ABSTRACT
The DTacP-sIPV-Hib combination vaccine can replace the single-component acellular pertussis, diphtheria, tetanus, polio, and Haemophilus influenzae type B vaccines. In this study, we evaluated the safety and immunogenicity of a newly developed DTacP-sIPV-Hib combination vaccine in animal models. We used 40 mice and 46 cynomolgus monkeys to evaluate acute and long-term toxicity. Thirty-six guinea pigs were used for sensitization assessment. For immunogenicity assessment, 50 NIH mice and 50 rats were equally randomized to receive 3 doses of 3 different batches of the tested vaccine at an interval of 21 d, or physiological saline solution (0.5 mL). Orbital blood was collected at an interval of 21 d post inoculation to detect related antibody titers or neutralizing antibody titers against poliovirus. Gross autopsy and histopathological examination revealed no abnormal toxicity or irritation in mice and cynomolgus monkeys. Sensitization assessment in guinea pigs indicated the lack of evident allergic symptoms in the high- and low-dose vaccine groups within 30 min after repeated stimulation. The DTacP-sIPV-Hib combination vaccine induced significant immune responses in mice, rats, and cynomolgus monkeys, with 100% seroconversion rates after 3 doses. The DTacP-sIPV-Hib combination vaccine is safe and immunogenic in animal models. Three doses of the vaccine elicited satisfactory antibody responses in mice, rats, and cynomolgus monkeys.
Introduction
Infectious diseases, such as diphtheria, pertussis, tetanus, Haemophilus influenzae type B, and poliomyelitis, pose serious health hazards to children. Vaccination is by far the most effective way to prevent these diseases. Pediatric combination vaccines have improved the vaccine coverage rates and timeliness of vaccination, which is important for maintaining the low prevalence of these childhood diseases.Citation1–3 Pentavalent and hexavalent vaccines derived from a diphtheria (D)-tetanus (T)-acellular pertussis (aP) backbone have been widely used worldwide with great success, and they are increasingly becoming the standard of pediatric prevention.Citation4,Citation5 Combining multiple antigens in a single vaccination can help allay concerns about the number of doses given in a crowded pediatric vaccination schedule. Consequently, combined vaccines have contributed to improved vaccination compliance, coverage rates, and reduced disease prevalence.Citation6–9
In the United States, vaccinations against 12 infectious agents are currently recommended, which requires about 24 injections by 18 months of age. As a result, the Advisory Committee on Immunization Practices (ACIP) recommends the use of combination vaccines whenever possible.Citation10 Therefore, the World Health Organization has been advocating combined vaccines to reduce the number of doses, improve the vaccination rate, and reduce the possibility of abnormal reactions.Citation11 Accordingly, the United States and European Union have guided the development and production of combined vaccines through guidelines,Citation12 allowing different marketing authorization holders to develop combined vaccines. Enterprises are allowed to conduct simultaneous vaccination trials for exploratory clinical research. The relevant vaccine legislation of China also states that the state supports the development of new vaccines, such as conjugate and multivalent vaccines.Citation13 However, although single DTaP-IPV/Hib vaccines combining DTaP-IPV and Hib are used worldwide, these vaccines are administered separately in China. Currently, China does not have a combined DTaP-IPV-Hib vaccine produced by its own enterprises, and thus they still need to import them.Citation14 Therefore, based on the technical guidelines for preclinical and clinical studies of combined vaccines in China,Citation15 we developed a novel combined DTacP-sIPV-Hib vaccine that differs in the production process from the combination vaccines available in the market and evaluated its safety and immunogenicity in animal models.
Materials and methods
Ethics statement
All animals involved in this study were housed and cared for in an Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited facility. All experimental procedures were conducted according to Chinese animal use guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC). All animals were anesthetized using isoflurane.
Animal models
The 4-5-weeks-old male and female mice (18–24 g) (ICR mice) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. The 5-8-weeks-old female guinea pigs (300–450 g) were obtained from China Academy of Food and Drug Research. The 2-5-years-old male and female cynomolgus monkeys (2.56–3.82 kg and 2.42–3.07 kg, respectively) were obtained from Yongfu County Xingui Wildlife Breeding Co.,Ltd. The NIH mice, female, 10–12 g, were obtained from SPF (Beijing) Biotechnology Co., Ltd. SPF Wistar rats, male and female, 175–250 g, were obtained from SPF (Beijing) Biotechnology Co., Ltd. All animals are bred and maintained in a specific pathogen-free (SPF) environment. All animals are fed and watered ad libitum and provided with a 12-hour light/dark cycle (temperature: 18–28°C, humidity: 40%–70%).
