?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Mumps reemergence has been reported in developed countries with high levels of two-dose mumps-containing vaccine (MuCV) coverage. The effectiveness of the two-dose MuCV may be compromised by limitations in the persistence of immunity. This prospective cohort study evaluated the persistence of immunity of a two-dose MuCV in children aged 3–7 years from 2015 to 2020. Persistence of antibody to mumps, determined as the geometric mean antibody concentration (GMC), and seropositivity were analyzed for both repeated measurements from three follow-ups and on each cross-section, respectively. A total of 105 eligible subjects were recruited. Their overall seropositivity rate was relatively high and stable (92.4%–84.8%), while the overall GMC decreased from 547.6 U/ml to 333.3 U/ml. Analysis of waning immunity in 91 participants showed a significant and consistent downward trend for GMC, which differed significantly in boys and girls. The overall seropositivity rate decreased slightly from 2015 (95.6%) to 2016 (92.3%) but both were significantly higher than in 2018 (84.6%). The rates in girls remained stable, while those in boys declined to 75% in 2018. The seropositivity rate of the cross-section level decreased from 95.4% to 86.4% in 4 years. Although two-dose MuCV may result in a high level of immunity, antibody concentrations decay over 2 years after the second dose. Children with waning immunity after receiving two doses, especially boys, require further surveillance at 4 years and later to avoid future mumps epidemics.
Clinical trial registration: NCT02901990.
Background
Mumps is an acute respiratory infectious disease caused by the mumps virus.Citation1 The clinical symptoms of mumps include fever and swollen parotid glands, with its most frequent complications being aseptic meningitis, orchitis, and viral encephalitis.Citation2 The high coverage of two doses of mumps-containing vaccine (MuCV) has greatly reduced the frequency and severity of mumps epidemics. Current MuCVs include anti-mumps monovalent, measles-mumps (MM), measles-mumps-rubella (MMR), and other multivalent combined vaccines.Citation3,Citation4 As recommended by the World Health Organization (WHO), many developed countries have introduced a two-dose MMR routine immunization schedule into their National Immunization Programs (NIPs), achieving a 97%–99% reduction in mumps incidence compared with the incidence prior to vaccine introduction.Citation5–7 Mumps resurgence, however, has been reported in children and adolescents who received at least a two-dose MuCV in countries with high vaccine coverage such as the United States, France, and Portugal.Citation8–10 Causes of resurgence of well-controlled mumps can include reductions in antibody titer, mumps virus evolution, and mismatches between vaccines and wild strain genotypes.Citation11–13
Beginning in 2008, the NIP in China began administering a single dose of the MMR vaccine to children aged 18–24 months.Citation14 This has led to a steady increase in coverage of the one dose MMR vaccine; however, the incidence of mumps decreased only during the first 2 years, but unexpectedly increased during the following years.Citation15 To improve immunity against the mumps virus and reduce the incidence of mumps, a second dose of MuCV was recommended for children in several provinces of China, with the number of cases of mumps decreasing dramatically due to earlier administration of the second dose of MMR to children aged 4–6 years.Citation16 Beginning in July 2020, the Chinese government implemented a two-dose MMR immunization schedule, at ages 8 and 18 months; however, data on the effect of the two-dose MMR regimen on mumps incidence remained limited.Citation17–19
The present study prospectively analyzed anti-mumps IgG antibodies in children aged 3–7 years vaccinated with a two-dose MuCV regimen and evaluated mumps antibody persistence in the next 4 years after vaccination. Persistence of antibody to mumps was analyzed by determining geometric mean antibody concentration (GMC) and seropositivity. Persistence of immunity was investigated to determine whether to be related to age at the second vaccination or gender based on repeated measurements. Mumps antibody GMC and seropositivity levels as a function of time were also compared at each follow-up visit. The results of this study may provide a reference for monitoring waning immunity in children who receive a two-dose MuCV and for strengthening the prevention and control of mumps.
