ABSTRACT
The increased dose of hepatitis B vaccine has been adopted for newborns since 2013 in Fujian, China. However, little is known about the impact of this measure on hepatitis B virus (HBV) prevention. We used the seroepidemiological surveys conducted in 2014 and 2020 to address the concern. Compared with subjects who received a 5 μg hepatitis B vaccine, participants who took a 10 μg hepatitis B vaccine were associated with a lower risk of HBV infection (adjusted odds ratio [OR] 0.26, 95% confidence interval [CI]: 0.10–0.68) and a marginal reduction risk of anti-HBc positive (OR, 0.37; 95% CI: 0.13–1.08; P = .07), but not for HBsAg carrier risk. The relation between vaccine dose and risk of anti-HBc positive (OR, 0.20; 95% CI: 0.05–0.81) became slightly stronger and significant among children investigated in 2020 who probably received universal vaccination. No significant association was found for subjects whose mothers were positive for HBsAg. The current 10 μg hepatitis B vaccines for universal vaccination for newborns are reasonable and effective in HBV prevention. More measures should be taken to reduce the risk of HBsAg carriers for infants whose mothers are positive for HBsAg.
Introduction
Hepatitis B virus (HBV) infection is a high prevalence in China and deemed as a major public health problem.Citation1,Citation2 According to the first Chinese national hepatitis sero-epidemiological survey conducted in 1992, the prevalence of HBV surface antigen (HBsAg) among the population aged 1–59 years old was 9.75%.Citation3 Hepatitis B vaccination is the most effective measure to prevent HBV infection.Citation4 To control and prevent HBV infection, the Chinese government has incorporated hepatitis B vaccine into the national immunization program in 1992 and further included it as a national immunization program vaccine series for newborns and infants in 2002.Citation5 These policies effectively improved the coverage of hepatitis B vaccination and then induced a reduction of HBV infection.Citation6 The HBsAg positive prevalence in the general population decreased to 7.18% in 2006Citation7 and was estimated to be 6.89% between 2013 and 2017.Citation1
The impact of the hepatitis B vaccination policy in reducing HBV infection was identified in high HBV pandemic areas of China, such as in Fujian province.Citation8 The achievement is particularly evident for children aged <5 years old. According to three hepatitis B sero-epidemiological surveys carried out in 1992, 2006, and 2014, respectively, the prevalence of HBV infection in children <5 years old hugely decreased from 21.67% in 1992 to 0.23% in 2014.Citation9
To achieve the goal of eliminating hepatitis B disease,Citation6 the government has also taken many supplementary measures on the basis of hepatitis B vaccination policy. These relevant measures that were conducive to improve hepatitis B vaccination and aimed to reduce HBV infection, which were studied in previous studies concerning routine coverage, surveillance and assessment,Citation10 supplementary immunization activities,Citation11 supply of free hepatitis B immunoglobulin for mothers positive for HBsAg,Citation12 and timely initiation of the first dose (e.g., within 24 h after birth) of hepatitis B vaccine.Citation13 In addition, the government has provided a 10 μg hepatitis B vaccine to replace the original 5 μg vaccine since 2013 to further improve the protective effect of hepatitis B vaccination in Fujian Province. However, little is known about the impact of this measure on HBV prevention.
We used the seroepidemiological surveys conducted in 2014 and 2020 in Fujian province to evaluate the impact of vaccine dose on HBV prevention. We hypothesized that the increased vaccine dose would help to reduce HBV infection.