Vaccine preparation
The DTacP-sIPV-Hib vaccine was developed by the Beijing Institute of Biological Products Company Limited, and contains pertussis toxoid (PT), filamentous hemagglutinin (FHA), pertactin (PRN), diphtheria toxoid (DT), tetanus toxoid (TT), inactivated poliomyelitis vaccine (type I, II, and III), and purified polyribosylribitol phosphate capsular polysaccharide (PRP) of Haemophilus influenzae type b (Hib) covalently bound to tetanus protein. The components of pertussis antigens, PT, FHA and PRN stock solutions were extracted from the culture of Bordetella pertussis and prepared by purification, detoxification, and aluminum hydroxide adsorption respectively. PT and FHA were purified with 0.05 ~ 1 M phosphate buffer, and the chromatographic column was hydroxyapatite. PRN was purified with 0.05 ~ 0.1 M sodium chloride in Tris-HCl buffer, and the chromatographic column was DEAE-Sepharose FF. PT and FHA were detoxified with glutaraldehyde. DT stock solution was extracted from Corynebacterium diphtheria, purified and detoxified by formaldehyde. TT stock solution was extracted from Clostridium tetani, detoxified by formaldehyde, and purified. sIPV stock solution was inoculated with Vero cells from Sabin strains I, II and III, respectively, and then cultured, harvested, concentrated, purified, and inactivated. Hib stock solution was made of purified Haemophilus influenzae type b capsular polysaccharide antigen, which is covalently combined with tetanus toxoid protein through adipic hydrazide (ADH). Hib was purified by ethanol precipitation. Each component was absorbed with aluminum hydroxide adjuvant. The final prepared bulk was formulated and packaged according to the proportion to obtain the DTacP-sIPV-Hib product, which is an all-liquid dosage form. Each human dose is 0.5 mL, containing 12.5 Lf Diphtheria toxoid, 3.5 Lf tetanus toxoid, 25 µg pertussis toxoid, 25 µg Filamentous Hemagglutinin (FHA), 8 µg pertactin (PRN), 15 DU type I Sabin polio Monovalent virus inactivated stock solution D antigen, 45 DU type II Sabin strain polio monovalent virus inactivated stock solution D antigen, 35 DU type III Sabin strain polio monovalent virus inactivated stock solution D antigen, 10 µg Haemophilus influenzae type b capsular polysaccharide antigen, 0.225 mg aluminum. We used four different batches of DTacP-sIPV-Hib vaccine in these studies. For safety in mice, guinea pigs and monkeys, the same batch S04 pentavalent vaccine was used. For immunogenicity testing in DTacP, Hib and IPV, we used three different batches of pentavalent vaccines numbered S01, S02 and S03. We used control Hib vaccine from Lanzhou Institute of Biological Products Company Limited (LIBP) and sIPV vaccine from Beijing Institute of Biological Products Company Limited (BIBP), both of which are commercially available vaccines.
Toxicity in mice
A total of 40 ICR mice were used in the toxicity experiment. Mice were randomly divided into 2 groups (10 mice/sex/group) and subgrouped according to sex, including a negative control group (sodium chloride injection) and test vaccine group (1 dose/mouse). The volume of each intramuscular injection was 0.5 mL, and the mice were observed for at least 4 h after D1 administration, and then once daily in the morning or afternoon for 14 consecutive days. The body weight of animals was measured on D1 (the day before administration), D8, and D15, and the food intake of animals was determined on D8 and D15. After the observation period, all animals were euthanized on D15, followed by gross dissection.
Immune-toxicity in cynomolgus monkeys
In this experiment, 46 cynomolgus monkeys were used. Monkeys were randomly divided into 5 groups according to sex: negative control group, adjuvant control group, Hib control group, low-dose test group, and high-dose test group, which were administered physiological saline injection (group 1, 2.5 mL, 5/sex), adjuvant control solution (group 2, 2.5 mL, 3/sex), Hib vaccine control solution (Hib vaccine injection, group 3, 2.5 mL, 5/sex), low-dose combination vaccine (DTacP-sIPV-Hib vaccine injection, group 4, 0.5 mL, 5/sex), and high-dose combination vaccine (DTacP-sIPV-Hib vaccine injection, group 5, 2.5 mL, 5/sex), respectively. Except for the Hib control group, which had 3 monkeys/sex, the other groups had 5 monkeys/sex. The administration volume of the test product was 0.5 mL per animal in the low-dose group, whereas it was 2.5 mL per animal in the other groups. The administration route was intramuscular. Animals were injected once every 3 weeks for 12 continuous weeks (5 times in total); that is, they were administered each injection at D1, D22, D43, D64, and D85. Animals were clinically evaluated throughout the duration of the experiment, including measurement of body weight, body temperature, electrocardiography, ophthalmological examination, determination of clinical pathology indices (blood count, coagulation function, blood biochemistry, and urinalysis), as well as estimation of T-lymphocyte subsets (CD3+, CD3+CD4+, CD3+CD8+, and CD3+CD4+/CD3+CD8+) and serum cytokines (TNF-α, IFN-γ, IL-2, IL-4, IL-5, and IL-6). In addition, we determined specific IgG antibodies by ELISA against TT, DT, PT, FHA, PRN and PRP, and neutralizing antibodies against poliovirus type I, II and III. The first 3 animals in each group (from each sex) were euthanized 3 d after the administration of the last dose (D88), whereas the remaining animals were euthanized at the end of the recovery period (D141, except group 2). All animals were observed for gross anatomy, the main organs were weighed, visceral body ratio and visceral brain ratio were calculated, and pathological examination of various tissues and organs was performed.
