ABSTRACT
The SARS-CoV-2 pandemic has posed a challenge for correctional facilities worldwide. People in such settings are more vulnerable to severe forms of infection and it is impossible to completely isolate inmates from the outside world. This study aimed to assess the antibody-mediated immune response in terms of neutralizing antibodies against Alpha, Beta, Gamma and Omicron (sub-lineage BA.1) variants of concern after two doses of mRNA vaccine in correctional officers and inmates from an Italian correctional facility. Most of the correctional officers (56.5%) and inmates (52.3% and 63.6%) retained their neutralizing activity toward the Alpha and Gamma variants, respectively. By contrast, the most striking reduction in comparison with the ancestral virus was found in the antibody response toward the Beta and Omicron variants, in both correctional officers (91.2% and 93.9%) and inmates (85.1% and 92.8%). In addition, subjects who had undergone primary vaccination and had previously been naturally infected had higher neutralizing antibody titers toward the 4 variants than negative subjects. Overall, our findings indicate that primary mRNA vaccination is able to induce neutralizing antibodies toward the ancestral virus, while titers toward variants may vary, depending on the mutations harboring by the variants. Although the correctional setting is often considered distinct or isolated from the wider society and sanitary system, the health of correctional workers and prisoners is inexorably linked to the public health of the country as a whole and it is of paramount importance to monitor the antibody response in these settings.
Introduction
Coronavirus Disease (COVID-19), which is caused by SARS-CoV-2, has posed a challenge for correctional facilities worldwide. Of the more than 8 million prison inmates in the world, 50,900 are in Italy,Citation1 which has one of the most overcrowded prison systems in Europe, with an occupation rate of 120%.Citation2,Citation3 Although the World Health Organization (WHO) has issued guidelines on “preparedness, prevention and control of COVID-19 in prisons and other places of detention,”Citation4 people in such settings are more vulnerable to severe forms of infection for several reasons. Firstly, the average level of health, prior to entry into prison, is lower than that of the general population, making these subjects more vulnerable and fragile. In addition, detainees often have comorbidities and high rates of substance abuse.Citation5,Citation6 In community settings such as prisons, it is almost impossible to respect measures aimed at limiting the spread of the virus, owing to overcrowding, limited access to hand sanitizers and face masks and poor ventilation. Indeed, it has been estimated that the incidence of COVID-19 among prisoners is nearly six times higher than among non-incarcerated subjects and increased crowding is associated with an increased incidence rate of COVID-19.Citation7–9
As prisons are not a closed system, it is impossible to completely isolate inmates from the outside world; indeed, visitors, prison staff, such as directors, administrative workers, healthcare personnel and correctional officers (CO), come and go daily, increasing the risk of bringing the virus into the facility.Citation10,Citation11 The most part of correctional workers, in fact, have been considered essential personnel during the COVID-19 response, and their jobs bring them into direct daily contact with a high-risk population. Apart of sharing all the risks of the physical environment as listed above, the CO are additionally exposed through uncontrolled physical contacts, moving prisoners or engaging in altercations, and the medical staff perform physical examinations and medical procedures. Because of transmission of COVID-19 through asymptomatic patients is nearly inevitable, and because conditions strongly favor contagion inside the institution, most correctional facilities will amplify the COVID-19 pandemic and act as a reservoir of illness to the wider community.Citation12
Vaccination has proved to be the most valuable and cost-effective healthcare intervention in the fight against SARS-CoV-2. Even if scientists and public health experts have recommended prioritization of prison and jail populations for deployment of COVID-19 vaccines, detainees have not been included in vaccine trial population.Citation13 Vaccines rollout has varied across prisons and countries, and only in some of them detainees and prison staff were among the priority targets of vaccination, resulting in lower infection rates among the former.Citation14 However, few studies have evaluated vaccination coverage in prisons.Citation14–16
The most commonly used COVID-19 vaccines are the mRNA-based vaccines (BNT162b2 and mRNA-1273), both of which are more than 90% effective against COVID-19.Citation14,Citation15
The mRNA-based vaccines were developed by encoding the spike (S) protein of the virus circulating early in the pandemic. Since then, however, several variants, some defined by the WHO as variants of concern (VOCs), have emerged worldwide, raising concerns regarding vaccine effectiveness. So far, five VOCs have emerged: Alpha (Pango lineage B.1.1.7), Beta (Pango lineage B.1.351), Gamma (Pango lineage P.1), Delta (Pango lineage B.1.617.2) and Omicron (Pango lineage B.1.1.529).Citation17 The Alpha variant, first documented in the United Kingdom in September 2020, harbored 17 mutations/deletions in the viral genome, including eight in the S protein, and was associated with a 50% increase in transmission and an increased risk of death. The Beta variant was isolated in South Africa in May 2020 and caused an increase in hospitalizations and deaths. The Gamma variant, first isolated in Brazil in November 2020, shared common mutations in the S protein with the Beta variant, increasing the possibility of evasion of the humoral response and enhancing transmissibility. The Delta variant emerged in India in October 2020 and rapidly became predominant throughout the world. This variant harbored 23 mutations compared to the Alpha variant, 12 of which in the S protein, allegedly making it the most transmissible variant. The currently circulating variant is Omicron, which is considered the most divergent; in November 2021, it was documented in many countries.Citation17–20 Omicron harbors more than 50 mutations, 30 of which in the S protein, and is able to escape the immunity elicited by vaccination and/or natural infection.Citation17–19–Citation21,Citation22 So far, many sub-lineages of the Omicron variant have emerged.