Methods
Study design and data collection
This study contained two surveys conducted in five national disease surveillance points in Fujian province in 2014 and 2020, respectively. Target populations were local residents who resided in the county for ≥6 months. The 2014 survey used a 2-stage cluster random sample. First, two or three towns were selected by probability proportional to the size method in each county. One village was randomly identified within each selected town. Second, potential subjects were then randomly recruited from local government lists of residents according to the age group strata (1–4 years, 5–14 years, and 15–29 years).Citation14 The 2020 survey focused on local residents aged 1–59 years old. Participants were selected by the stratified 3-stage cluster random sampling method according to the survey protocol that was the same with the 2006 survey.Citation8 Briefly, 12 towns were randomly selected for the first stage and then 12 villages (one village per township) at random for the second stage. A total of 5873 eligible subjects were recruited from the list of village residents by stratified age groups of 1–4, 5–14, and 15–59 years.Citation8,Citation14
Trained professionals were responsible for the field investigation using a unified questionnaire.Citation14 Basic characteristics (e.g., birth year, gender, ethnicity, birth place, and education level), mother’s HBsAg status (negative, positive, or unknown), and history of hepatitis B vaccination (e.g., yes, no, or unknown) were collected for all subjects. Immunization information of hepatitis B vaccine (e.g., vaccination date, vaccine dose, and type) was further investigated from the vaccination cards or was self-reported for all subjects in the 2014 survey. For children aged <15 years in the 2020 survey, these messages were extracted from the inoculation card or certificate. Participants aged ≥15 years in 2020 and those without hepatitis B vaccination were excluded from the analysis. This study was approved by the ethics committee of the Chinese Center for Disease Control and Prevention (CDC) (No. 201811). Written informed consent was obtained before the survey.
Group of hepatitis B vaccines
We classified the surveyed subjects into three groups according to the dosage of the first dose of hepatitis B vaccine (5 μg, 10 μg, or unknown). A total of 278 subjects who received unknown dosage of the vaccine were classified by their birth year. Children who received the vaccine before 2013 were recognized as the 5 μg vaccine group, while those vaccinated in or after 2013 were deemed as the 10 μg group. Two hundred and ten children who reported unknown hepatitis B vaccination were grouped as an unknown group. In addition, eight persons who received 20 μg vaccine were classified as the 10 μg group.
Outcomes
The primary outcome was HBV infection, which was defined according to the history of hepatitis B vaccination. Participant positive for HBsAg or antibody to hepatitis B core antigen (anti-HBc) was deemed as HBV infection for those with a history of hepatitis B vaccination.Citation8 An additional positive for anti-HBs was further considered as HBV infection for subjects without hepatitis B vaccination. A large-scale leak detection and revaccination was carried out for children born since 1992,Citation15 therefore, for residents with an unknown history of hepatitis B vaccination, we recognized subjects born ≥1992 as having a hepatitis B vaccination, while as unvaccinated people who were born before 1992.
Given that the reduction in anti-HBc positive status may not be proportional to the reduction in HBsAg status Citation14 and that the HBV infection and clearance may have occurred prior to vaccination leading to the anti-HBc positive status, we also separately considered HBsAg and anti-HBc positive as the secondary outcomes.
Laboratory measurement
Blood samples of the subjects were collected and processed by the laboratory physician during the field investigation. Separated serums were stored at a temperature of −80°C or lower in the municipal CDC after the specimen disposal. After the field survey, all specimens were transported to the laboratory of Fujian CDC and tested by enzyme-linked immunosorbent assay (ELISA) using Fujian CDC using detection reagents issued by China CDC (e.g., HBsAg, anti-HBs, or anti-HBc).Citation14 Institute of Viral Diseases of China CDC was responsible for the detection of HBeAg and anti-HBe for those positive for HBsAg using Abbott microparticle ELISA.Citation14
Covariates
In the present study, we collected covariates, such as gender (male or female), ethnicity (Han or non-Han), birth place (hospital, home, or unknown), and mother’s HBsAg status (positive, negative, or unknown), as potential confounders. In addition, five counties were divided into urban (Jiaocheng and Meilie) or rural (Huian, Yongding, and Jianou) by the administrative division.Citation8 According to the time nodes for the implementation of the free hepatitis B vaccination policy (e.g., 2002), we divided the participants into two birth cohorts (<2002 or ≥2002).Citation8 All these covariates were included in the adjusted models as potential confounders.
Data analysis
Distribution (frequents and %) of general characteristics and outcomes was displayed by category variables across the three groups. Differences across the groups or between the 5 μg and 10 μg groups were compared by chi-square or Fisher’s exact test.
Logistic regression was constructed to estimate the association between hepatitis B vaccine dose and the risk of HBV infection, HBsAg carrier, and anti-HBc positive. Odds ratio (OR) and 95% confidence interval (CI) were calculated for the risk in subjects with 10 μg vaccine compared with those with 5 μg vaccine. Adjusted ORs and 95% CI were measured after controlling for potential covariates.
