countries. Finally, 12 articles Citation31–46 with 6326 study participants were included as criteria for this systematic review and meta-analysis study ().
The cross-sectional study design was applied to all included studies. Three of these were cross-sectional studies conducted at institutions, while the remaining nine studies were community-based. Three studies conducted in Ethiopia,Citation37,Citation47 two studies in Kenya,Citation39,Citation40 two in Nigeria,Citation42 two in Ghana,Citation31,Citation43 one study in Cameroon,Citation44 one study Mozambique Citation45 and one study in Somalia.Citation46 The sample size ranged from 32 to 1116. The prevalence of intention to use maternity waiting home ranged from 5.6% to 63.7%. All studies were assessed by using Joanna Briggs Institute (JBI) quality appraisal checklist and yielded low risk ().
Table 1. Characteristics of the included studies in the systematic review and meta-analysis for the prevalence of vaccination dropout in Sub-Saharan Africa.
Meta-analysis
Prevalence of dropout of vaccination in Sub-Saharan Africa
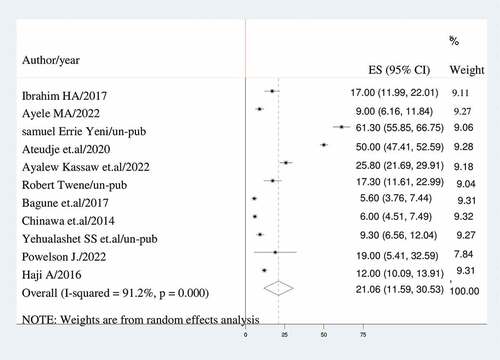
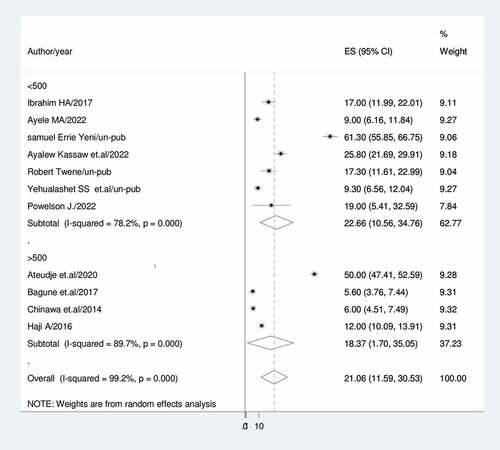
A DerSimonian and Laird random-effects model was used to determine the overall estimate of dropout of vaccination. Accordingly, the pooled prevalence of dropout of vaccination among children with a random-effects model was 21.06% (95% CI: 11.59, 30.53) with a heterogeneity index (I2) of 91.2% (p < 0.001) ().
Subgroup analysis
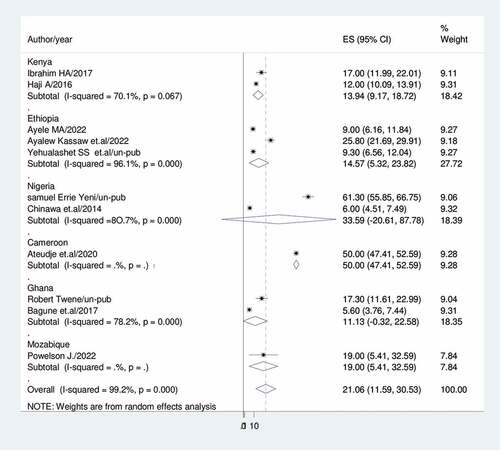
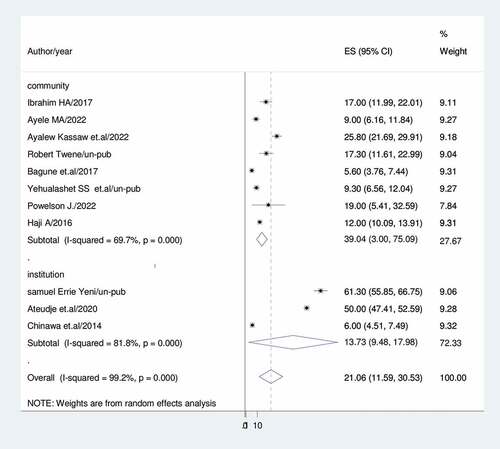
Subgroup analysis was conducted based on country, setting, publication, and sample size because this meta-analysis demonstrated a notable degree of heterogeneity. Accordingly, Ghana had the lowest prevalence of vaccine dropout (11.13%; 95%CI: 0.32, 22.58; I2 = 80.6%) while Nigeria had the highest (33.59%; 95%CI: 20.61, 87.78) (). For community-based studies and institutional-based studies, the prevalence of vaccination dropout was (39.04; 95% CI:3.00, 75.09), I2 = 69.7%, and (13.73%; 95%CI:9.48,17.98), I2 = 81.8%, respectively) ().