This study aimed to assess the antibody-mediated immune response in terms of neutralizing antibodies against Alpha, Beta, Gamma and Omicron (sub-lineage BA.1) VOCs after two doses of mRNA vaccine in CO and inmates from an Italian correctional facility.
Materials and methods
Study population
A total of 342 serum samples were collected at the Bari correctional facility (Apulia, Italy) 21 days after the 2nd dose (primary vaccination) of mRNA vaccine (BNT162b2). One hundred forty-seven (147) serum samples were from CO and 195 from inmates.Citation23 Subjects were recruited on a voluntary basis and provided informed consent to participate in the study and data processing prior to the start of the study and after receiving a briefing on the study by medical personnel. All subjects were informed about the results of the study and received two doses of BNT162b2 vaccine. All subjects included in the study were administered by adequately trained medical staff a questionnaire on the general characteristics, work activity, and the recent and remote pathological history. The same day of the administration of the questionnaire a blood sampling was collected by adequately trained medical staff.
All samples were tested by means of commercial ELISAs for the detection of antibodies against the S protein and the nucleoprotein (NP), which is indicative of previous infection. Samples were further tested in duplicate by means of the virus neutralization (VN) assay, using authentic live viruses.
The research protocol was approved by the Ethics Committee of the University Hospital of Bari (n. 6955, prot. N. 0067544–02082021).
ELISAs
A double-antigen ELISA kit was used to detect antibodies against the SARS-CoV- 2 NP and S proteins in human serum samples. IgG antibodies binding to the SARS-CoV-2 NP protein were determined by means of the ERADIKIT COVID-19 MULTISPECIES (In3diagnostic, Turin, Italy). The results were defined according to the calculated ratio described in the following formula, and expressed as a percentage: PR (%) = (OD test sample – OD negative control)/(OD positive control – OD negative control). Values ≥20% were considered positive for the presence of antibodies against SARS-CoV-2. IgG antibodies binding to the SARS-CoV-2 S protein were determined by using the IDK anti-SARS-CoV-2 IgG ELISA kit (Immundiagnostik AG, Bensheim, Germany). The results were defined by using a linear ordinate for OD and a logarithmic abscissa for concentration. The numbers obtained were multiplied by the dilution factor of 101 to get the actual concentrations in ng/mL. Values ≥175 ng/mL were considered positive for the presence of antibodies against the SARS-CoV-2 S protein.
Viruses
Authentic ancestral SARS-CoV-2 2019 (2019-nCov/Italy-INMI1 strain) virus (hereinafter referred to as ancestral or Wuhan virus) was purchased from the European Virus Archive goes Global (EVAg, Spallanzani Institute, Rome). The Alpha variant, named England/MIG457/2020, and the Beta variant, named hCoV-19/Netherlands/NoordHolland_10159/2021, next strain clade 20 H, wild-type viruses were purchased from EVAg. The Gamma variant (next strain 20J/501Y.V3) (lineage B.1.1.28.1) was kindly provided by the University of Siena, Department of Medical Biotechnology. The live Omicron SARS-CoV-2 variant, sub-lineage BA.1, was kindly provided by Prof. Piet Maes, NRC UZ/KU Leuven (Leuven, Belgium). The Omicron sequence has been deposited on GISAID with the following ID: EPI_ISL_6794907.
Virus neutralization assay
The VN assay was performed as previously described.Citation24 Briefly, a 2-fold serial dilution of heat-inactivated serum samples was prepared and mixed with an equal volume of SARS-CoV-2 viral solution containing 100 tissue culture infective dose 50% (TCID50) of each virus. After 1 hour of incubation at room temperature, 100 μL of virus – serum mixture was added to a 96-well plate containing an 80% confluent Vero E6 cell monolayer. Plates were incubated for 72 hours (ancestral virus) and 96 hours (Alpha, Beta, Gamma and Omicron variants) at 37°C and 5% CO2 in a humidified atmosphere and then checked for presence/absence of cytopathic effect (CPE) by means of an inverted optical microscope. A CPE higher than 50% indicated infection.