Sensitization in guinea pigs
We randomly divided 36 female guinea pigs into 4 groups (9 animals each): negative control group, positive control group, low-dose test group (0.1× dose/sensitization, 0.2× dose/stimulation), and high-dose test group (1× dose/sensitization, 2× dose/stimulation). The negative control group was administered a sodium chloride injection. The positive control group was administered human serum albumin, with the sensitizing dose being 20 mg per animal and stimulating dose being 40 mg per animal. The sensitizing doses for the administered DTacP-sIPV-Hib combined vaccine were 0.1 and 1 dose/guinea pig for the low-dose and high-dose groups, respectively, whereas the stimulation doses for the administered DTacP-sIPV-Hib combined vaccine were 0.2 and 2 dose/guinea pig for the low-dose and high-dose groups, respectively. The sensitization dose was administered thrice via intramuscular injection, once every alternative day. D1, D3, and D5 sensitization. The stimulating dose was administered via foot intravenous injection, 14 d after the last sensitization (D19), with the exception of the first 3 animals in each group and 6 animals in the high-dose test group that were stimulated 21 d after the last sensitization (D26).
Vaccine immunogenicity analysis and neutralization assay
DT, TT, PT, FHA, and PRN immunogenicity analysis and ELISA assay
NIH mice were used to study the immunogenicity of DT, TT, PT, FHA, and PRN. A total of 40 NIH mice were randomly divided into 4 groups, vaccine group (3 batches, 1 group for each batch) and normal saline group, which received intraperitoneal (i.p.) injections of 1/10 human doses of vaccine formulations on a 3-dose schedule at 0, 21, and 42 d. Serum samples were collected 21 d after the administration of each dose and antigen-specific antibodies were quantified in diluted sera using ELISA.Citation16 The ELISA method we used was jointly developed by the BIBP/CNBG/Sinopharm and the National Institutes for Food and Drug Control. The vaccine homologous antigens (DT, TT, PT, FHA and PRN) manufactured by BIBP/CNBG/Sinopharm. Briefly, the purified PT, FHA, PRN, TT, and DT antigens were added to microtitration plates (Greiner Bio-one, Germany) and allowed to react overnight at 4°C. The plates were washed 4 times with a washing buffer (PBS supplemented with 0.05% Tween 20). For blocking, 100 μL of blocking buffer (1% bovine serum albumin in PBS) was added to each well and reacted for 1 h at 37°C. The remaining solution was completely removed from the plates. Subsequently, B. pertussis antiserum (mouse) (97/642, WHO Reference Reagent, NIBSC) and serum samples diluted to a specific concentration were added to the wells, followed by the addition of a pre-prepared KPL peroxidase-labeled antibody (Sera Care, USA). The absorbance of each well was read using a Multiskan FC reader (Thermo Fisher Scientific) at 450/630 nm. The antibody levels of PT, FHA and PRN were expressed in IU/mL. DT, TT and Hib are determined by the cutoff value, and the highest dilution ratio higher than the cutoff value is judged as the positive well, and the dilution ratio of the sample corresponding to the well is the antibody titer of the sample. The geometric mean value of the antibody titer of each group of serum samples is calculated, and then the logarithm is calculated as the antibody titer of the serum DT, TT and Hib.
IPV immunogenicity analysis and neutralization assay
A total of 50 rats were randomly divided into 5 groups (5 male and 5 female rats per group). The DTacP-sIPV-Hib vaccine was used in the experimental group (3 batches of vaccine in the pentavalent vaccine group, 10 rats in each batch), whereas sabin-IPV (with phenol red) was administered to the control group, and PBS to the blank control group. All groups were intramuscularly injected in a 3-dose schedule at 0, 21, and 42 d. Serum samples were collected 21 d after administration of each dose, and neutralizing antibody titers against the 3 types of poliovirus were determined using a microneutralization test according to the method recommended by WHO. Briefly, a 4-fold dilution series of each serum sample was heated for 30 min at 56°C. The reciprocal of the highest serum dilution that inhibited 50% of the viral cytopathic effect was considered as the neutralizing antibody titer against the related poliovirus. Determination of neutralizing antibody titer in this virus neutralization assay followed the same principle as that used for a single-antigen vaccine (Sabin strain). Seroconversion was defined as an increase in antibody titer of the pre- to post-vaccination values by a factor of at least 4. If rat had an antibody titer <1:8 before vaccination, seroconversion was defined as an antibody titer ≥1:8 after vaccination.
Hib immunogenicity analysis and ELISA assay
Methods according to the Chinese Pharmacopoeia (Volume III, 2020 edition), NIH mice were used to elucidate the immunogenicity of Hib. A total of 50 NIH mice were randomly divided into 5 groups (3 batches of vaccine in the pentavalent vaccine group, 10 mice in each batch, the Hib vaccine group and the 0.85% sodium chloride group), which received a subcutaneous injection of the respective solution on a 3-dose schedule at 0, 14, and 28 d. Serum samples were collected 14 d after the second or third immunization and the levels of anti – PRP IgG were quantified using indirect ELISA. Briefly, the 3 batches of pentavalent vaccines were diluted with 0.85% sodium chloride solution to 10 µg Hib polysaccharide per milliliter, 10 NIH mice weighing 12-14 g were subcutaneously injected with each batch of vaccine (0.25 mL per mouse, contains 2.5 μg Hib polysaccharide). Ten NIH mice of the same batch were taken as negative control and injected with 0.85% sodium chloride solution. Another 10 NIH mice of the same batch were taken as positive control and injected with Hib conjugate vaccine containing 2.5 μg polysaccharide. Blood samples were collected from the retro orbital vein on the 21st to 28th day, and anti-Hib IgG antibody was determined by ELISA. The Cutoff value was calculated from the absorbance value of serum of mice in the control group with 0.85% sodium chloride solution, and the serum anti-Hib IgG antibody level of mice in the vaccine group should be higher than the Cutoff value.