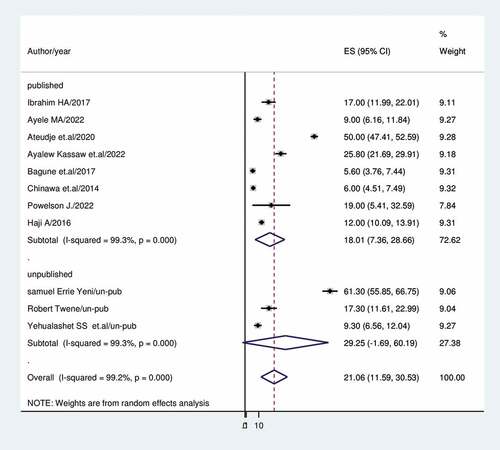
In studies with sample sizes higher than 500, the prevalence of vaccine dropout was 18.37% (95%CI: 1.70, 35.05); I2 = 89.7%; in studies with sample sizes less than 500, it was 22.66% (95%CI: 10.56, 34.76); I2 = 78.2% (). In contrast to published studies, which show a rate of vaccine dropout of (18.01%), unpublished studies indicate a prevalence of vaccination dropout of (29.25%); 95%CI: 1.69, 60.19), I2 = 99.3 ().
Heterogeneity and publication bias
To adjust the reported heterogeneity of this study (I2 = 91.2%), we computed a Sub-group analysis based on country, study setting, sample size and publication. Univar ate meta-regression was also done to identify the source of heterogeneity using sample size and year as a covariate. It showed that no effect of sample size and year on heterogeneity between studies ().
Table 2. Meta-regression analysis of factors affecting between-study heterogeneity.
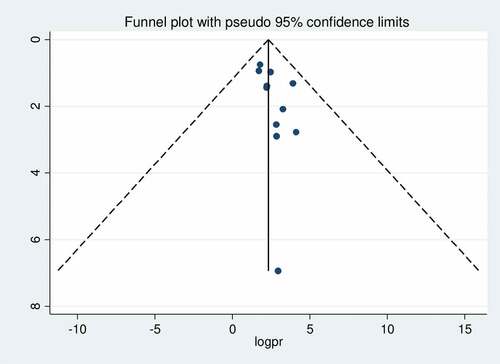
The presence of publication bias was assessed by funnel plot visually and by Egger’s test and Begg’s test objectively. The funnel plot shows asymmetrical distribution of studies by visual inspection (). Therefore, the presence of publication bias was also assessed by Egger’s regression test (p = .081) and Begg’s rank correlation test (p = .161) with no evidence of publication bias.
Leave –one-out-sensitivity analysis
A leave-one-out sensitivity analysis was carried out to detect the effect of each study on the overall prevalence of vaccination dropout by excluding one study at a time. As a result, studies omitted at a time did not show a significant change on the overall prevalence of vaccination dropout ().
Table 3. The pooled prevalence of vaccination dropout in Sub-Saharan Africa when one study omitted from the analysis a step at a time.