The VN titer was expressed as the reciprocal of the highest serum dilution showing protection from viral infection and CPE.
Statistical analysis
Median VN titers were calculated along with their interquartile range (IQR). For each variant, a decrease in median VN titers in comparison with the Wuhan strain was calculated as a reduction factor (RF). Comparisons between median VN titers were performed by using the Friedman test or Kruskal-Wallis test with Dunn’s correction for multiple comparisons. Statistical significance was set at p < .05, two tailed. All statistical analyses were performed by means of GraphPad Prism version 9.0 for Windows (GraphPad Software, San Diego, California, USA, www.graphpad.com).
Results
The 147 CO had a median age of 53.9 years (range 23.9–72.2 years); 34 (23.1%) were female and 113 (76.9%) were male. Eighteen (18) (12.2%) had at least one disease (cancer, transplants, HIV, hepatitis C or hepatitis B virus chronic infection, diabetes, autoimmune disease) and/or were on immunosuppressive therapy. The 195 inmates had a median age of 46.3 years (range 20.0–81.2 years) and were all male. Twenty-seven (27) (13.85%) had at least one disease and/or were drug users and/or were on immunosuppressive therapy.
A median time of 21 days after the 2nd dose of mRNA vaccine, 98.6% of CO and 99.0% of inmates had antibodies against the S protein.
shows individual VN titers against the Wuhan strain in CO () and inmates (). Among CO, the median VN titer against the Wuhan strain was 40.0 (IQR 20.0–80.0); 8 subjects (5.4%) were negative. Among inmates, the median VN titer against the Wuhan strain was 40.0 (IQR 14.1–80.0), with 14 subjects (7.2%) negative. On comparing median VN titers against the Wuhan strain, no differences were found between CO and inmates.
Figure 1. Virus neutralization (VN) titers to SARS-CoV-2 virus after 2 doses of mRNA vaccine in correctional officers and inmates. Panel a: VN titers against Wuhan strain in correctional officers; Panel b: VN titers against Wuhan strain in inmates; Panel c: VN titers against Wuhan and Alpha, Beta, Gamma, and Omicron BA.1 variants in correctional officers; Panel d: VN titers against Wuhan and Alpha, Beta, Gamma, and Omicron BA.1 variants in inmates. Dot plots show individual values. Tukey boxplots show outlier values (dots), medians (middle line), third and first quartiles (boxes), while the whiskers display the minimum and maximum values. Horizontal dashed line represents the Lower Limit of Quantification (LLOQ) of VN assay. Statistically significant differences were analyzed by Friedman and Dunn’s multiple comparisons test (p < .05).
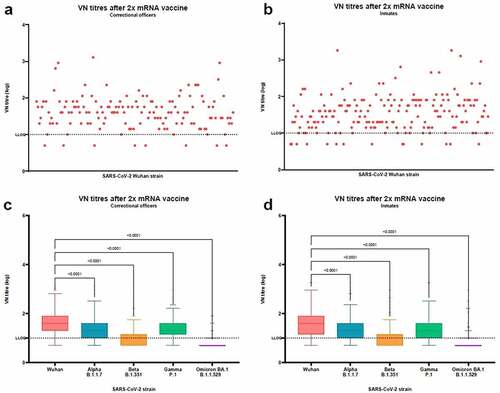
When tested for Alpha, Beta, Gamma, and Omicron BA.1 variants (, ), a significant decrease in median VN titers was observed (p < .0001 for both CO and inmates).
Table 1. Fold decrease in virus neutralization titers to SARS-CoV-2 variants with respect to Wuhan strain in correctional officers, by variant.
Table 2. Fold decrease in virus neutralization titers to SARS-CoV-2 variants with respect to Wuhan strain in inmates, by variant.