We conducted a sensitivity analysis, in which the surveyed but not treated hepatitis B vaccine dose was used to test the robust of the associations. We further conducted stratification analyses according to the mother’s HBsAg status (negative or positive) or survey time (2014 or 2020) to find potential disparities of the association among specific populations.
All analyses were conducted by IBM SPSS Statistics version 26.0 (IBM Corp., Armonk, NY, USA). A two-side P value <.05 was considered statistical significance.
Results
Basic characteristics
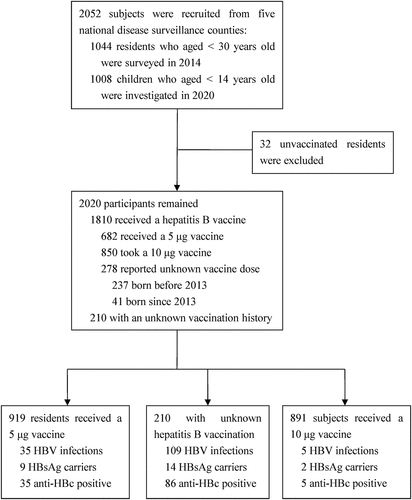
A total of 2052 residents, including 1044 subjects who aged <30 years surveyed in 2014 and 1008 children with less than 14 years investigated in 2020, were recruited in the present study. Among them, 32 participants without a hepatitis B vaccination history were excluded from the study. The remaining 2020 were included in the analysis (). Majority of the participants were Han ethnicity (98.9%), born in hospital (85.9%), and since 2002 (93.8%). 51.3% of them were males and 40.0% lived in urban.
Among the included subjects, according to the protocol, 919 were classified as having received a 5 μg hepatitis B vaccine, 891 as the 10 μg hepatitis B vaccine group, and 210 participants with uncertain vaccination history as unknown group (). No significant difference for residential area, gender, and ethnicity was found across the hepatitis B vaccine dose groups. However, unbalanced distributions were identified for the other characteristics ().
Table 1. General characteristics and hepatitis B viral infection outcomes by dose of hepatitis B vaccine.
Association between vaccine dose and risk of viral infection
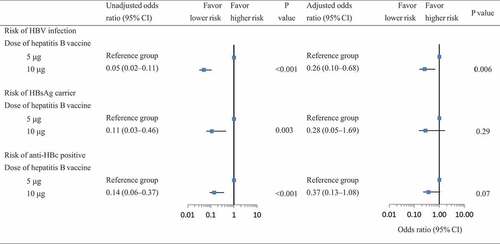
The risk of viral infection, including HBV infection, HBsAg, and anti-HBc positive was the highest in the unknown dose group, followed by the 5 μg dose group, and the lowest among those who received a 10 μg dose hepatitis B vaccine (). Logistic regression models indicated that, compared with subjects who received a 5 μg dose of hepatitis B vaccine, the participants vaccinated with a 10 μg dose vaccine had a declined risk of HBV infection, HBsAg carrier, and anti-HBc positive.
The significant association of vaccine dose with the risk of HBsAg carriers and anti-HBc positive disappeared in multivariate regression models in which resident (urban or rural), birth cohort (<2002 or ≥2002), gender (male or female), birth place (hospital, home, or unknown), mother’s status of HBsAg (negative, positive, or unknown) were controlled. However, subjects who received a 10 μg vaccine remained had a lower risk of HBV infection relative to those with a 5 μg dose vaccine. The adjusted OR was 0.26 (95% CI: 0.10–0.68, P = .006) ().
Figure 2. Association of vaccine dose with the risk of HBV infection, HBsAg carrier, and anti-HBc positive.
Sensitivity and stratification analyses
In the sensitivity analysis that we included the surveyed dose of the first hepatitis B vaccine as the exposure variable, no different results were produced. People who had a 10 μg hepatitis B vaccine were significantly associated with a lower risk of HBV infection (adjusted OR = 0.33, 95% CI: 0.11–0.98) but not for the risk of HBsAg and anti-HBc positive (P for adjusted OR was 0.21 and 0.067, respectively) ().