Discussion
Immunization is a cost-effective and affordable method to decrease maternal and child morbidity and mortality. Several immunogenicity studies demonstrated that pregnant women who received the vaccine developed protective antibodies against the disease.Citation48 Various evidences also showed that maternal death decline secondary to tetanus vaccine,Citation49 DTP vaccine,Citation50–52 and influenza vaccine during pregnancy.Citation53
Pentavalent vaccines (a combination vaccine which protects against five killer diseases: diphtheria, pertussis, tetanus, hepatitis B and Hib) have been launched in by 2011.Citation54,Citation55 The pentavalent vaccines provide a golden opportunity to curb Hib disease and hepatitis B along with (DPT) in the developing countries. If the vaccines are provided individually, the coverage of hepatitis B and Hib vaccines usually lags behind DPT coverage. This gap can be filled by using pentavalent vaccine in routine immunization programs.
The first dosage of the pentavalent vaccine is used as a tracer indicator for subsequent doses (often the third). Low dropout rates imply good access to and utilization of vaccination services. The World Health Organization (WHO) suggested using the indicators of immunization dropout to be DTP1 to DTP3, BCG to measles-containing virus (MCV1), and MCV1 to MCV2.Citation56 A high dropout rate between Penta1 and the measles vaccination shows a service usage issue if an infant defaults to the three doses of the pentavalent vaccine.Citation57 If the dropout rate is greater than 10%, the World Health Organization (WHO) indicates that many people are not utilizing the services.Citation58
Determining the overall prevalence of immunization dropouts among children in Sub-Saharan Africa was the goal of the current study. Thus, in Sub-Saharan Africa, the combined frequency of vaccination nonuse was 21.06% (95%.CI: 11.59, 30.53). This result is consistent with research conducted in Iraq (19.3%).Citation59 The socioeconomic similarities of the research areas where both studies were conducted in low- and middle-income countries might be a reason for the explanation.
The findings of this research is significantly higher than a study conducted in India,Citation60 which found that the dropout rates for BCG-DPT3, DPT1-DPT3, and BCG-Measles were 16.1%, 12.9%, and 8%, respectively. This might be brought on by differences in sample size, study participant makeup, health system architecture, and policy. For instance, the prior study had a total sample size of 550 individuals, but the current study had 6326 study participants. The time gap between the studies may also possibly provide an explanation for this disparity.
Comparatively, our study’s findings were lower than those of research conducted in Nepal (28.5%) Citation61 and India (26.1%).Citation62 The study setting, time gap and the sample size across the studies of may be contributing factors to this variation. For instance, the current study was carried out on a sample size of 6326 whereas previous conducted on a sample size of 140 and 550 respectively.
Sub-group analysis was done based on country, study setting, sample size and publication. As a result, Ghana (11.13%) had the lowest prevalence of dropouts while Nigeria had the highest (33.6%).Studies conducted in the community (39%) revealed a higher rate of vaccination dropout than studies conducted in institutions (13.7%). This may be because women living in institutions have access to sufficient health information. Previous research that addressed attitudes and beliefs that might affect a mother’s intention to vaccinate was supported by this conclusion. Healthcare professionals frequently serve as parents’ major source of vaccine information and have been demonstrated to have an impact on uptake.Citation63,Citation64 Studies with fewer than 500 participants reported a rate of vaccination dropout of 22.7%, while studies with more than 500 participants had a prevalence of (18.37%).The prevalence of immunization dropout rate was 29.25% in unpublished research and 18.01% in published studies.
To handle a large variance that occurred in between-study heterogeneity, a random-effect model was used in this research. We conducted leave-one-out sensitivity, and the results reveal that no single study had a substantial effect on the overall prevalence of incomplete immunization. We assessed the possible variability source via sub-group analysis using the study sub-region, sample size, publication and age of children. The high heterogeneity might be due to differences in the sample populations, paper qualities, or socio-cultural, ethnic, and regional differences. This study has some limitations. First, the study protocol was not registered. Second, articles were restricted to only being published in the English language. Third, all of the included studies were cross-sectional, which might affect the outcome variable because of other confounding factors. However, this research has also some strength. First, compressive electronic online international searching engines were used. Second, our review incorporated gray literature as part of the primary studies.