Among CO, the median VN titer was 20.0 (IQR 10.0–40.0, RF of 2.0) toward the Alpha variant, with 43.5% of subjects showing a ≥ 2-fold reduction in the median VN titer; 10.0 (IQR 5–14.1, RF of 4.0) toward the Beta variant, with 91.2% of subjects showing a ≥ 2-fold reduction in the median VN titer; 20.0 (IQR 14.1–40.0, RF of 2.0) toward the Gamma variant, with 43.5% of subjects showing a ≥ 2-fold reduction in the median VN titer; and 5.0 (IQR 5–5, RF of 8.0) toward the Omicron BA.1 variant, with 93.9% of subjects showing a ≥ 2-fold reduction in the median VN titer. Among inmates, the median VN titer was 20.0 (IQR 10.0–40.0, RF of 2.0) toward the Alpha variant, with 47.7% of subjects showing a ≥ 2-fold reduction in the median VN titer; 5.0 (IQR 5–14.1, RF of 8.0) toward the Beta variant, with 85.1% of subjects showing a ≥ 2-fold reduction in the median VN titer; 20.0 (IQR 10.0–40.0, RF of 2.0) toward the Gamma variant, with 36.4% of subjects showing a ≥ 2-fold reduction in the median VN titer; and 5.0 (IQR 5–5, RF of 8.0) toward the Omicron BA.1 variant, with 92.8% of subjects showing a ≥ 2-fold reduction in the median VN titer. No differences were observed between CO and inmates on comparing median VN titer toward the same strain.
1Fifteen (15) (4.4%) subjects among CO and inmates tested positive to ELISA antibodies against the NP. Subjects were divided according to their NP ELISA results, as shown in . Among NP-positive subjects, the median VN titer against the Wuhan strain was 640.0 (IQR 226.3–1280.0); this was significantly higher than the median VN titer among NP negatives (40.0, IQR 20.0–566, p = .0007). On comparing median VN titers between NP-positive and NP-negative subjects, differences were significant for all the variants tested (p < .0001).
Figure 2. Virus neutralization (VN) titers to SARS-CoV-2 virus after 2 doses of mRNA vaccine in nucleoprotein (NP) positive and NP negative subjects. Panel a: VN titers against Wuhan strain in NP positive subjects; Panel b: VN titers against Wuhan strain in NP negative subjects; Panel c: VN titers against Wuhan and Alpha, Beta, Gamma, and Omicron BA.1 variants in NP positive subjects; Panel d: VN titers against Wuhan and Alpha, Beta, Gamma, and Omicron BA.1 variants in NP negative subjects. Dot plots show individual values. Tukey boxplots show outlier values (dots), medians (middle line), third and first quartiles (boxes), while the whiskers display the minimum and maximum values. Horizontal dashed line represents the Lower Limit of Quantification (LLOQ) of VN assay. Statistically significant differences were analyzed by Friedman and Dunn’s multiple comparisons test (p < .05).
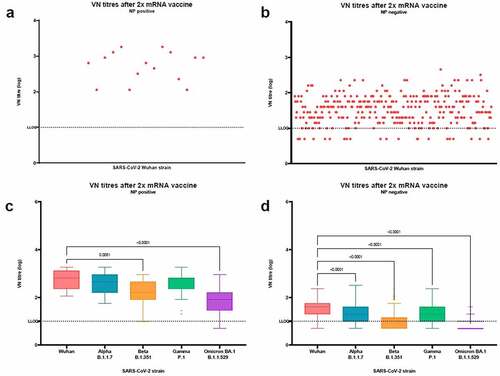
While the decrease in median VN titers toward all the variants tested was significant (p < .0001) in NP-negative subjects, in NP-positive subjects the decrease was significant toward the Beta (median VN titer 160.0, IQR 80.0–452.5, p = .0081) and Omicron BA.1 (median VN titer 80.0, IQR 28.3–160.0, p < .0001) variants, but not toward the Alpha (median VN titer 452.5, IQR 160.0–905.1) and Gamma (median VN titer 226.3, IQR 226.3–640.0) variants.
No differences were found comparing NP-positive CO and NP-positive inmates toward the Wuhan strain (median VN titer 772.5, IQR 198.0–998.8 for CO and median VN titer 640.0, IQR 216.6–1545.0 for inmates) and all the variants tested.
Discussion
Since the beginning of the SARS-CoV-2 pandemic, on 11 March 2020, 5 VOCs harboring mutations in the S protein have emerged, which may jeopardize current vaccine effectiveness.
In this study, we evaluated the antibody response in terms of neutralizing antibodies toward the Alpha, Beta, Gamma and Omicron (sub-lineage BA.1) variants in 342 serum samples collected from CO and inmates at the Bari correctional facility (Apulia, Italy) 21 days after the 2nd dose of mRNA vaccine (BNT162b2).
Vaccination in correctional facilities has specific challenges, such as the coordination of the administration of the second and the booster doses among inmates, without loss to follow up, considering the high turnover of inmates, often with individuals being released in the community before their second or third dose is due.Citation25 In addition, incarcerated subjects are at high risk for COVID-19 transmission and severe disease, they have higher rates of many physical and mental health comorbidities, usually more common than in community subjects of similar age. Studies aimed at evaluating the antibody response in correctional settings provide the opportunity to increase data on the effectiveness of the vaccination strategy in a context different from the general population and potentially to identify susceptible cohorts to COVID-19.