Table 2. Sensitivity analysis for the risk of HBV infection and HBsAg carriage.
Stratification analyses according to HBsAg status of the mother (positive or negative) indicated that neither we included the treated nor the surveyed vaccine dose groups into the model, no significant relation between vaccine dose and the risk of HBV infection (e.g., HBV infection, HBsAg carrier, or anti-HBc positive) was found. Similar results were identified in residents surveyed in 2014. However, for subjects investigated in 2020, receipt of 10 μg vaccine was related to a lower risk of HBV infection and anti-HBc positive ().
Table 3. Stratification analyses on the risk of HBV infection and HBsAg carriage according to mother’s HBsAg status and survey time.
Discussion
In the present study, we identified that people who received a 10 μg hepatitis B vaccine were associated with a lower risk of HBV infection, compared with those vaccinated with a 5 μg vaccine. In addition, higher vaccine dose also declined the risk of anti-HBc positive for residents surveyed in 2020. However, no significant relation between vaccine doses with the risk of HBsAg carriers was found.
HBV infection is a serious health concern and contributes to an extremely high burden of liver diseases worldwide, such as hepatic cirrhosis and hepatocellular carcinoma.Citation16 Due to the limitations of antiviral agents against HBV that have been widely applied to treat chronic hepatitis B, vaccination is the most important strategy to control HBV infection.Citation4 In HBV high epidemic areas, mother-to-child transmission is the main route of HBV transmission and is responsible for an estimated 30%-50% new infections in China.Citation17 Focusing on cutting off this main transmission, China prioritized implementing the hepatitis B vaccination for newborns since 1992 and guaranteed by multiple measures, including switching the dosage to 10 μg for all infants regardless of the maternal HBsAg status.Citation4
The dosage of hepatitis B vaccine depends on vaccine type, immunogenicity, and protective efficacy as well as the cost and restrictive supply.Citation4 In the early stage of vaccination, low dosage of hepatitis B vaccine was popular and an option for newborns since reduced vaccine dosage appeared to be effective and considered high cost and restrictive supply.Citation18 However, low dose of hepatitis B vaccine was no longer a wise choice since the improvement of vaccine production technology and the breakthrough of supply capacity.Citation19 Although the 10 μg dose vaccine was thought to contribute to HBV prevention relative to low vaccine dose (e.g., 5 μg vaccine), few literatures focus on comparing the difference of preventive effect between the two dose vaccines.
Despite HBV vaccination has been extensively promoted worldwide, available evidence on HBV vaccination effectiveness is still controversial.Citation20 Hepatitis B vaccine induced protective antibody levels and persist for long-term after primary immunization were critical for HBV prevention.Citation21 The age of primary immunization and the time since vaccination are important factors implicated in the long-term persistence of an anti-HBs titer ≥10 mIU/mL.Citation20 In addition, higher vaccine dosage might also contribute to this benefit than a lower dose.Citation20 Previous studies have identified that high dosage (e.g., 20 μg) of hepatitis B vaccine improved seroprotection than low vaccine dosage (e.g., 10 μg) in adults,Citation22–24 as well as in specific populations, such as chronic kidney disease patients and drug users.Citation25,Citation26 The present study extended the benefit of higher vaccine dose on HBV prevention among residents in Fujian Province, especially for those investigated in 2020 (children aged <14 years). These findings are in line with the results of previous studies.Citation20,Citation27 Higher vaccine doses might stimulate a stronger immune response of the body and persist for long-term with a higher anti-HBs titer, thereby lowering the risk of HBV infection.