Statistical analysis
Statistical analyses were performed with two-tailed analysis, and the significance level was set at 0.05 or p ≤ .05. The data of antibody titers, cytokines, body weight, body temperature, food intake and other data were statistically analyzed by using software SAS (9.2) and GraphPad Prism (8.0), and calculate the mean and standard deviation (mean ± s.d.). The male and female animals were counted separately, and the differences between the test group and the control group were compared.
Results
Safety in mice
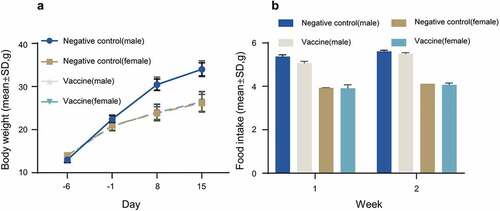
We first performed a single intramuscular injection in ICR mice to evaluate the acute toxicity of the combined DTacP-sIPV-Hib vaccine. In this experiment, we divided 40 mice into 2 groups (n = 20, 10/sex) and intramuscularly injected them with a single dose (0.5 mL) of combined DTacP-sIPV-Hib vaccine or physiological saline (control). After inoculation, we continuously observed all mice for 14 d and euthanized them on Day 15 for gross observation and systematic anatomy evaluation. We did not observe any deaths, impending deaths, or apparent clinical signs in either group over the 14 consecutive days after vaccine inoculation. Moreover, we did not detect any significant differences in weight or feeding state between the experimental and control groups (). Additionally, we did not observe any histopathological changes after euthanasia (not shown). Except for local irritation associated with the combined vaccine, we did not observe any deaths or apparent systemic toxicity in any of the mice. Conclusively, we found that the maximum tolerated dose (MTD) was equal to or greater than 1 dose per mouse.
Safety in guinea pigs
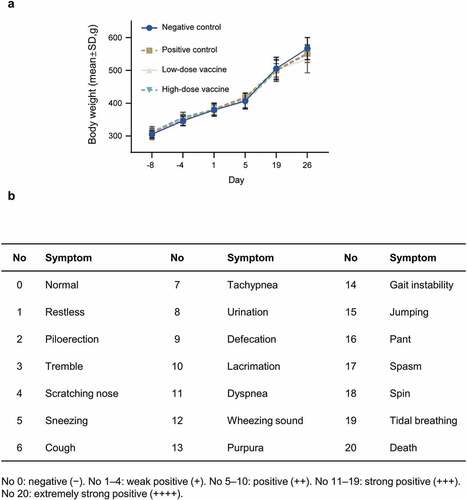
We subsequently evaluated the potential systemic anaphylaxis due to the combined DTacP-sIPV-Hib vaccine by performing intramuscular and intravenous injections in guinea pigs. We did not find any abnormal reactions during the sensitization period based on clinical observations and measurements of the body weights of guinea pigs (). We evaluated the occurrence and intensity of anaphylaxis based on the systemic sensitization evaluation criteria released by the Center for Drug Evaluation of China ().Citation17 In the positive control group, 3 guinea pigs that were stimulated 14 d after the last sensitization (D19) were strongly positive for anaphylaxis (3/3 animals were strongly positive). Similarly, the 6 guinea pigs that were stimulated 21 d after the last sensitization (D26) exhibited strongly positive to extremely positive allergic reactions (2/6 animals showed strongly positive and 4/6 animals showed extremely positive reactions). However, we did not detect any allergic reaction symptoms in the negative control or low-dose experimental groups on D19 or D26. Additionally, in the high-dose experimental group, 3 guinea pigs that were challenged 14 d after the last sensitization (D19) exhibited weak to strongly positive allergic reactions (1/3 animals showed weakly positive reactions, whereas 2/3 animals showed strongly positive reactions). We further performed supplemental testing on 6 guinea pigs on the same day (D19) after exclusion of anaphylaxis-like symptoms. Similarly, these animals showed weak to strongly positive allergic reactions (2/6 animals showed weakly positive reactions, whereas 1/6 and 3/6 animals showed positive and strongly positive reactions, respectively).