In the correctional facility under study, the prevalence of inmates that have received two vaccine doses was similar to that observed in the Italian general population, whereas the percentage of those receiving the third dose was lower.Citation16
As reported in other studies,Citation16–36 the most striking reduction in comparison with the ancestral virus was found in the antibody response toward the Beta and Omicron variants, in both CO and inmates. Indeed, 91.2% and 93.9% of CO and 85.1% and 92.8% of inmates showed a ≥ 2-fold reduction in the median VN titer against the Beta and Omicron variants, respectively. The Beta variant, like the Gamma, harbors 3 mutations in the RBD region of the S protein, which may enable these variants to evade the neutralizing antibodies against the ancestral virus. The Omicron variant, which is derived from the Alpha lineage, is characterized by several mutations in the S protein; these mutations, and especially those at the top of the protein, in regions accessible to antibodies, may increase the likelihood of immune evasion.Citation27,Citation34,Citation36 Together, the mutations carried by the variants that have emerged may enable these variants to evade the antibodies induced by primary vaccination.Citation37–41 These findings suggest that two doses of mRNA could be insufficient to provide adequate levels of protection against variants with critical mutations. Thus, a booster dose could enhance the immune response, as reported for the Omicron variant.Citation29
By contrast, 56.5% of CO and 52.3% and 63.6% of inmates retained their neutralizing activity toward the Alpha and Gamma variants, respectively. Our findings regarding the Alpha variant are consistent with previous observations that mutations carried by this variant have little or no effect on neutralizing activity in samples from vaccinated subjects.Citation42–45 Moreover, neutralization of the Gamma variant was not compromised, since most of the vaccinated subjects retained an antibody titer similar to that against the ancestral virus, as reported in other studies.Citation30,Citation46,Citation47
However, no significant difference was observed between CO and inmates, indicating that the latter does not seem to have a different response to the vaccine as neutralizing activity.
Furthermore, we evaluated the antibody response toward the 4 VOCs on considering positivity to the NP protein, which is indicative of previous infection. Our findings showed that subjects who had undergone primary vaccination and had previously been naturally infected had higher neutralizing antibody titers toward the 4 variants than negative subjects. These NP-positive subjects had also developed an immune response against the Omicron variant. Consistently with previous reports, our results provide evidence that previous exposure to SARS-CoV-2 enhances the production of neutralizing antibodies in vaccinated individuals against both the ancestral virus and VOCs.Citation26,Citation29,Citation34,Citation48
These findings taken together support the need for a rapid and synchronized widespread deployment of additional mRNA vaccine doses as public health preventive measure to increase the protection of inmates and correctional workers against COVID-19. Notably, all these subjects are at risk to contract the COVID-19 infection and therefore, it is of paramount importance to monitor the antibody response in these settings.
A key strength of this study was the use of a VN assay with authentic live viruses rather than a surrogate neutralization assay. A long assay incubation time of the virus-sample mixture in cell cultures can enable us to identify more precisely the antibody titer that best correlates with real protection, since this titer is based on the complete inhibition of CPE in the cell monolayer.
This study also has some limitations. Firstly, we did not evaluate the same serum samples after the third dose. Secondly, gender distribution was not balanced, since most of the subjects enrolled were male. Thirdly, we did not evaluate the antibody response against the Delta variant. Lastly, we did not evaluate other branches of immunity, such as T-cell responses, which could contribute to protection even if neutralizing antibodies are absent or reduced. In addition, it might be interesting to evaluate the antibody response and adverse events after breakthrough infection after a longer period of time.
Overall, our findings indicate that primary mRNA vaccination is able to induce neutralizing antibodies toward the ancestral virus, while titers toward variants may vary, depending on the mutations harboring by the variants. Critical mutations may lead to an almost complete loss of neutralizing activity in some cases, such as that of the Omicron variant. However, additional exposure to viral antigens through natural infection increases and enhances neutralizing activity even against the most divergent variant.
In conclusion, although the correctional setting is often considered distinct or isolated from the wider society and sanitary system, the health of correctional workers and prisoners is inexorably linked to the public health of the country as a whole. The finding of this study highlights that completing the vaccination course by administering a booster dose in particular settings such as prisons must be considered as an essential and effective measure for the COVID-19 prevention.