Materials and methods
Study design and participants
A prospective observational cohort study of children aged 3–7 years who had received two doses of MuCV was initiated from October 2015 to 2020 in Jiangsu Province, China. A specific sampling protocol was conducted to guarantee that sufficient participants with mumps seropositivity were recruited. The sample size (N) for observational study was calculated by EquationEquation [1](1)
(1) :
Where the primary endpoint driving the need for the sample size calculation was the mumps seropositivity (p) in children of China, which was set as p = .8 based on a review of previously published studies and expert opinions (15, 17, 18). The allowable error (ε) was set as 0.1 and α = 0.05, Z1-α/2 = 1.96. Then, with 95% confidence and assumptions that results falling within 10% of the true rate, the minimum sample size (N) was estimated to be 96 subjects. To account for loss to follow-up, additional 10% subjects were added, making the enrolled sample size 105 subjects.
In consideration of geographic characteristics, socioeconomic status and feasibility of follow-up, stratified multi-stage random sampling was adopted. North and south of Jiangsu Province were taken as two stratums and at the first stage, for each stratum, one county was randomly selected. In the second stage, in each selected county, all the kindergartens and primary schools were added in the sampling frame, and one kindergarten and one primary school were randomly drawn. At last, children were randomly selected from junior kindergarten and first-grade of primary school to visit.
Children were included if they were (1) aged 3–7 years; (2) physically healthy; (3) local residents with no emigration plan for the next 3 years; and (4) documented having been vaccinated with two doses of MuCV, including anti-mumps monovalent vaccine, MM, MMR, and with the first dose of MMR at age 8–12 months. Children were excluded if they: (1) had been infected with the mumps virus; (2) had not been vaccinated or received only one dose of MuCV; (3) refused blood sample collection; (4) had a serious illness or were not recommended participating in the study for other reasons after clinical evaluation; or (5) their parent/guardian did not sign the informed consent form. Besides, pediatricians were also involved in the recruitment of participants. Parents or guardians of children enrolled were asked to fill out a questionnaire and be interviewed by the pediatrician. Due to the typical clinical symptoms of mumps, children who had been infected with the mumps virus can be easily identified by the pediatrician and excluded.
Cohort follow-up and specimen collection
Mumps was diagnosed according to diagnostic criteria.Citation20 In addition, subjects were evaluated for mumps by follow-up surveillance every 2 months or through immediate reports by parents, teachers, and/or doctors, with the incidence of mumps assessed until June 30, 2020. Personal information of all the participants, including date of birth, gender, and immunization history, was collected. MuCV immunization information for each child was retrospectively collected from their vaccination certificate or the Jiangsu Provincial Vaccine and Immunization System.
Venous blood samples for the detection of serum anti-mumps IgG antibodies were collected from all participants on October 11, November 8, 2015, 2016, and November 20, 2018. The collected blood samples were centrifuged at 3000 rpm for 10 min within 24 h, and the serum samples were transferred to a sterilized EP tube and stored at −70ºC after labeling with a unique code. All samples were transported to the laboratory of the Institute of Immunization Planning of the Center for Disease Control and Prevention of Jiangsu Province.
Ethical issues
This study was approved by the Medical Ethics Committee of the Jiangsu Provincial Center for Disease Control and Prevention (NO: SL2015-B015-02). Written informed consent was provided by the parents/guardians of all enrolled participants. This study was registered at ClinicalTrials.gov under the number NCT02901990.
Laboratory assays
Anti-mumps IgG antibody in serum was quantitatively measured by ELISA. To reduce any laboratory bias and ensure the stability of test results, all serum samples obtained in 2015, 2016, and 2018 were assayed by the same staff members using commercial ELISA kits from InstitutVirion\Serion GmbH (SERION ELISA classic anti-mumps virus IgG, batch number: 2015, SBF.DX; 2016 SLF.CL. 2018, SBF.DF). As defined by the manufacturer, concentrations ≥108 U/ml were considered positive, and concentrations <108 U/ml were considered negative. Samples with concentrations between 90 and 107 U/ml were retested and reclassified.