Safety in cynomolgus monkeys
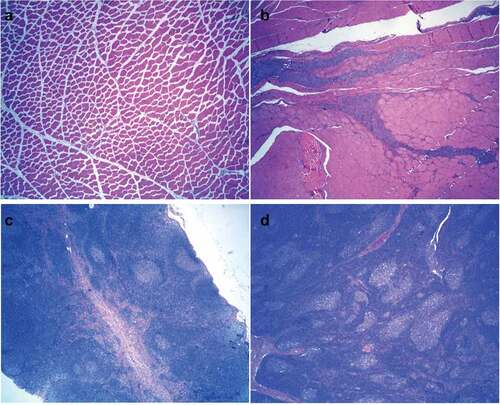
We further evaluated the long-term toxicity of the combined DTacP-sIPV-Hib vaccine in cynomolgus monkeys. We found that during the experiment, the body weight of animals in each group fluctuated within a small range; however, we did not detect any significant abnormal changes related to drug administration. Moreover, we did not observe any statistical differences in the body weight of animals in each group compared with the same period and sex in the negative control group (). After drug administration, the body temperature of animals in each group fluctuated within a small range; however, we did not find any significant abnormal changes related to drug administration (). Additionally, we did not observe any cases of death, impending death, or significant abnormalities in clinical physiological and pathological indicators, lymphocyte subgroup distribution (CD3+, CD4+, CD8+, CD4+/CD8+, and CD20+), or levels of cytokines (tumor necrosis factor alpha [TNF-α], interferon [IFN]-γ, interleukin [IL]-2, IL-4, IL-5, and IL-6) in any of the 5 groups (). Likewise, we did not observe any abnormalities in the gross anatomical evaluation of euthanized animals in any of the experimental groups on D88 and D141. We did not detect any evident systemic toxicity or pathological changes related to drug administration in any group of animals; however, we did observe local irritant reactions in the local muscle tissue of animals in the adjuvant control and low-dose and high-dose combined vaccine groups, which were probably attributed to the aluminum adjuvant. In both the low-dose and high-dose combined vaccine groups, we observed hyperplasia of the skin epidermis, dermal inflammation, and subcutaneous abscess at the injection site, as well as an increase in lymphatic follicles in the inguinal lymph nodes or an increase in sinus wall tissue cells, in animals (). However, 8 weeks post inoculation, the above changes were recovered to some extent. The level of no observed adverse effects was 5 doses/monkey in this trial.
Figure 3. Safety in cynomolgus monkeys.
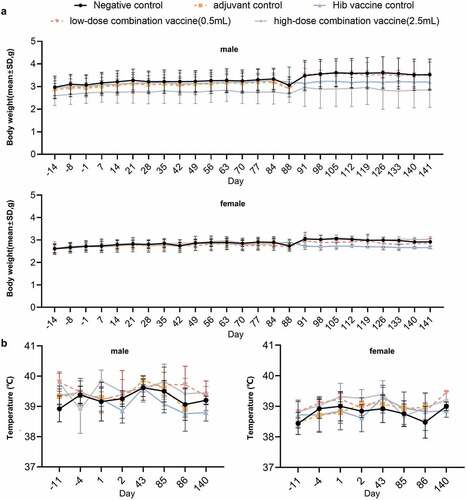
Figure 4. Hematological analysis of cynomolgus monkey in all five groups. The percentages of the lymphocyte subsets CD3+, CD3+CD4+ (labeled CD4+), CD3+CD8+ (labeled CD8+), and CD3+CD4+/CD3+CD8+ (labeled CD4+/CD8+) were monitored at day −11(11 days before vaccination), day −4 (4 days before vaccination), day 4 (3 days after the first dose), day 88 (3 days after the last dose), and day 141 (8 weeks after the recovery period, except for the group 2). Values are shown as the mean ± SD.
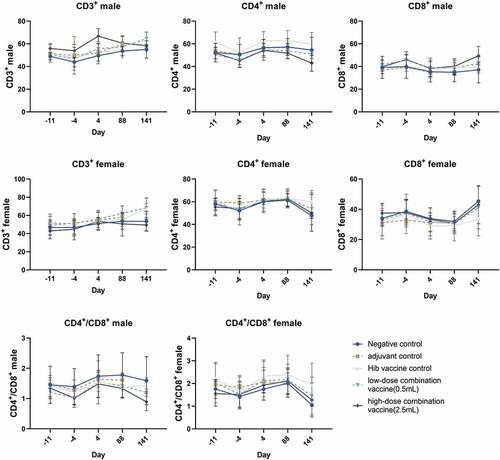
Figure 5. The key cytokines TNF-α, IFN-γ, IL-2, IL-4, IL-5, and IL-6 were examined at days −11, −4, 1, 2, 4, 85, 86, 88 and 141, respectively. Values are shown as the mean ± SD.
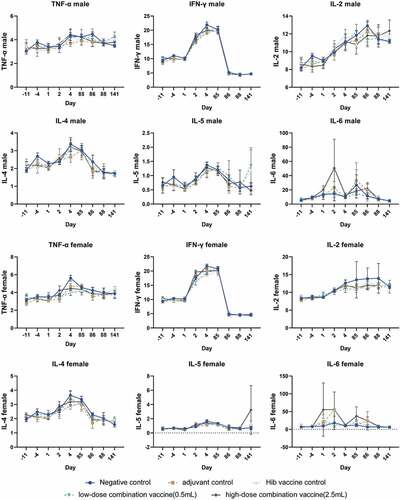
Figure 6. Histopathological results of male cynomolgus monkeys 81 days (a–d) after administration (3 days after last dose). HE staining. Magnification: 40x.