Conclusion
In conclusion, Sub-Saharan Africa has a high prevalence of vaccine dropout. Vaccination dropout rates varied by country, study setting, publication, and sample size. As a result, Ghana had the lowest prevalence of vaccine dropout, compared to Nigeria’s greater prevalence. The prevalence of immunization dropout was also shown to be greater in community-based studies and unpublished research. Studies with sample sizes under 500 also showed a higher occurrence.
To prevent immunization dropout, community education about the value of vaccinations should be prioritized.
Authors’ contributions
NAG conceptualized the study: NAG and KDT contributed during data extraction and analysis: NA, KA and GA, wrote result interpretation: NAG, BB and KDT, Prepared the first draft: NAG, BB and GAA contributed during the conceptualization and interpretation of results and substantial revision: NAG, KDT,BB and GAA. Revised and finalized the final draft manuscript. All the authors read and approved the final version of the manuscript.
Authors’ information
NAG: School of Midwifery, College of Medicine and Health Sciences, Wolaita Sodo University, Sodo, Ethiopia.
KDT: Department of Comprehensive Nursing, College of Medicine and Health Science, Wollo University, Dessie, Ethiopia
GAA: Department of Reproductive Health, College of Medicine and Health Science, Wolaita Sodo University, Sodo, Ethiopia
BB: Department of Psychiatric Nursing, College of Medicine and Health Sciences, Wolaita Sodo University, Sodo, Ethiopia
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All relevant data are within the Manuscript and its Supporting Information files.
Additional information
Funding
References
- World Health Organization. Immunization coverage. Fact sheets; 2020. https://wwwS.who.int/news-room/fact-sheets/detail/immunization-coverage
- United Nations Children Fund (UNICEF). Immunization. UNICEF data: monitoring the situation of children and women. 2020. https://data.unicef.org/topic/child-health/immunization/
- DL SS, Lydon P, Gacic-Dobo M, Eggers R, Conklin L. Global routine vaccination coverage, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1252–11. doi:10.15585/mmwr.mm6444a5.
- Patel MK, Dumolard L, Nedelec Y, Sodha SV, Steulet C, Gacic-Dobo M, Kretsinger K, McFarland J, Rota PA, Goodson JL. Progress toward regional measles elimination — worldwide, 2000–2018. Morb Mortal Wkly Rep. 2019;68(48):1105–11. doi:10.15585/mmwr.mm6848a1.
- World Health Organization (WHO). National immunization coverage score cards estimates for 2018 [Internet]. World Health Organization; 2018:24. [accessed 2020 May 2]. http://www.who.int/immunization/monitoring_surveillance/data/en
- Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–87. doi:10.1016/S0140-6736(10)60549-1.
- World Health Organization. Child mortality 2019. Levels & Trends in Child Mortality. 2019a.
- Services USDoHaH. The state of the National vaccine plan 2013 annual report, protecting the Nation’s health through immunization. 2013.
- Greenwood B. The contribution of vaccination to global health: past, present and future. Philos Trans R Soc B. 2014;369(1645):20130433. doi:10.1098/rstb.2013.0433.
- Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O’Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhea 2013. Lancet. 2013;381(9875):1405–16. doi:10.1016/S0140-6736(13)60222-6.
- Clark A, Sanderson C. Timing of children’s vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet. 2009;373(9674):1543–49. doi:10.1016/S0140-6736(09)60317-2.
- Rainey JJ, Watkins M, Ryman TK, Sandhu P, Bo A, Banerjee K. Reasons related to non-vaccination and under-vaccination of children in low and middle income countries: findings from a systematic review of the published literature, 1999–2009. Vaccine. 2011;29(46):8215–21. doi:10.1016/j.vaccine.2011.08.096.
