ABSTRACT
The safety profile of the 9-valent human papillomavirus vaccine (9vHPV) was evaluated based on the reporting rate of adverse events following immunization (AEFI) obtained from the passive surveillance data in Zhejiang. The 9vHPV AEFI reports in Zhejiang were collected and reviewed from the National Adverse Event Following Immunization Surveillance System (NAEFISS) from 2019 to 2021. Reporting rates of AEFI were analyzed under multiple aspects, including age, city, number of vaccinations, AEFI categories, and diagnosis categories. This study used the reporting odds ratio (ROR) for anomalous signal assessment. The NAEFISS collected 331 AEFI reports after administering 1,064,851 doses of 9vHPV, with a crude AEFI rate of 3.12/10,000 doses. The third dose had the highest reporting rate of minor vaccine-related reaction (n = 80, 3.06 per 10,000), followed by the first dose (n = 134, 2.98 per 10,000), and second dose (n = 76, 2.15 per 10,000). Fever/redness/induration was the most common minor adverse event (281 records, 2.64/10,000 doses). Nine cases of urticaria, ten cases of allergic rash, and ten cases of syncope were recorded. This study found a positive signal association between 9vHPV immunization and adverse events such as syncope, encephalitis, sterile abscess, and urticaria. This study did not identify any new emerging safety concerns. In the future, more research is needed to validate and further explore adverse reactions associated with 9vHPV.
Introduction
Human papillomaviruses (HPVs) are the major pathogens contributing to precancerous lesions of several cancers, including cervical, oropharyngeal, anal, and penile cancers. According to current estimates, infections induced by high-risk types of HPV cause more than 90% of cervical cancer.Citation1 HPV-16 and HPV-18 are the most common high-risk HPV types, accounting for 70% of cervical cancer cases.Citation2 The 9-valent human papillomavirus vaccine (9vHPV) protects against not only the quadrivalent HPV vaccines’ HPV 6, 11, 16, and 18, but also the additional five high-risk types (HPV 31, 33, 45, 52, 58). HPV58, which is associated with cervical cancer, is uncommon elsewhere in the world, but it ranks third among all HPV types in China.Citation3 The China Drug Administration approved the 9vHPV vaccine, Merck’s Gardasil®9, in May 2018, and it is currently recommended for females aged 16–26.
Vaccination is an effective strategy for preventing a wide range of contagious and fatal diseases. However, adverse reactions to vaccination are quite common and are on the rise due to improved adverse event reporting networks; consequently, this may also increase public concern about vaccine safety.Citation4,Citation5 In clinical trials, the most frequent adverse events (AEs) of the 9vHPV vaccine were injection-site pain, swelling, and erythema, which were more prevalent in comparison to the quadrivalent HPV vaccine; there were also seven reported deaths, none of which were considered vaccine-related.Citation6 Recently, the safety issue was regarded as a primary concern in surveys carried out from parents about HPV vaccination.Citation7,Citation8 According to a systematic review of HPV vaccination in Europe, the most common patient concerns prior to vaccination were about vaccine safety.Citation9 Moreover, the Nordic Cochrane Center criticized approaches to handling the safety assessment of HPV vaccination by the European Medicines Agency.Citation10 Unlike in clinical trials, where rare or delayed onset adverse events are more difficult to detect, the safety data gathered by the adverse events following immunization (AEFI) surveillance system were derived from real-world adverse events.Citation6 Therefore, continuous AEFI monitoring in the general population is critical for identifying and evaluating the safety profile of the 9vHPV vaccine.Citation11
This study aimed to assess the reporting rate of AEFIs following 9vHPV based on real- world data derived from the National Adverse Event Following Immunization Surveillance System (NAEFISS) database from 2019 to 2021 in Zhejiang, China.
Methods
Study area
Zhejiang province, located in eastern China, is an economically prosperous region with a population of around 70 million people. Immunization data was dynamically collected and maintained on the Zhejiang provincial Immunization Information System (ZJIIS).Citation12 With the assistance of immunization physicians, 11 types of vaccines and over 25 million inoculations have been archived in the ZJIIS to date.