Immunogenicity
We used 4 batches of the prepared DTacP-sIPV-Hib vaccine and control sIPV vaccine in accordance with the requirements of the Chinese Pharmacopoeia (Volume III, 2020 edition).
DTacP
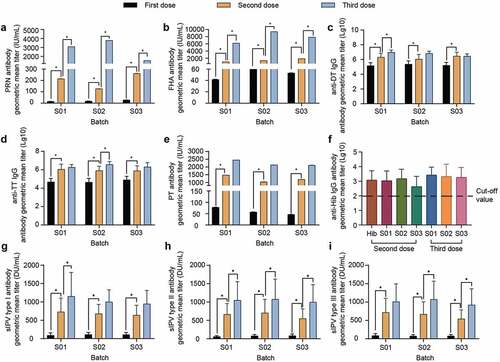
The anti-PT, FHA, PRN, DT, and TT antibodies of the pentavalent vaccine (S01, S02, and S03) after immunization and the antigen-specific antibody titer showed an upward trend with the increase in immunization times as indicated by ELISA, revealing a significant enhancement effect. We specifically detected that the titers of anti-PT, FHA, PRN, DT, and TT antibodies in each group were significantly higher after the second injection compared with those after the first injection (p < .05) (). In addition to the PT antibody, the titers of anti-FHA and anti-PRN antibodies were significantly higher after the third injection relative to those after the second injection (p < .05). However, we did not observe any significant difference in the titer of anti-PT antibody at the corresponding immunization times among the groups (). It is worth noting that the levels of antibody against DT and TT induced by the third injection of S01 and S03 were not significantly higher than those following the second injection (p > .05), whereas the levels of antibody against TT induced by the third injection of S02 were significantly higher than those after the second injection (p < .05) ().
Figure 7. Antibody response in vaccinated mice and rats.
Hib
The anti-Hib IgG seroconversion rate in all experimental groups was 100%. In particular, the levels of anti-Hib IgG antibodies after 3 doses of pentavalent vaccine were higher than those after 2 doses of the vaccine; however, there was no significant difference (p > .05). Additionally, we did not detect any significant differences in the levels of Hib antibodies between groups immunized with 2 and 3 injections of the 3 batches of pentavalent vaccines and those immunized with the Hib stock solution (p > .05). Likewise, there was no significant difference (p > .05) in the levels of Hib antibodies between groups after 2 and 3 injections of these 3 batches of pentavalent vaccine (S01, S02, and S03) ().
IPV
Except for the normal saline group, the GMTs of neutralizing antibodies against poliovirus types I, II, and III were increased in each group. In particular, the seropositivity rates were 100% for the sabin-inactivated poliovirus antigen in both the DTacP-sIPV-Hib and control sIPV groups. In addition, the GMT of all experimental groups was increased by more than 6 times after the second injection time compared with that after the first immunization. Similarly, the GMT of all types of IPV was increased after the third immunization compared with that after the second immunization. It is worth noting that the neutralizing antibodies of all types in the sIPV control group were slightly higher than those in the DTacP-sIPV-Hib group, but the difference was not significant (p > .05) ( and ).
Table 1. Seroconversion rate of neutralizing antibody and GMT results of type I poliovirus after immunization of rats in each group.
Table 2. Seroconversion rate of neutralizing antibody and GMT results of type II poliovirus after immunization of rats in each group.
Table 3. Seroconversion rate of neutralizing antibody and GMT results of type III poliovirus after immunization of rats in each group.
Discussion
Pertussis, diphtheria, tetanus, poliomyelitis, and H. influenzae type B are major causes of infant mortality and are included in the national Expanded Program on Immunization (EPI). However, immunization against all of these diseases requires the administration of more than 10 injections. To improve the timing of multiple inoculation and avoid incorrect or missed inoculations, the provided liquid vaccine composition could simultaneously prevent against all of the above-mentioned infectious diseases. According to such an inoculation procedure, the liquid vaccine composition is injected 4 times (3 times for basic immunization and 1 time for enhanced immunization). This greatly reduces the workload of vaccination and burden on children. In the present study, we developed a novel combined DTacP-sIPV-Hib vaccine that differs in its production process from the combined vaccines available in the market.
Before a new type of vaccine is approved for human clinical trials, appropriate animal models need to be used to support its clinical development and licensing of the product, and safety information must be provided.Citation18–20 In our study, comprehensive preclinical safety assessments of a combined DTacP-sIPV-Hib vaccine, including toxicity, irritation, and sensitization were conducted in mice, guinea pigs, and cynomolgus monkeys. We found that the combined DTacP-sIPV-Hib vaccine had good safety profiles in all tested animals. Although safety evaluation in cynomolgus monkeys showed that 3 d after the last dose (D88) and at the end of the recovery period (D141, except for group 2), the animals in the low-dose and high-dose experimental groups showed epidermal hyperplasia, dermal inflammation, increased lymphoid follicles in inguinal lymph nodes, or increased cells in the sinus wall, these effects were ameliorated to some extent after 8 weeks post inoculation. This change did not fully recover, we may think that either because the dose of antigen we injected was too high, or because the recovery time of 2 months was too short and a longer recovery time was needed. In combination with similar clinical trials of vaccines, adverse reactions such as redness, tenderness and inflammation were present.