- Larson HJ, De Figueiredo A, Xiahong Z, Schulz WS, Verger P, Johnston IG, Cook AR, Jones NS. The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMedicine. 2016 Oct 1;12:295–301. doi:10.1016/j.ebiom.2016.08.042.
- Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1736–88. doi:10.1016/S0140-6736(18)32203-7.
- World Health Organization (WHO). Immunization coverage. Fact Sheet. WHO; 2019:1.
- World Health Organization (WHO). Annex to the global vaccine action plan review and lessons learned report [Internet]. World Health Organization; 2018. [accessed 2020 May 18]. http://apps.who.int/bookorders
- Burton A, Monasch R, Lautenbach B, Gacic-Dobo M, Neill M, Karimov R, Wolfson L, Jones G, Birmingham M. WHO and UNICEF estimates of national infant immunization coverage: methods and processes. Bull World Health Organ. 2009;87:535–41. doi:10.2471/BLT.08.053819.
- Okwo-Bele J-M, Cherian T. The expanded programme on immunization: a lasting legacy of smallpox eradication. Vaccine. 2011;29(Suppl 4):D74–9. doi:10.1016/j.vaccine.2012.01.080.
- Chard AN. Routine vaccination coverage — worldwide, 2019. MMWR Morb Mortal Wkly Rep. [Internet]. 2020;69(45):1706. doi:10.15585/mmwr.mm6945a7.
- VanderEnde K, Gacic-Dobo M, Diallo MS, Conklin LM, Wallace AS. Global routine vaccination coverage - 2017. MMWR Morb Mortal Wkly Rep. 2018;67(45):1261–64. doi:10.15585/mmwr.mm6745a2.
- WHO. 2009. Global elimination of measels. World Health Organization.
- UNICEF & WHO. Immunization summary – a statistical reference containing data through 2011. Geneva. 2012 Nov.
- WHO. WHO recommended vaccines BCG, Hepatitis B, Polio, DTP, Hib, pneumococcal, rotavirus,measles, rubella,hpv. [accessed. 2014 Oct 4]. http://www.who.int/Immunization/policy/Immunization_routine_table2.pdf
- World Health Organization (WHO). Progress towards measles control in WHO’s African region, 2001–2008. Weekly Epidemiol Rec. 2009;39(84):397–404.
- Gentile A. Pediatric disease burden and vaccination recommendations: understanding local differences. Int J Infect Dis. 2010;30:1019–29.
- World Health Organization (WHO). Immunization agenda 2030: a global strategy to leave no one behind. WHO. 2019;1–29. https://www.who.int/publications/m/item/immunization-agenda-2030-a-globalstrategy-to-leave-no-one-behind
- Machingaidze S, Wiysonge CS, Hussey GD. Strengthening the expanded program on immunization in Africa: looking beyond 2015. PLoS Med. 2013;10(3):e1001405. doi:10.1371/journal.pmed.1001405.
- WHO. Levels and trends in child mortality: report 2019. The World Bank. 2019.
- Shen AK, Fields R, McQuestion M. The future of routine immunization in the developing world: challenges and opportunities. Glob Health: Sci Pract. 2014;2(4):381–94. doi:10.9745/GHSP-D-14-00137.
- Thapa K, Adhikary P, Faruquee MH, Suwal BR. Associated Factors for Dropout of First Vs Third Doses of Diphtheria Tetanus Pertussis (DPT) vaccination in Nepal. Adv Prev Med. 2021;2021:2021. doi:10.1155/2021/1319090.
- Baguune B, Ndago JA, Adokiya MN. 2017. Immunization dropout rate and data quality among children 12–23 months of age in Ghana. Arch Public Heal. 75(1). doi:10.1186/s13690-017-0186-8.
- Usman HR, Akhtar S, Habib F, Jehan I. Redesigned immunization card and center-based education to reduce childhood immunization dropouts in urban Pakistan: a randomized controlled trial. Vaccine. 2009;27(3):467–72. doi:10.1016/j.vaccine.2008.10.048.