Sources of AEFI data
NAEFISS is a national passive surveillance system to detect common and rare AEFIs by collecting AEFI reports nationwide.Citation13 Reporting and investigation procedures were described in previous studies.Citation14 Due to the time required for data correction and cleaning, we collected AEFI data of women who received 9vHPV from the NAEFISS between 2019 and 2021. The national surveillance system included variables of relevant attributes such as age, date of vaccination, manufacturer, outcome, and so on. The administered doses of 9vHPV were acquired from the ZJIIS over the same period.
Category of AEFI
All AEFIs are classified into 5 categories in accordance with the national guidelines launched by the Health Authorities of China.Citation15 (1) vaccinal error; (2) coincidental events; (3) anxiety reactions; (4) vaccine product-related reactions (minor reaction and severe reaction); (5) vaccine quality defect-related reactions. The definition of vaccine product-related reactions (minor reaction and severe reaction), also called vaccine reaction following immunization (common and rare vaccine reaction), was described in the previous publication in detail.Citation15 Briefly, minor reaction occurs within a few hours of injection, including local pain, swelling, or redness at the injection site, which could be resolved quickly and pose little danger. Severe reactions may result in tissue or organ damage, impaired function, disabling, or life-threatening, but rarely. AEFI severities were assessed as two types, serious and non-serious, according to the national guidelines: (1) non-serious, with no additional intervention or with hospital visit or event interfering with daily activities or loss of working hours. (2) serious, with untoward medical occurrences resulting in birth defect, life-threatening, death, persistent or significant incapacity/disability, hospitalization or prolonged hospital stay. Serious AEFIs include, but are not limited to, allergic shock, allergic laryngeal edema, allergic purpura, thrombocytopenic purpura, systemic disseminated BCG infection, localized allergic necrotic reaction (Arthus reaction), febrile convulsion, encephalitis and meningitis, epilepsy, brachial neuritis, polyneuritis, vaccine-associated paralytic poliomyelitis, Guillain–Barre syndrome, encephalopathy, BCG osteomyelitis, toxic shock syndrome, syncope, and systemic purulent infection.
Data analysis
The AEFI incidence was calculated per 10,000 doses of 9vHPV administered. Data collation was done using Microsoft Excel 2019, and descriptive analysis was conducted using R software 3.5.0. The trend of reporting rates was depicted in months using graphical representation. Onset interval refers to the time interval between vaccination time, and the reported onset of earliest symptoms and the percentages of onset intervals were investigated in this study. Disproportionality analysis was conducted based on this study’s algorithm of reporting odds ratio (ROR).Citation16 The algorithm compared the reporting ratio of AEFI reported for one specific vaccine with the reporting ratio for all other vaccines. Generally, the cutoff value is defined as a value of ROR-1.96SE > 1 (standard error [SE]). If the ROR is higher than the threshold value, it is defined as a positive signal.
Results
After excluding duplicated records, a total of 331 AEFI records following 9vHPV were registered in the NAEFISS. Of which, 329 cases (99.40%) were classified as non-serious, while 2 (0.60%) were classified as serious. 290 (87.61%) were categorized as minor vaccine product-related reactions, 19 (5.74%) were serious adverse reactions, 6 (1.81%) were coincidental events, and 16 (4.83) were psychogenic reactions. The majority of AEFI reports were observed in women aged 22–24 (45.02%) and 25–26 (25.08%). The time interval of most symptoms onset from vaccination was within 24 hours (61.63% accounting for all AEFI records) ().
Table 1. Characteristics of adverse events following immunization (AEFI) with 9-valent HPV vaccine.
During the study’s duration, 1,064,851 doses of 9vHPV were given in Zhejiang, with an overall AEFI reporting rate of 3.12/10,000 doses. The peak reporting rate was in Feb 2020 with 7.47/10,000 doses (). The reporting rate was highest in Jiaxing city (4.26/10,000 doses) and lowest in Quzhou (1.73/10,000 doses) besides Zhoushan due to no AEFI reporting. The reporting rate of severe vaccine product-related reaction was the highest in Jiaxing city (0.39/10,000 doses), while no severe vaccine-related reaction was observed in the other three cities, including Quzhou, Zhoushan and Lishui ().