Asymptomatic infection was defined as (1) a negative result for mumps IgG antibody in one serum specimen, followed by a positive result for mumps IgG antibody in the next serum specimen with no clinical manifestations; or (2) a positive result for mumps IgG antibody in one serum specimen and a fourfold or greater increase in mumps IgG antibody concentration in the next serum specimen with no clinical manifestations.
Statistical analysis
The database was established using EpiData v3.1 software, with statistical analyses performed using R v4.0.4 software. Immunity to mumps virus in the enrolled children who received two doses MuCV was described in terms of seropositivity rates and GMC with 95% confidence intervals (CIs). Persistence of immunity was evaluated in children without asymptomatic mumps infection. Changes in mumps seropositivity rates from 2015 to 2018 were compared by the McNemar test. The declining trends of log transformed GMC levels from 2015 to 2018 were analyzed by repeated measures analysis of variance (RMANOVA) for boys and girls as well as for children with different vaccination ages at the second dose. Mumps antibody GMC and seropositivity levels as a function of time were also compared by Wilcoxon rank sum tests and Fisher’s exact tests, respectively, at each follow-up. In these analyses, vaccination age at the second dose was defined as the years between the date of second dose of MuCV and the date of birth. The vaccination interval (function of time) was defined as the period between the date of the second dose of MuCV and the date of blood collection. All hypothesis tests were two-sided with α = 0.05 defined as statistically significant.
Results
A total of 105 children aged 3–7 years from Jiangsu Province was included in the study cohort. Before the first blood collection in 2015, all participants received two doses of MuCV, with their first dose of MMR at age 8–12 months. Eighty-one subjects (77.1%) received their second dose at age 2–3 years, and 24 (22.9%) at age 4–6 years. Of these subjects, 87 (82.9%) received mumps monovalent vaccine, 14 (13.3%) received MMR, and (3.8%) received MM. None of these subjects were clinically diagnosed with mumps during the 4-year follow-up period, whereas six (5.7%) and eight (7.6%) asymptomatic infections during follow-up from 2015 to 2016 and from 2016 to 2018, respectively. Immunity profiles were analyzed in all 105 subjects, whereas waned immunity was analyzed in 91 (86.7%) participants without subsequent mumps infection ().
Baseline immunity to mumps virus in 2015 for 105 children who received two-dose MuCV is shown in . The total seropositivity rates was 92.4%, whereas the total mumps antibody GMC was 547.6 U/ml. For different subgroups, the immunity level of girls seems higher than that of boys. And children receiving the second dose at 2–3 years old showed higher immunity levels than those receiving the second dose at 4–5 years old.
Table 1. Baseline immunity to mumps virus in 105 enrolled children who received two-dose MuCV in 2015 in Jiangsu Province, China.
shows the persistence of immunity analysis in 91 children without asymptomatic mumps infection. The total seropositivity rates remained relatively stable in the first 2 years and declined to 84.6% in 2018, whereas the total mumps antibody GMC decreased from 641.7 U/ml to 342.2 U/ml over the 4 years. For different subgroups, the seropositivity rates in girls remained stable, whereas it declined significantly in boys from 2016 to 2018. For the children receiving the second dose at 2–3 years old and at 4–5 years old, both the antibody GMC and seropositivity rate showed a declining trend. The McNemar test showed that the overall seropositivity rate for mumps decreased slightly from 2015 to 2016 (p = .250) but significantly in 2018 (p2015 vs 2018 = .002; p2016 vs 2018 = .016). A declining trend was also found for the total GMC level, with that of 2015 significantly higher than 2018. RMANOVA showed that antibody levels differed significantly in boys and girls (F = 6.279, p = .014) but with a consistent downward trend (F = 0.014, p = .987 for the interaction effect between gender and time point). Additionally, children receiving the second dose at 2–3 years possess a higher GMC level than those vaccinated the second dose at 4–5 years (F = 7.100, p = .009) but with a different changing trend in the 4 years (F = 3.132, p = .046). Dot-plots of individual subjects’ GMC level by gender and by vaccination age (years) at the second dose are shown in .