Author’s contributions
Conceptualization: C.M.T., N.D., P.L.; Formal Analysis: S.M.; Investigation: C.M.T., S.M., M.L., E.L.; Resources: C.M.T., A.S., N.B., N.D., P.L.; Writing – original draft preparation: C.M.T.; Writing – review and editing: S.M., M.L., N.B., A.S., E.L., E.M., N.D., P.L.; Visualization: S.M.; Supervision: C.M.T. and P.L.; Project administration: C.M.T., N.D., P.L.; Funding acquisition. C.M.T., N.D., P.L.
Disclosure statement
C.M.T. is an external consultant of VisMederi Research srl.
M.L. is an employee of VisMederi Research srl.
E.M. is an external consultant and Chief Scientific Officer of VisMederi srl and VisMederi Research srl.
The other authors declare no competing interests to declare.
Additional information
Funding
References
- Ministero della Giustizia. Detenuti presenti - aggiornamento al 30 giugno 2022. 2022 Jun 30.
- Pattavina A, Palmieri MJ. Fears of COVID-19 contagion and the Italian prison system response. Victims Offenders. 2020;15(7–8):1124–8. doi:10.1080/15564886.2020.1813856.
- Tavoschi L, Monarca R, Giuliani R, Saponaro A, Petrella S, Ranieri R, Alves da Costa F, Ferreira-Borges C, Montanari L. Prevention and control of COVID-19 in Italian prisons: stringent measures and unintended consequences. Front Public Health. 2020;8:559135. doi:10.3389/fpubh.2020.559135.
- WHO. Preparedness, prevention and control of COVID-19 in prisons and other places of detention. 2021 Feb 8.
- Wright NM, Hearty P, Allgar V. Prison primary care and non-communicable diseases: a data-linkage survey of prevalence and associated risk factors. BJGP Open. 2019;3(2). doi:10.3399/bjgpopen19X101643.
- di Giacomo E, de Girolamo G, Peschi G, Fazel S, Clerici M. Italian prisons during the COVID-19 outbreak. Am J Public Health. 2020;110(11):1646–47. doi:10.2105/AJPH.2020.305896.
- Kinner SA, Young JT, Snow K, Southalan L, Lopez-Acuna D, Ferreira-Borges C, O’Moore E. Prisons and custodial settings are part of a comprehensive response to COVID-19. Lancet Public Health. 2020;5(4):e188–89. doi:10.1016/S2468-2667(20)30058-X.
- Leibowitz AI, Siedner MJ, Tsai AC, Mohareb AM. Association between prison crowding and COVID-19 incidence rates in Massachusetts prisons, April 2020-January 2021. JAMA Intern Med. 2021;181(10):1315–21. doi:10.1001/jamainternmed.2021.4392.
- Brinkley-Rubinstein L, Peterson M, Martin R, Chan P, Berk J. Breakthrough SARS-CoV-2 infections in prison after vaccination. N Engl J Med. 2021;385(11):1051–52. doi:10.1056/NEJMc2108479.
- Cingolani M, Caraceni L, Cannovo N, Fedeli P. The COVID-19 epidemic and the prison system in Italy. J Correct Health Care. 2021;27(1):3–7. doi:10.1089/jchc.20.04.0026.
- Neufeld M, Alves da Costa F, Ferreira-Borges C. Prisons need to be included in global and national vaccinations effort against COVID-19. Lancet Reg Health Eur. 2021;4:100088. doi:10.1016/j.lanepe.2021.100088.
- Montoya-Barthelemy AG, Lee CD, Cundiff DR, Smith EB. COVID-19 and the correctional environment: the American prison as a focal point for public health. Am J Prev Med. 2020;58(6):888–91. doi:10.1016/j.amepre.2020.04.001.
- Strassle C, Jardas E, Ochoa J, Berkman BE, Danis M, Rid A, Taylor HA. Covid-19 vaccine trials and incarcerated people - the ethics of inclusion. N Engl J Med. 2020;383(20):1897–99. doi:10.1056/NEJMp2025955.
- Vella R, Giuga G, Piizzi G, Alunni Fegatelli D, Petroni G, Tavone AM, Potenza S, Cammarano A, Mandarelli G, Marella GL. Health management in Italian prisons during COVID-19 outbreak: a focus on the second and third wave. Healthcare (Basel). 2022;10(2). doi:10.3390/healthcare10020282.
- Esposito M, Salerno M, Di Nunno N, Ministeri F, Liberto A, Sessa F. The risk of COVID-19 infection in prisons and prevention strategies: a systematic review and a new strategic protocol of prevention. Healthcare (Basel). 2022;10(2). doi:10.3390/healthcare10020270.
- Stufano A, Buonvino N, Trombetta CM, Pontrelli D, Marchi S, Lobefaro G, De Benedictis L, Lorusso E, Carofiglio MT, Vasinioti VI, et al. COVID-19 outbreak and BNT162b2 mRNA vaccination coverage in a correctional facility during circulation of the SARS-CoV-2 Omicron BA.1 variant in Itlay. Vaccines (Basel). 2022;10(7). doi:10.3390/vaccines10071137.