Figure 1. Reporting rate of adverse event following immunization of 9vhpv from 2019 to 2021, Zhejiang province (/10,000 doses).
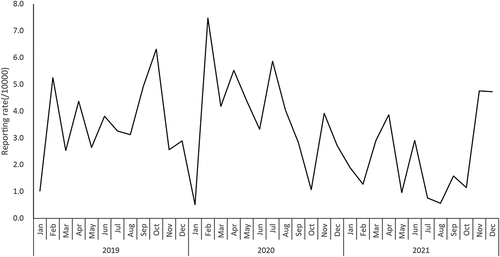
Table 2. Distribution of 9vHPV AEFI reports by city.
The results showed that the reporting rate of different AEFI categories differed depending on the dose received. For minor vaccine product-related reactions, the reporting rate was 2.98 per 10,000 following the first vaccination; 2.15 per 10,000 after the second; 3.06 per 10,000 after the third. The reporting rate for severe vaccine-related reactions was 0.27 per 10,000 after the first injection; 0.14 per 10,000 after the second; 0.08 per 10,000 after the third injection. The reporting rate of the psychogenic reaction was 0.31 per 10,000 after the first injection ().
Table 3. Reporting rate of the 9vHPV AEFI by doses.
After reviewing the clinical diagnosis and classifications of the 331 AEFI records, 19 were classified as severe vaccine product-related reactions. There were ten cases of anaphylactic rash (0.09/10,000 doses), six cases of urticaria (0.06/10,000 doses), one case of angioedema (0.01/10,000 doses), and one case of sterile abscess formation (0.01/10,000 doses). Among the 290 cases of minor vaccine-related reactions, the most common AEFI reported was fever/redness/induration with 281 cases (2.64/10,000 doses), followed by urticaria and other reactions. The abnormal signals with according to ROR-1.96SE were detected for encephalitis (5.45), sterile abscess (2.43), urticaria (1.16), apsychia (43.96), and other reactions (1.03) ().
Table 4. Clinical diagnosis of AEFI reports following human papillomavirus 9-valent vaccine (9vHPV) from 2019–2021.
Minor vaccine-related reactions (62.41%) appeared within 24 hours of administration, and 19.66% appeared within 1–2 days. In contrast, most severe vaccine-related reactions (42.11%) occurred within 1–2 days of vaccination and 26.32% within 24 hours ().
Table 5. Interval between AEFI onset and immunization.
Discussion
Over a 3-year period (2019–2021) of post-licensure safety surveillance for 9vHPV vaccine in Zhejiang, unusual AEFI reports were not identified in the NAEFISS. Despite inherent limitations of the NAEFISS as a passive surveillance system, rare AEFIs could be potentially observed in the post-licensure monitoring with an increase in administered doses. After the administration of 1,064,851 doses, the 9vHPV overall rate of adverse reporting was 3.12 per 10,000 doses, which is higher than the AEFI reporting rate of 4vHPV (2.68/10,000 doses).Citation13 This could partly be attributed to the higher aluminum-based adjuvant concentration of 9vHPV versus 4vHPV (0.5 vs. 0.225 mg). The majority of AEFIs were non-serious, which was similar to post-vaccine surveillance reported by Shimabukuro TT in the United States at 97.4%,Citation17 with a serious AEFI reporting rate of 0.60 per 10,000 doses, which is 3-fold the serious AEFI reporting rate (0.20/10,000 doses) of the US Vaccine Adverse Event Reporting System (2007–2017).Citation18 This disparity could be partly explained by differences in definitions of serious AEFI or the sensitivity of the surveillance system to AEFI.Citation19 Overall, the most common AEFIs of 9vHPV were the same general adverse events that had previously been reported in clinical trials and epidemiological studies.