Figure 2. Immunity to mumps virus from 2015 to 2018 in children Aged 3–7 years who received the Two-dose MuCV regimen. (a) GMC level by gender; (b) GMC level by Vaccination age (years) for the second dose.
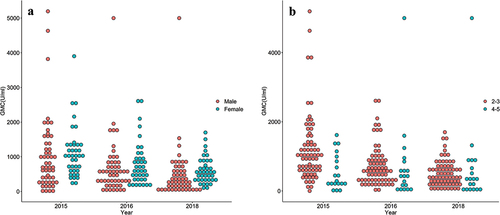
Table 2. Mumps antibody responses among 91 enrolled children without subsequent mumps infection in Jiangsu Province, China.
shows the mumps antibody GMC and seropositivity rate for children during each cross-sectional year (2015, 2016, and 2018) as a function of time after second dose of MuCV administration. Mumps antibody GMC was significantly higher in children assessed 1 year than ≥2 years after the second dose. Although GMC did not differ significantly in children assessed 2 and ≥3 years after the second dose of MuCV, the seropositivity rate was significantly higher at 2 years than at ≥3 years, and still significantly decreased from 4 years after the second dose to ≥5 years.
Table 3. Mumps antibody GMC and seropositivity levels in children as a function of time since the second dose of MuCV administration each year as each cross section.
Discussion
The present study described the immunity profile of mumps antibody in a cohort of 105 children aged 3–7 years who received a two-dose MuCV in Jiangsu Province, China. From 2015 to 2020, none of these children developed clinically diagnosed mumps and 14 asymptomatic infections were observed. In general, the results of this study showed that the overall seropositivity rate remained relatively high and stable levels during the 4-year study period (84.8%–92.4%). This rate was close to the seropositivity rate for herd immunity to mumps (88%–92%), a rate considered the threshold for interrupting mumps transmission.Citation21,Citation22 In addition, the mumps antibody GMC was significantly higher with two-dose than one-dose MuCV,Citation23 suggesting that the two-dose MuCV schedule can provide more protection against mumps infection. Receiving two doses of MuCV at a suitable age can greatly reduce the risk of infection in children and reduce the burden on epidemic control.
Persistence of immunity analysis shows that seropositivity rates declined significantly in 2018 (p = .02), which retained a stable high level in girls but decreased to 75% in boys in 2018. Although the overall mumps antibody GMC for children who received two-dose MuCV remained above the positive threshold (108 U/ml) from 2015 to 2018, the mumps antibody GMC decayed significantly but similarly after 2015 for both boys and girls.
The reasons underlying the difference in mumps antibody GMC in boys and girls remain unclear. Although several studies have reported no statistically significant differences in mumps antibody GMC between genders,Citation8,Citation12,Citation15 the present study found that the mumps antibody GMC was significantly lower in boys than in girls. Moreover, some mumps epidemics occurring in Chinese schools showed that more boys than girls were affected.Citation16 A national study in Finland, a country in which mumps has been eliminated, found no difference in antibody levels between boys and girls during the first few years after second-dose MMR vaccination; however, antibody levels were significantly lower in boys than in girls 8 years after MMR.Citation24 Several reports of mumps outbreaks in schools in the United States indicated that more girls than boys were infected with the mumps virus.Citation8,Citation25,Citation26 Additional studies may be required to assess the mechanisms underlying the differences between boys and girls in immune responses to mumps.