- Moher D, Liberati A, Tetzlaff J, Altman DG. The preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed1000097.
- Chanie MG, Ewunetie GE, Molla A, Muche A. Determinants of vaccination dropout among children 12-23 months age in north Gondar zone, northwest Ethiopia, 2019. PLoS ONE. 2021;16(2):e0246018. doi:10.1371/journal.pone.0246018.
- Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K et al, Chapter 7: Systematic reviews of etiology and risk. Joanna Briggs Institute Reviewer‘s Manual. The Joanna Briggs Institute. 2017 Jul 17;5.
- Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of inter-rater agreement. J Clin Epidemiol. 2012;65(9):934–39. doi:10.1016/j.jclinepi.2011.11.014.
- Kassaw A, Gebere Mariam A, Kebede A, Kebede F. Magnitudes of immunization dropout rate and predictors for 12-23 months aged children in Abobo District southwest Ethiopia. Int J Child Health Nutr. 2022;11(1):25–29. doi:https://doi.org/10.6000/1929-4247.2022.11.01.3.
- Abebe AM, Wudu Kassaw M, Zemariam AB, Estifanos Shewangashaw N. Coverage, opportunity, and challenges of expanded program on immunization among 12–23-month-old children in woldia town, northeast Ethiopia, 2018. Biomed Res Int. 2019Dec 31;2019. doi:10.1155/2019/5302307.
- Shewasinad Yehualashet S, Dessie Nigussie Y, Yilema TD, Mengiste ST, Alemu YT. Vaccine dropout rate and associated factors among children age 12-23 month in Shewa Robit Town, North Shewa Zone, Amahra Region, Ethiopia, a community based cross-sectional study design.
- Adam Haji SL, Ngan’Ga Z, Gura Z, Tabu C, Sandhu H, Arvelo W. Reducing routine vaccination dropout rates: evaluating two interventions in three Kenyan districts. BMC Public Health. 2016;16:15. doi:10.1186/s12889-016-2823-5.
- Ibrahim A, Some E, Otieno O. Dropout from Routine Immunization Among Children 12-23 Months of Age in Garissa Sub County, Kenya. 2017;3(3):1–16.
- Chinawa JM. Immunization dropout rates in Ihe, Awgu Local Government Area, Enugu State, South East Nigeria: a 1 year review. Ann Med Health Sci Res. 2014 Jul-Aug;4(4):642. doi:10.4103/2141-9248.139360.
- Errie Yenyi S. Influence of low rate of reporting of adverse events following immunization on immunization dropout. 2019.
- Robert Twene. 2000. Why high dropout on immunisation programme in the Ashanti Akim North District. University of Ghana http://ugspace.ug.edu.gh
- Ateudjieu J, Ndinakie Yakum M, Pascal Goura A, Maureen Tembei Douanla Koutio Ingrid A, Bita’a Landry B, Bita’a Landry B, Kenfack B, Amada L, Tadzong I, Bissek AC, et al. EPI immunization coverage, timeliness and dropout rate among children in a West Cameroon health district: a cross sectional study. BMC Public Health. 2020;20(1):228. doi:https://doi.org/10.1186/s12889-020-8340-6.
- Powelson J, Magadzire BP, Draiva A, Denno D, Ibraimo A, Benate BBL, Jahar LC, Marrune Z, Chilundo B, Chinai JE, et al. Determinants of immunisation dropout among children under the age of 2 in Zambézia province, Mozambique: a community-based participatory research study using Photovoice. BMJ Open. 2022;12(3):e057245. doi:10.1136/bmjopen-2021-057245.
- Ali Mohamud I. 2018. Study on determinants of the immunization dropout status among under five children in Mogadishu. Somalia: Doctoral dissertation, Daffodil International University.