According to the temporal analysis, one peak of the reported AEFI rate was observed in February 2020, with a fluctuating trend over this period and then gradually declining after reaching the peak. The peak was most likely caused by reporting sensitivity, which is generally high in the first few months after a new vaccine is introduced, then decreases and stabilizes as the vaccination campaign progresses.Citation20 According to the findings, the age range of 22–26 years accounted for 70% of all reported AEFIs. The main reasons may be related to the sensitivity of this age group of 9vHPV recipients to the safety of HPV vaccines Citation21 and the cognition degree of HPV vaccine knowledge acquired from information sources.Citation22 The majority of AEFI cases occurred after the first inoculation, with 164 cases occurring after the first dose, 84 cases following the second, and 83 cases following the third. However, the third dose had the highest reporting rate of the administered dose with the minor vaccine product-related reaction (n = 80, 3.06 per 10,000), followed by the first dose (n = 134, 2.98 per 10,000), and second dose (n = 76, 2.15 per 10,000). Fever, redness and induration were the most common minor adverse reactions, similar to other vaccines. In parallel, the reporting onset time for most reactions was short after vaccinations.
The 9vHPV reporting rates for serious vaccination-related reactions and total AEFI varied across cities in this study, possibly due to the different surveillance sensitivities across cities.Citation23,Citation24 For instance, some cities had a high overall reporting AEFI rate but had no severe vaccination-related reaction reports (e.g., Quzhou and Lishui). This could be attributed to differences in cognitive abilities between investigation and notification procedures. Thus, further in-depth evaluations and comparisons between the AEFI sensitivities among cities are warranted to eliminate this issue.
Similar to previous findings, positive signals and cases of encephalitis, sterile abscess, urticaria, and syncope were reported in this study.Citation25 Regarding syncope, the association with 9vHPV vaccines had a high ROR (ROR = 43.96). The vaccine-related reaction was consistent with previous CDC survey research findings, indicating that syncope occurs when the patient reacts to pain or anxiety during the vaccination procedure.Citation26 Another vaccine-related reaction worthy of note is the formation of sterile abscess (ROR = 2.43). One study found a link between aluminum adjuvant and sterile abscesses and their recurrence.Citation27 The higher aluminum content in the 9vHPV versus 4vHPV vaccine could be one explanation. Nonetheless, more observations and research are needed to better understand this phenomenon.
Due to the inadequacy of the passive surveillance system, this study had several limitations. The NAEFISS is a passive reporting system that is susceptible to limitations such as underreporting, reporting biases, and data completeness. For example, non-serious AEFI cases may result in possible underreporting, which leads to an underestimate of reported AEFIs. The surveillance data may be incomplete during investigation procedures of AEFI. The majority of reports are recorded simply as “Fever/redness/induration” in the system categories, and no further distinction could be made between the severity of injection site pain/redness and systemic events such as fever. The positive signals between vaccination and the AEFI detected through the system did not necessarily indicate causality. As a result, more research and evidence are required to identify and evaluate the relationships. However, there are also some strengths to this study; all AEFI reports were inspected and evaluated in a standardized manner according to the national AEFI surveillance guidelines. Unlike other studies that used distributed doses as the denominator, the administered doses were captured systematically from the vaccination systems to calculate AEFI rates, which tend to be more accurate than the rate of detections.
Conclusion
The efficacy of 9vHPV in preventing HPV-related diseases and infections, including cervical cancer, is significant. Our findings revealed that most AEFIs that appeared after receiving the 9vHPV from 2019 to 2021 were not serious, and no new safety issues were identified in the report, which is consistent with that of the 4vHPV vaccine, indicating that the vaccine was comparatively safe. For data monitoring, the anomalous signal observed made it difficult to infer a causal relationship between the AEFI and the 9vHPV vaccine. As a result, more research is needed to explore the relationship further.
Author contributions
Y.H. and X.J.P. conceived and designed the experiments; H.L. and Y.P.C. performed the experiments; H.L. and F.X.C. analyzed the data; L.Z.S. and Y.W. contributed reagents/materials/analysis tools; F.X.C. and Y.H. wrote the paper.
Ethics approval and consent to participate
This study was approved by the ethical review board of Zhejiang provincial CDC. All the data were exported anonymously and kept confidential without identifying information.