We further found that the protective effect of 10 μg dosage vaccine was mainly reflected in reducing the risk of anti-HBc positive. However, no significant relation was produced for the risk of HBsAg carriers. In addition, no association was linked in children whose mother was positive for HBsAg. This was consistent with the findings in a prospective and multicenter cohort study of mothers positive for HBsAg that was conducted to compare the immune response among infants who received 10 μg with those with a 20 μg vaccine.Citation28 Despite an increased high-response and decreased low-response rates for infants who received three doses of 20 μg hepatitis B vaccine were found, however, these differences were restricted to infants born to mothers with HBV DNA <5 log 10 IU/mL.Citation28 This study further concluded that the 20 μg hepatitis B vaccine did not reduce immunoprophylaxis failure of infants from HBsAg positive mothers. Similar null associations were found among patients receiving methadone maintenance treatment or those using immunomodulatory drugs.Citation29,Citation30 The reasons of the phenomenon are still unknown, but might be related to the state of maternal immune function, the transplacental transfer of HBsAg,Citation31 and the mother’s load of HBV DNA.Citation32
To our knowledge, this is the first study on the association between vaccine dose and the long-term risk of HBV infection in China. Our findings have some important implications. Firstly, the increased dose of hepatitis B vaccine helps to reduce the risk of HBV infection and anti-HBc positive. These benefits are mainly attributed to the universal vaccination for newborns, since the relation was identified in subjects investigated in 2020 rather than in those surveyed in 2014. In this study, participants in 2020 aged <14 years and probably received universal vaccination, while the 2014 subjects (aged <30 years old) possibly took the vaccine for catch-up vaccination.Citation4 Thus, a 10 μg hepatitis B vaccine for universal vaccination for newborns is reasonable since the risk of HBV infection was low (0.56%, 95% CI: 0.07–1.05%). Secondly, the protective effect of higher dose of hepatitis B vaccine was not related to a lower risk of HBsAg carriers for infants whose mother is positive for HBsAg. Mother positive for HBsAg remains the biggest risk factor of HBsAg carriers in infants, although specific measures were implemented, such as vaccinated free hepatitis B immunoglobulin. Therefore, some additional measures should be considered for this specific population since the prevalence of HBsAg positive in the general population remains high. A higher dosage of hepatitis B vaccine and post-vaccination serologic testing might be the preferred options.Citation33,Citation34
There are some limitations in the present study. First, although a significant association was found, we acknowledged that the robust of these results might be affected due to the small number of outcomes. Secondly, some subjects in the 2014 survey (e.g., a proportion of participants in the 15–29 age group) verbally reported their hepatitis B vaccination history that was used in the present study. We are not able to check the message with the surveillance system or the vaccination card/certificate. Thus, there might be potential misclassification biases. Finally, we surveyed subjects from five national monitoring points in a high hepatitis B prevalence area. The results might be different from those in areas of moderate and low prevalence of the disease. Therefore, more studies on this topic were warranted.
Conclusion
In the present study, compared with subjects with a 5 μg hepatitis B vaccine, participants who received a 10 μg hepatitis B vaccine were associated with a lower risk of HBV infection and anti-HBc positive, but not for HBsAg carrier risk. The relation disappeared for infants whose mother was positive for HBsAg. The current 10 μg hepatitis B vaccines for universal vaccination for newborns are reasonable and effective in HBV prevention. More measures should be taken to reduce the risk of HBsAg carriers for infants whose mother was positive for HBsAg.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
The authors gratefully thank the assistance and cooperation of the local field investigators and the surveyed subjects. We appreciate staff in the China CDC for technical guidance in field epidemiological investigation and support of laboratory testing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The datasets used in the present study are available from the corresponding author ([email protected] or [email protected]) on reasonable request only.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Wang H, Men P, Xiao Y, Gao P, Lv M, Yuan Q, Chen W, Bai S, Wu J. Hepatitis B infection in the general population of China: a systematic review and meta-analysis. BMC Infect Dis. 2019;19:811. doi:10.1186/s12879-019-4428-y.
- Li J, Wang J, Nicholas S, Maitland E, Fei T. Regional differences of hepatitis B discrimination in rural China. Hum Vaccin Immunother. 2021;17(7):2257–7. doi:10.1080/21645515.2020.1853999.
- Xia GL, Liu GB, Cao HL, Bi S, Zhan M, Su C, Nan J, Qi X. Prevalence of hepatitis B and C virus infections in the general Chinese population. Results from a nationwide cross-sectional seroepidemiologic study of hepatitis A, B, C, D, and E virus infections in China, 1992. Int Hepatol Commun. 1996;5:62–73. doi:10.1016/S0928-4346(96)82012-3.