- Sumaya CV, Gibbs RS. Immunization of pregnant women with influenza A/New Jersey/76 virus vaccine: reactogenicity and immunogenicity in mother and infant. J Infect Dis. 1979;140(2):141e6. doi:10.1093/infdis/140.2.141.
- Thwaites CL, Loan HT. Eradication of tetanus. Br Med Bull. 2015;116:69e77. doi:10.1093/bmb/ldv044.
- Donegan K, King B, Bryan P. Safety of pertussis vaccination in pregnant women in UK: observational study. BMJ. 2014;349(jul11 1):4219. doi:10.1136/bmj.g4219.
- Kharbanda EO, Vazquez-Benitez G, Lipkind HS, Klein NP, Cheetham TC, Naleway A, Omer SB, Hambidge SJ, Lee GM, Jackson ML, et al. Evaluation of the association of maternal pertussis vaccination with obstetric events and birth outcomes. J Am Med Assoc. 2014;312(18):1897e904. doi:10.1001/jama.2014.14825.
- Berenson AB, Hirth JM, Rahman M, Laz TH, Rupp RE, Sarpong KO. Maternal and infant outcomes among women vaccinated against pertussis during pregnancy. Hum Vaccines Immunother. 2016;12(8):1965e71. doi:10.1080/21645515.2016.1157241.
- Webb SA, Seppelt IM, Investigators AI. Pandemic (H1N1) 2009 influenza (“swine flu”) in Australian and New Zealand intensive care. Crit Care Resusc. 2009;11:170e2.
- http://www.gavialliance.org/support/nvs/hib/
- http://www.unicef.org/supply/files/8._DTP-containing_vaccines_including_pentavalent_and_hexavalent.pdf
- World Health Organization (WHO). Guidance for immunization programme managers. Geneva, Switzland; 2020:1–30.
- Health Education and Training (HEAT). Immunization module: monitoring your immunization programme n.d. [accessed 2019 Jan 20]. http://www.open.edu/openlearncreate/mod/oucontent/view.php?id=53371§i on=1.4.2
- Mmanga K, Mwenyenkulu TE, Nkoka O, Ntenda PAM. 2021. Tracking immunization coverage, dropout and equity gaps among children ages 12–23 months in Malawi – bottleneck analysis of the Malawi Demographic and Health Survey. Int Health. doi:10.1093/inthealth/ihab038.
- Abdalsaid EM, Alhilfi RA, Maki ZT. Immunization Coverage and its determinants in children aged 12-23 months in Basrah. Med J Basrah Univ: MJBU. 2017;35(2):No.2. doi:10.33762/mjbu.2017.134239.
- Singh J, Neki NS. Evaluation of vaccination coverage and Dropout rates among children of age 0–5 years in slums of Amritsar city. Int J Curr Res Biol Med. 2018;3(3):16–22.
- Shrestha SR, Shakya B, Oli R. Assessment of factors associated with dropout for pentavalent vaccine in tertiary care hospital of Kathmandu, Nepal. Nepal Med Coll J. 2020;22(3):106–10. doi:10.3126/nmcj.v22i3.32624.
- Singh J, Deepti SS, Mahajan S, Lal M, Singh T, Neki NS. Evaluation of vaccination coverage and Dropout rates among children of age 0-5 years in slums of Amritsar city. Int J Curr Res Biol Med. 2018;3:16–22.
- Siddiqui M, Khan AA, Varan AK, Esteves-Jaramillo A, Sultana S, Ali AS, Zaidi AKM, Omer SB. Intention to accept pertussis vaccine among pregnant women in Karachi, Pakistan. Vaccine. 2017;35(40):5352–59. doi:10.1016/j.vaccine.2017.08.033.
- Varan AK, Esteves-Jaramillo A, Richardson V, Esparza-Aguilar M, Cervantes-Powell P, Omer SB. Intention to accept Bordetella pertussis booster vaccine during pregnancy in Mexico City. Vaccine. 2014;32(7):785–92. doi:10.1016/j.vaccine.2013.12.054.