Acknowledgments
We thank all physicians for submitting AEFI reports in the NAEFISS concerning the 9vHPV vaccines of Zhejiang province. We appreciate the efforts of our CDC colleagues at the county and municipal levels who collected and compiled the data.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–6. doi:10.1002/ijc.30716. PMID: 28369882.
- Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121(3):621–32. doi:10.1002/ijc.22527. PMID: 17405118.
- Yin F, Wang Y, Chen N, Jiang D, Qiu Y, Wang Y, Yan M, Chen J, Zhang H, Liu Y. A novel trivalent HPV 16/18/58 vaccine with anti-HPV 16 and 18 neutralizing antibody responses comparable to those induced by the Gardasil quadrivalent vaccine in rhesus macaque model. Papillomavirus Res. 2017;3:85–90. doi:10.1016/j.pvr.2017.02.005. PMID: 28720462.
- Baxter P. Pertussis vaccine encephalopathy: ‘oh! Let us never, never doubt. Dev Med Child Neurol. 2010;52(10):883–84. doi:10.1111/j.1469-8749.2010.03781.x. PMID: 21175453.
- Jegede AS. What led to the Nigerian boycott of the polio vaccination campaign? PLoS Med. 2007;4(3):e73. doi:10.1371/journal.pmed.0040073. PMID: 17388657.
- Moreira ED Jr, Block SL, Ferris D, Giuliano AR, Iversen OE, Joura EA, Kosalaraksa P, Schilling A, Van Damme P, Bornstein J, et al. Safety profile of the 9-valent HPV vaccine: a combined analysis of 7 phase III clinical trials. Pediatrics. 2016;138(2). doi: 10.1542/peds.2015-4387. PMID: 27422279.
- Beavis A, Krakow M, Levinson K, Rositch AF. Reasons for lack of HPV vaccine initiation in NIS-teen over time: shifting the focus from gender and sexuality to necessity and safety. J Adolesc Health. 2018;63(5):652–56. doi:10.1016/j.jadohealth.2018.06.024. PMID: 30348283.
- Gilkey MB, Mohan D, Janssen EM, McRee AL, Kornides ML, Bridges JFP. Exploring variation in parental worries about HPV vaccination: a latent-class analysis. Hum Vaccin Immunother. 2019;15(7–8):1745–51. doi:10.1080/21645515.2019.1574157. PMID: 30951396.
- Karafillakis E, Larson HJ, consortium A. The benefit of the doubt or doubts over benefits? A systematic literature review of perceived risks of vaccines in European populations. Vaccine. 2017;35(37):4840–50. doi:10.1016/j.vaccine.2017.07.061. PMID: 28760616.
- Nordic Cochrane Centre. Complaint to the European Medicines Agency (EMA) over maladministration at the EMA. [ accessed 2022 Nov 30]. https://www.deadlymedicines.dk/wp-content/uploads/2019/02/10.-2016-05-26-Complaint-to-EMA-over-EMAs-handling-of-safety-of-the-HPV-vaccines.pdf
- Alicino C, Merlano C, Zappettini S, Schiaffino S, Della Luna G, Accardo C, Gasparini R, Durando P, Icardi G. Routine surveillance of adverse events following immunization as an important tool to monitor vaccine safety. Hum Vaccin Immunother. 2015;11(1):91–94. doi:10.4161/hv.34360. PMID: 25483520.
- Hu Y, Liang H, Wang Y, Chen Y. Inequities in childhood vaccination coverage in Zhejiang, Province: evidence from a decomposition analysis on two-round surveys. Int J Environ Res Public Health. 2018;15(9):2000. doi:10.3390/ijerph15092000. PMID: 30217080.
- Hu Y, Pan X, Shen L, Chen F, Wang Y, Liang H, Chen Y, Lv H. Post-licensure safety monitoring of quadrivalent human papillomavirus vaccine using the national adverse event following immunization surveillance system from Zhejiang province, 2018–2020. Hum Vaccin Immunother. 2021;17(12):5447–53. doi:10.1080/21645515.2021.1978793. PMID: 34613883.
- Pan X, Lv H, Chen F, Wang Y, Liang H, Shen L, Chen Y, Hu Y. Analysis of adverse events following immunization in Zhejiang, China, 2019: a retrospective cross-sectional study based on the passive surveillance system. Hum Vaccin Immunother. 2021;17:3823–30. PMID: 34170800.