- World Health Organization. Tracking SARS-CoV-2 variants. 2020. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/.
- Duong D. Alpha, beta, delta, gamma: what’s important to know about SARS-CoV-2 variants of concern? CMAJ. 2021;193(27):E1059–60. doi:10.1503/cmaj.1095949.
- Shiehzadegan S, Alaghemand N, Fox M, Venketaraman V. Analysis of the delta variant B.1.617.2 COVID-19. Clin Pract. 2021;11(4):778–84. doi:10.3390/clinpract11040093.
- World Health Organization. Enhancing response to Omicron SARS-CoV-2 variant: technical brief and priority actions for Member States. 2020.
- Trombetta CM, Marchi S, Viviani S, Manenti A, Benincasa L, Ruello A, Bombardieri E, Vicenti I, Zazzi M, Montomoli E. Serum neutralizing activity against B.1.1.7, B.1.351, and P.1 SARS-CoV-2 variants of concern in hospitalized COVID-19 patients. Viruses. 2021;13(7). doi:10.3390/v13071347.
- Thakur V, Ratho RK. OMICRON (B.1.1.529): a new SARS-CoV-2 variant of concern mounting worldwide fear. J Med Virol. 2022;94(5):1821–24. doi:10.1002/jmv.27541.
- Stufano A, Buonvino N, Cagnazzo F, Armenise N, Pontrelli D, Curzio G, De Benedictis L, Lovreglio P. Efficacy of the measures adopted to prevent COVID-19 outbreaks in an Italian correctional facility for inmates affected by chronic diseases. Front Public Health. 2021;9:694795. doi:10.3389/fpubh.2021.694795.
- Manenti A, Maggetti M, Casa E, Martinuzzi D, Torelli A, Trombetta CM, Marchi S, Montomoli E. Evaluation of SARS-CoV-2 neutralizing antibodies using a CPE-based colorimetric live virus micro-neutralization assay in human serum samples. J Med Virol. 2020;92(10):2096–104. doi:10.1002/jmv.25986.
- Berk J, Del Pozo B, Rich JD, Lee JD. Injecting opioid use disorder treatment in jails and prisons: the potential of extended-release buprenorphine in the carceral setting. J Addict Med. 2022;16(4):396–98. doi:10.1097/ADM.0000000000000942.
- Payne RP, Longet S, Austin JA, Skelly DT, Dejnirattisai W, Adele S, Meardon N, Faustini S, Al-Taei S, Moore SC, et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell. 2021;184(23):5699–714 e11. doi:10.1016/j.cell.2021.10.011.
- Furukawa K, Tjan LH, Kurahashi Y, Sutandhio S, Nishimura M, Arii J, Mori Y. Assessment of neutralizing antibody response against SARS-CoV-2 variants after 2 to 3 doses of the BNT162b2 mRNA COVID-19 vaccine. JAMA Netw Open. 2022;5(5):e2210780. doi:10.1001/jamanetworkopen.2022.10780.
- Sanchez-Sendra B, Albert E, Zulaica J, Torres I, Gimenez E, Botija P, Beltran MJ, Rodado C, Geller R, Navarro D. Neutralizing antibodies against SARS-CoV-2 variants of concern elicited by the comirnaty COVID-19 vaccine in nursing home residents. Sci Rep. 2022;12(1):3788. doi:10.1038/s41598-022-07849-2.
- Trombetta CM, Piccini G, Pierleoni G, Leonardi M, Dapporto F, Marchi S, Andreano E, Paciello I, Benincasa L, Lovreglio P, et al. Immune response to SARS-CoV-2 Omicron variant in patients and vaccinees following homologous and heterologous vaccinations. Commun Biol. 2022;5(1):903. doi:10.1038/s42003-022-03849-0.
- van Gils MJ, Lavell A, van der Straten K, Appelman B, Bontjer I, Poniman M, Burger JA, Oomen M, Bouhuijs JH, van Vught LA, et al. Antibody responses against SARS-CoV-2 variants induced by four different SARS-CoV-2 vaccines in health care workers in the Netherlands: a prospective cohort study. PLoS Med. 2022;19(5):e1003991. doi:10.1371/journal.pmed.1003991.
- Garcia-Beltran WF, Lam EC, St Denis K, Nitido AD, Garcia ZH, Hauser BM, Feldman J, Pavlovic MN, Gregory DJ, Poznansky MC, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184(9):2372–83 e9. doi:10.1016/j.cell.2021.03.013.