This might have been due to the associated local irritant reaction caused by the aluminum adjuvant or antigen and did not affect the overall safety of the vaccine.Citation21–24
In terms of immunogenicity assessment, specific antibodies against pertussis components (PT, FHA, and PRN), DT, TT, and Hib components were induced after immunization in 3 batches of prepared pentavalent vaccines. The levels of antibodies against pertussis components (PT, FHA, and PRN), DT, and TT were significantly increased with increasing immunization doses. The levels of antibodies against FHA and PRN induced by 3 immunization doses were significantly higher than those induced by 2 doses, showing an evident enhancement effect. The levels of antibodies against other components, such as PT, DT, and TT, were also increased after 3 doses of immunization; however, there was no significant difference compared with that after 2 immunization doses. In this study, we investigated the immunogenicity of PT, FHA, PRN, DT and TT by intraperitoneal injection. Although this route of administration may be inconsistent with clinical studies, but it can also reflect the ability of the vaccine to induce antibody production in preclinical stage. In the Hib efficacy study, the levels of Hib antibodies induced in the experimental groups was 100% positive after 2 and 3 doses of immunization. Likewise, the levels of Hib antibodies after 2 and 3 doses of immunization with the 3 batches of the 5-conjugate vaccine were comparable to those of the Hib stock solution, thus, meeting the release standard of the Hib efficacy test in the third part of the Chinese Pharmacopoeia, 2020 edition. The level of neutralizing antibodies against each poliovirus type induced by sIPV components in animals was comparable to that of a commercial sIPV vaccine containing phenol red, with good consistency between batches, showing good immunogenicity. We found that the polio neutralizing Abs observed in the rats immunized with the positive control sIPV vaccine were slightly higher than the three test groups, but the difference was not significant (p > .05). Our previous study also found that when the sIPV vaccine was formulated as a pentavalent vaccine, the level of sIPV neutralizing antibodies in the pentavalent vaccine may be somewhat disturbed and slightly lower than that of the positive control sIPV vaccine, but there was no statistical difference between them. We consider that further study is needed. In cynomolgus monkeys, the immunogenicity of each component of pentavalent vaccine was detected. The results showed that the immunogenicity of each component was good and the results were not shown. In conclusion, the 3 batches of prepared pentavalent vaccines showed good immunogenicity in animal models. However, our study had some limitations. For instance, the selection of a control group for immunogenicity assessment in mice and rats was not appropriate. When this study was performed, we did not use the imported DTaP-IPV/Hib vaccine as a control.Citation25 This issue will be considered in subsequent clinical trials. We also need to refer to existing publications of control vaccines.
In conclusion, the combined DTacP-sIPV-Hib vaccine exhibited good consistency between batches. The combined DTacP-sIPV-Hib vaccine was safe in mice, guinea pigs, and cynomolgus monkeys, and immunogenic in mice, rats, and cynomolgus monkeys (data not shown). The results of this study provide evidence for the future evaluation of the combined DTacP-sIPV-Hib vaccine candidate in subsequent clinical trials in humans.
Author contributions
Yuntao Zhang, Hui Wang, Yuxiu Zhao and Xiaoming Yang designed the study and contributed to the revision of the manuscript. Chongyang Wu, Baifeng Yang, Yadan Zhang, and Yunchao Huang contributed to drafting of the manuscript and data interpretation. Yancen Guo, Shihui Li, and Shouzhi Yu provided the relevant resources. Yuan Dong, Yingwei Liu, and Wanli Li performed laboratory tests. Chongyang Wu, Baifeng Yang, Yuan Dong, and Xiaoli Wei contributed to statistical analyses. Each author listed in the manuscript has seen and approved the submission of this manuscript.
Acknowledgments
We thank all participants who contributed to this study.
Disclosure statement
All the authors are employees of the manufacturer of the DTacP-sIPV-Hib combination vaccine subject of the manuscript.
Additional information
Funding
References
- Bogaerts H. The future of childhood immunizations: examining the European experience. Am J Manag Care. 2003;9(1 Suppl):S30–12. doi:10.1023/A:1023536527428.
- Koslap-Petraco MB, Judelsohn RG. Societal impact of combination vaccines: experiences of physicians, nurses, and parents. J Pediatr Health Care. 2008;22(5):300–09. doi:10.1016/j.pedhc.2007.09.004.
- Wenger J. Vaccines for the developing world: current status and future directions. Vaccine. 2001;19(13–14):1588–91. doi:10.1016/s0264-410x(00)00356-x.
- Bar-On ES, Goldberg E, Hellmann S, Leibovici L. Combined DTP-HBV-HIB vaccine versus separately administered DTP-HBV and HIB vaccines for primary prevention of diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae B (HIB). Cochrane Database Syst Rev. 2012;4:CD005530. doi:10.1002/14651858.CD005530.pub3.