- Liu D, Wu W, Li K, Xu D, Ye J, Li L, Wang H. Surveillance of adverse events following immunization in China: past, present, and future. Vaccine. 2015;33(32):4041–6. doi:10.1016/j.vaccine.2015.04.060. PMID: 25936727.
- Sakaeda T, Tamon A, Kadoyama K, Okuno Y. Data mining of the public version of the FDA adverse event reporting system. Int J Med Sci. 2013;10(7):796–803. doi:10.7150/ijms.6048. PMID: 23794943.
- Shimabukuro TT, Su JR, Marquez PL, Mba-Jonas A, Arana JE, Cano MV. Safety of the 9-valent human papillomavirus vaccine. Pediatrics. 2019;144(6). doi:10.1542/peds.2019-1791. PMID: 31740500.
- Bonaldo G, Vaccheri A, D’Annibali O, Motola D. Safety profile of human papilloma virus vaccines: an analysis of the US vaccine adverse event reporting system from 2007 to 2017. Br J Clin Pharmacol. 2019;85(3):634–43. doi:10.1111/bcp.13841. PMID: 30569481.
- Joshi J, Das MK, Polpakara D, Aneja S, Agarwal M, Arora NK. Vaccine safety and surveillance for adverse events following immunization (AEFI) in India. Indian J Pediatr. 2018;85(2):139–48. doi:10.1007/s12098-017-2532-9. PMID: 29170922.
- Dey A, Wang H, Quinn H, Pillsbury A, Glover C, Hickie M, Wood N, Beard F, Macartney K. Surveillance of adverse events following immunisation in Australia annual report, 2019. Commun Dis Intell. 2018;2021:45. doi:10.33321/cdi.2021.45.23. PMID: 33934694.
- Shah PD, Calo WA, Gilkey MB, Boynton MH, Alton Dailey S, Todd KG, Robichaud MO, Margolis MA, Brewer NT. Questions and concerns about HPV vaccine: a communication experiment. Pediatrics. 2019;143(2). doi:10.1542/peds.2018-1872. PMID: 30670584.
- Costa AS, Gomes JM, Germani A, da Silva MR, Santos EFS, Soares Junior JM, Baracat EC, Sorpreso ICE. Knowledge gaps and acquisition about HPV and its vaccine among Brazilian medical students. PloS One. 2020;15(3):e0230058. doi:10.1371/journal.pone.0230058. PMID: 32191725.
- Nzolo D, Ntetani Aloni M, Mpiempie Ngamasata T, Mvete Luemba B, Bazundama Marfeza S, Bothale Ekila M, Nsibu CN, Tona NL. Adverse events following immunization with oral poliovirus in Kinshasa, Democratic Republic of Congo: preliminary results. Pathog Glob Health. 2013;107(7):381–84. doi:10.1179/2047773213Y.0000000113. PMID: 24392682.
- Wu W, Liu D, Nuorti JP, Li K, Xu D, Ye J, Zheng J, Cao L, Wang H. Deaths reported to national surveillance for adverse events following immunization in China, 2010–2015. Vaccine. 2019;37(9):1182–87. doi:10.1016/j.vaccine.2019.01.009. PMID: 30709723.
- Yih WK, Kulldorff M, Dashevsky I, Maro JC. A broad safety assessment of the 9-valent human papillomavirus vaccine. Am J Epidemiol. 2021;190(7):1253–59. doi:10.1093/aje/kwab022. PMID: 33558897.
- Centers for Disease C, Prevention. Syncope after vaccination—United States, January 2005–July 2007. MMWR Morb Mortal Wkly Rep. 2008;57(17):457–60. PMID: 18451756.
- Klein NP, Edwards KM, Sparks RC, Dekker CL, Clinical Immunization Safety Assessment N. Recurrent sterile abscesses following aluminium adjuvant-containing vaccines. BMJ Case Rep. 2009;2009. doi:10.1136/bcr.09.2008.0951. PMID: 21686546.