- Zhao H, Zhou X, Zhou YH. Hepatitis B vaccine development and implementation. Hum Vaccin Immunother. 2020;16(7):1533–44. doi:10.1080/21645515.2020.1732166.
- Lu FM, Zhuang H. Management of hepatitis B in China. Chin Med J. 2009;122:3–4. doi:10.1111/j.1365-2893.2010.01266.x.
- Liu J, Liang W, Jing W, Liu M. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ. 2019;97(3):230–38. doi:10.2471/BLT.18.219469.
- Liang XF, Bi SL, Yang WZ, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. Epidemiological serosurvey of hepatitis B in China—declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27:6550–57. doi:10.1016/j.vaccine.2009.08.048.
- Wu JN, Wen XZ, Zhou Y, Lin D, Zhang SY, Yan YS. Impact of the free-vaccine policy on timely initiation and completion of hepatitis B vaccination in Fujian, China. J Viral Hepat. 2015;22(6):551–60. doi:10.1111/jvh.12359.
- Huang L, Zhou Y, Yang X. Epidemiological characteristics of hepatitis B infection in 1–29 year old population in Fujian Province. Strait J Pre Med. 2018;24:32–33.
- Wu JN, Zhou Y, Zhang DJ, Zheng JF, Pan WY, Cai ZK, Yan YS. Study on the authenticity of immunization coverage of the routine immunization coverage surveillance system of Fujian. Chin J Epidemiol. 2011;32(9):946–48.
- Hutton DW, So SK, Brandeau ML. Cost-effectiveness of nationwide hepatitis B catch-up vaccination among children and adolescents in China. Hepatology. 2010;51(2):405–14. doi:10.1002/hep.23310.
- Tang J, Luo YQ, Zhou YH. Elimination of hepatitis B virus infection in children: experience and challenge in China. Chinese Med J. 2021;134(23):2818–24. doi:10.1097/CM9.0000000000001791.
- Wu JN, Li DJ, Zhou Y. Association between timely initiation of hepatitis B vaccine and completion of the hepatitis B vaccine and national immunization program vaccine series. Int J Infect Dis. 2016;51:62–65. doi:10.1016/j.ijid.2016.08.018.
- Cui F, Shen L, Li L, Wang H, Wang F, Bi S, Liu J, Zhang G, Wang F, Zheng H, et al. Prevention of chronic hepatitis B after 3 decades of escalating vaccination policy, China. Emerg Infect Dis. 2017;23(5):765–72. doi:10.3201/eid2305.161477.
- Fujian Provincial Health Commission, Fujian Center for Disease Control and Prevention. 40th Anniversary of Immunization Program in Fujian province. Fujian Science and Technology Press; 2019 Sept.
- Liao X, Liang Z. Strategy vaccination against hepatitis B in China. Hum Vaccin Immunother. 2015;11(6):1534–39. doi:10.4161/21645515.2014.980206.
- Xu Y, Liu H, Wang Y, Hao R, Li Z, Song H. The next step in controlling HBV in China. BMJ. 2013 Jul 16;347(1):f4503. doi:10.1136/bmj.f4503.
- Moyes CD, Milne A, Dimitrakakis M, Goldwater PN, Pearce N. Very-low-dose hepatitis B vaccine in newborn infants: an economic option for control in endemic areas. Lancet. 1987;1(8523):29–31. doi:10.1016/S0140-6736(87)90712-4.
- Kane M. Reduced doses of hepatitis B vaccines: is it a good idea? Bull World Health Organ. 1995;73:529–30.
- Pileggi C, Papadopoli R, Bianco A, Pavia M. Hepatitis B vaccine and the need for a booster dose after primary vaccination. Vaccine. 2017;35:6302–07. doi:10.1016/j.vaccine.2017.09.076.
- McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, Bulkow L, Fiore A, Bell B, Hennessy T. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis. 2009;200:1390–96. doi:10.1086/606119.
- ul-Haq N, Hasnain SS, Umar M, Abbas Z, Valenzuela-Silva C, Lopez-Saura P. Immunogenicity of 10 and 20 microgram hepatitis B vaccine in a two-dose schedule. Vaccine. 2003;21(23):3179–85. doi:10.1016/S0264-410X(03)00232-9.