- Stanley AP, Walter AO, Paul AO, Kathryn ME. Section 2: Licensed Vaccines and Vaccines in Develpment. 15. Combination vaccines. Plotkin’s vaccines. 7th ed. Philadelphia, PA: Elsevier; 2017.
- Kalies H, Grote V, Verstraeten T, Hessel L, Schmitt HJ, von Kries R. The use of combination vaccines has improved timeliness of vaccination in children. Pediatr Infect Dis J. 2006;25(6):507–12. doi:10.1097/01.inf.0000222413.47344.23.
- Marshall GS, Happe LE, Lunacsek OE, Szymanski MD, Woods CR, Zahn M, Russell A. Use of combination vaccines is associated with improved coverage rates. Pediatr Infect Dis J. 2007;26(6):496–500. doi:10.1097/INF.0b013e31805d7f17.
- Meyerhoff A, Jacobs RJ, Greenberg DP, Yagoda B, Castles CG. Clinician satisfaction with vaccination visits and the role of multiple injections, results from the COVISE study (Combination vaccines impact on satisfaction and epidemiology). Clin Pediatr (Phila). 2004;43(1):87–93. doi:10.1177/000992280404300112.
- Woodin KA, Rodewald LE, Humiston SG, Carges MS, Schaffer SJ, Szilagyi PG. Physician and parent opinions. Are children becoming pincushions from immunizations? Arch Pediatr Adolesc Med. 1995;149(8):845–49. doi:10.1001/archpedi.1995.02170210019003.
- Sara E, Oliver MD, Kelly L, Moore MD. Licensure of a Diphtheria and Tetanus Toxoids and Acellular Pertussis, Inactivated Poliovirus, Haemophilus influenzae Type b Conjugate, and Hepatitis B Vaccine, and guidance for use in infants. MMWR. 2020 Feb 7;69(5):136–39. doi:10.15585/mmwr.mm6905a5.
- WHO recommendations for routine immunization-summary tables; 2021 Nov. https://www.who.int/teams/immunization-vaccines-and-biologicals/policies/who-recommendations-for-routine-immunization—summary-tables.
- U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research. Guidance for industry for the evaluation of combination vaccines for preventable diseases: production, testing and clinical studies; 1997 Apr. https://www.fda.gov/media/77191/download.
- Vaccine Administration Law of the People’s Republic of China. Chapter I general provisions article 14. 2019.
- Zheng J, Liu D. Chinese Prevention Medicine Association. Chinese preventive medicine association technical guide for the application of acellular diabetic, inactivated polio and Haemophilus influenzae (conjugate) combined vaccine (DTaP-IPV/Hib five-unit vaccine). South China J Prev Med. 2011; 37(2): 67–74.
- Center for Drug Evaluation, China National Medical Products Administration. Guidelines. Technical guidelines for preclinical and clinical studies of combined vaccines; 2005 Oct 14. https://www.cde.org.cn/zdyz/domesticinfopage?zdyzIdCODE=0e94e3802cf31d2434d4a387b4cee4c5.
- Wang LC, Chao Z, Wei C, Yan W, Luo P, Ma X. Analysis of immune responses induced in mice by two different types of acellular pertussis vaccines. Prog Microbiol Immunol. 2018;46(4):14–18. doi:10.13309/j.cnki.pmi.2018.04.003.
- Center for Drug Evaluation, NMPA. Technical guidelines of drug irritation, sensitization and hemolytic study; [accessed 2020 Dec 10]. http://www.cde.org.cn/zdyz.do?method=largePage&id=188.
- Forster R. Study designs for the nonclinical safety testing of new vaccine products. J Pharmacol Toxicol Methods. 2012;66(1):1–7. doi:10.1016/j.vascn.2012.04.003.
- Levast B, Schulz S, Hurk S, Gerdts V. Animal models for neonatal diseases in humans. Vaccine. 2013;31(21):2489–99. doi:10.1016/j.vaccine.2012.11.089.
- Golding H, Khurana S, Zaitseva M. What is the predictive value of animal models for vaccine efficacy in humans? The importance of bridging studies and species-independent correlates of protection. Cold Spring Harb Perspect Biol. 2018;10(4):a028902. doi:10.1101/cshperspect.a028902.
- Baldrick P. Dose site reactions and related findings after vaccine administration in safety studies. J Appl Toxicol. 2016;36(8):980–90. doi:10.1002/jat.3314.
- Hogenesch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol. 2012;3:406. doi:10.3389/fimmu.2012.00406.
- Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004;82(5):497–505. doi:10.1111/j.0818-9641.2004.01286.x.
- Gherardi RK, Aouizerate J, Cadusseau J, Yara S, Authier FJ. Aluminum adjuvants of vaccines injected into the muscle: normal fate, pathology and associated disease. Morphologie. 2016;100(329):85–94. doi:10.1016/j.morpho.2016.01.002.
- Plotkin SA, Liese J, Madhi SA, Ortiz E. A DTaP–IPV//PRP∼T vaccine (Pentaxim™): a review of 16 years’ clinical experience. Expert Rev Vaccines. 2011;10(7):981–1005. doi:10.1586/erv.11.72.