- Wibmer CK, Ayres F, Hermanus T, Madzivhandila M, Kgagudi P, Oosthuysen B, Lambson BE, de Oliveira T, Vermeulen M, van der Berg K, et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med. 2021;27(4):622–25. doi:10.1038/s41591-021-01285-x.
- Carreno JM, Alshammary H, Tcheou J, Singh G, Raskin AJ, Kawabata H, Sominsky LA, Clark JJ, Adelsberg DC, Bielak DA, et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2022;602(7898):682–88. doi:10.1038/s41586-022-04399-5.
- Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, Berrios C, Ofoman O, Chang CC, Hauser BM, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185:457–66.e4. doi:10.1016/j.cell.2021.12.033.
- Tseng HF, Ackerson BK, Luo Y, Sy LS, Talarico CA, Tian Y, Bruxvoort KJ, Tubert JE, Florea A, Ku JH, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and delta variants. Nat Med. 2022;28(5):1063–71. doi:10.1038/s41591-022-01753-y.
- Liu C, Zhou D, Nutalai R, Duyvesteyn HME, Tuekprakhon A, Ginn HM, Dejnirattisai W, Supasa P, Mentzer AJ, Wang B, et al. The antibody response to SARS-CoV-2 Beta underscores the antigenic distance to other variants. Cell Host Microbe. 2022;30(1):53–68 e12. doi:10.1016/j.chom.2021.11.013.
- Ao D, Lan T, He X, Liu J, Chen L, Baptista-Hon DT, Zhang K, Wei X. SARS-CoV-2 Omicron variant: immune escape and vaccine development. MedComm (2020). 2022;3(1):e126. doi:10.1002/mco2.126.
- Chakraborty C, Sharma AR, Bhattacharya M, Lee SS. A detailed overview of immune escape, antibody escape, partial vaccine escape of SARS-CoV-2 and Their emerging variants with escape mutations. Front Immunol. 2022;13:801522. doi:10.3389/fimmu.2022.801522.
- Mengist HM, Kombe Kombe AJ, Mekonnen D, Abebaw A, Getachew M, Jin T. Mutations of SARS-CoV-2 spike protein: implications on immune evasion and vaccine-induced immunity. Semin Immunol. 2021;55:101533. doi:10.1016/j.smim.2021.101533.
- Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, Ludden C, Reeve R, Rambaut A, Consortium C-GU, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409–24. doi:10.1038/s41579-021-00573-0.
- Lazarevic I, Pravica V, Miljanovic D, Cupic M. Immune evasion of SARS-CoV-2 emerging variants: what have we learnt so far? Viruses. 2021;13(7). doi:10.3390/v13071192.
- Collier DA, De Marco A, Ferreira I, Meng B, Datir RP, Walls AC, Kemp SA, Bassi J, Pinto D, Silacci-Fregni C, et al. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature. 2021;593(7857):136–41. doi:10.1038/s41586-021-03412-7.
- Edara VV, Hudson WH, Xie X, Ahmed R, Suthar MS. Neutralizing antibodies against SARS-CoV-2 variants after infection and vaccination. JAMA. 2021;325(18):1896–98. doi:10.1001/jama.2021.4388.
- Hernandez-Luis P, Aguilar R, Pelegrin-Perez J, Ruiz-Olalla G, Garcia-Basteiro AL, Tortajada M, Moncunill G, Dobano C, Angulo A, Engel P. Decreased and heterogeneous neutralizing antibody responses against RBD of SARS-CoV-2 variants after mRNA vaccination. Front Immunol. 2022;13:816389. doi:10.3389/fimmu.2022.816389.
- Shen X, Tang H, McDanal C, Wagh K, Fischer W, Theiler J, Yoon H, Li D, Haynes BF, Sanders KO, et al. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021;29(4):529–39 e3. doi:10.1016/j.chom.2021.03.002.
- Dejnirattisai W, Zhou D, Supasa P, Liu C, Mentzer AJ, Ginn HM, Zhao Y, Duyvesteyn HME, Tuekprakhon A, Nutalai R, et al. Antibody evasion by the P.1 strain of SARS-CoV-2. Cell. 2021;184(11):2939–54 e9. doi:10.1016/j.cell.2021.03.055.
- Cromer D, Steain M, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Kent SJ, Triccas JA, Khoury DS, Davenport MP. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3(1):e52–61. doi:10.1016/S2666-5247(21)00267-6.
- Perez-Then E, Lucas C, Monteiro VS, Miric M, Brache V, Cochon L, Vogels CBF, Malik AA, De la Cruz E, Jorge A, et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med. 2022;28(3):481–85. doi:10.1038/s41591-022-01705-6.