- Chiaramonte M, Majori S, Ngatchu T, Moschen ME, Baldo V, Renzulli G, Simoncello I, Rocco S, Bertin T, Naccarato R, et al. Two different dosages of yeast derived recombinant hepatitis B vaccines: a comparison of immunogenicity. Vaccine. 1996;14(2):135–37. doi:10.1016/0264-410X(95)00148-T.
- Schiff GM, Sherwood JR, Zeldis JB, Krause DS. Comparative study of the immunogenicity and safety of two doses of recombinant hepatitis B vaccine in healthy adolescents. J Adolesc Health. 1995;16(1):12–17. doi:10.1016/1054-139X(94)00105-N.
- Feng Y, Yao T, Han Y, Shi J, Dong S, Wu Y, Shao Z, Liu H, Guo H, Chai G, et al. Immunogenicity and safety of a high-dose and prolonged-schedule hepatitis B vaccine among chronic kidney disease patients: a randomized, parallel-controlled trial. Expert Rev Vaccines. 2021;20(6):743–51. doi:10.1080/14760584.2021.1915777.
- Feng Y, Shi J, Gao L, Yao T, Feng D, Luo D, Li Z, Zhang Y, Wang F, Cui F, et al. Immunogenicity and safety of high-dose hepatitis B vaccine among drug users: a randomized, open-labeled, blank-controlled trial. Hum Vaccin Immunother. 2017;13(6):1–7. doi:10.1080/21645515.2017.1283082.
- Schönberger K, Riedel C, Rückinger S, Mansmann U, Jilg W, Kries RV. Determinants of long term protection after hepatitis B vaccination in infancy: a meta-analysis. Pediatr Infect Dis J. 2013;32:307–13. doi:10.1097/INF.0b013e31827bd1b0.
- Zhang X, Zou H, Chen Y, Zhang H, Tian R, Meng J, Zhu Y, Guo H, Dai E, Zhu B, et al. The effects of increased dose of hepatitis B vaccine on mother-to-child transmission and immune response for infants born to mothers with chronic hepatitis B infection: a prospective, multicenter, large-sample cohort study. BMC Med. 2021;19:148. doi:10.1186/s12916-021-02025-1.
- Solay AH, Eser F. High dose hepatitis B vaccine is not effective in patients using immunomodulatory drugs: a pilot study. Hum Vaccin Immunother. 2019;15(5):1177–82. doi:10.1080/21645515.2019.1574151.
- Shi J, Feng Y, Gao L, Feng D, Yao T, Shi S, Zhang Y, Liang X, Wang S. Immunogenicity and safety of a high-dose hepatitis B vaccine among patients receiving methadone maintenance treatment: a randomized, double-blinded, parallel-controlled trial. Vaccine. 2017;35(18):2443–48. doi:10.1016/j.vaccine.2017.03.034.
- Wang J, He Y, Jin D, Liu J, Zheng J, Yuan N, Bai Y, Yan T, Yang Y, Liu Y, et al. No response to hepatitis B vaccine in infants born to HBsAg (+) mothers is associated to the transplacental transfer of HBsAg. Infect Dis (Lond). 2017;49(8):576–83. doi:10.1080/23744235.2017.1292541.
- Wang B, Xu XX, Wen HX, Hao HY, Yang ZQ, Shi XH, Fu ZD, Wang XF, Zhang F, Wang SP. Influencing factors for non/low-response to hepatitis B vaccine in infants of HBsAg positive mothers. Chin J Epidemiol. 2017;38(7):911–15.
- Zhou YH, Wu C, Zhuang H. Vaccination against hepatitis B: the Chinese experience. Chin Med J. 2009;122:98–102.
- Zhou Y, Lu Z, He H, Yan R, Deng X, Tang X, Zhu Y, Xu X. Influencing factors and necessity of post-vaccination serologic testing follow-up for HBsAg-positive mothers and their infants: a 5-year prospective study in Zhejiang Province, China (2016–2020). J Viral Hepat. 2021;28(10):1413–21. doi:10.1111/jvh.13581.