Although many countries have adopted two-dose MMR immunization strategies to prevent and control mumps, the effectiveness of these strategies may be limited.Citation7,Citation27 In most countries in Europe and the United States, where the first dose of MMR is administered at age 12–15 months and the second dose at age 4–6 years,Citation28,Citation29 similar trends of decreasing mumps antibody levels were observed, with these children reported to be at higher risk of mumps infection approximately 13 years after completing the two doses of MMR regimen.Citation6,Citation30 The present study found that mumps antibody GMC was significantly higher in children 1 year than ≥2 years after the second dose of MuCV, that seropositivity rate decreased each year after immunization. Because the observation period in this study was only 4 years, longer-term studies are needed to determine the most appropriate timing of mumps vaccine booster. The repeated cross-sectional analysis indicates the need to evaluate the seropositivity rate ≥4 years after two-dose MuCV administration.
None of these children developed clinically diagnosed mumps during the follow-up surveillance period. Asymptomatic infection following two doses of MuCV was observed in six (5.7%) children from 2015 to 2016 and in eight (7.6%) from 2016 to 2018. About 7%–10% of school-aged children who received two doses of MuCV were reported likely to develop an asymptomatic infection in primary schools with intermediate vaccine coverage during a mumps outbreak,Citation31 indicating that lower antibody concentrations can protect children from clinical symptoms and complications. Notably, the persistent co-occurrence of asymptomatic infection and seronegative can partly explain the relatively stable seropositivity rate in the population over a short period of time.Citation24 This finding demonstrates that the two-dose MuCV strategy is effective in controlling low-level epidemics of mumps virus in primary school-aged children.
The present study had several limitations. First, the follow-up times for the vaccinated children were relatively short, and serum was collected only three times. Second, the efficiency of the two-dose MuCV regimen against mumps could not be evaluated, because none of these children developed mumps. Prospective studies, with mumps cases surveillance of children who develop mumps, are needed to determine the immune persistence of two-dose MuCV.
Conclusions
The present study described the immunity profile of mumps antibody in a cohort of 105 children aged 3–7 years old who received a two-dose MuCV, which provided a useful reference for evaluating the effect of two doses of MuCV. This study found that a two-dose MuCV strategy can protect children from clinically diagnosed mumps for at least 5 years post vaccination. Two-dose MuCV vaccination induced a high level of immunity to mumps but seropositivity rates and antibody level decayed significantly 2 years after the second dose. Both seropositivity rates and antibody level may differ by gender or vaccinated age for the second dose, which requires further verification. It is suggested to have two-dose MuCV vaccination for children, and further surveillance of waning immunity beyond 4 years is needed to avoid future mumps epidemics.
Author contributions
Jinfang Sun, Mingma Li, and Yuanbao Liu were involved in the study design, data analysis, article drafting, critical revision of the article and final approval. Zhiguo Wang, Xiang Sun, and Lei Zhang were involved in fieldwork, including the recruitment and follow-up of participants and data collection. Xiuying Deng, Ying Hu, and Qiang Chen were involved in the laboratory experiments and in critical revision of the manuscript.
Acknowledgments
We would like to express our sincere appreciation to all staff members of the Centers for Disease Control and Prevention in Jiangsu Province, who participated in this study for their responsible field and laboratory work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the authors under reasonable request and with permission of the Jiangsu Provincial Center for Disease Control and Prevention.
Additional information
Funding
References
- Hviid A, Rubin S, Muhlemann K. Mumps. Lancet. 2008;371(9616):932–7. doi:10.1016/S0140-6736(08)60419-5.
- Grennan D. Mumps. JAMA. 2019;322(10):1022. doi:10.1001/jama.2019.10982.
- Galazka AM, Robertson SE, Kraigher A. Mumps and mumps vaccine: a global review. Bull World Health Organ. 1999;77(1):3–14.
- Marshall HS, Plotkin S. The changing epidemiology of mumps in a high vaccination era. Lancet Infect Dis. 2019;19(2):118–19. doi:10.1016/S1473-3099(18)30541-3.
- Levine DA. Vaccine-preventable diseases in pediatric patients: a review of measles, mumps, rubella, and varicella. Pediatr Emerg Med Pract. 2016;13(12):1–20.
- Vygen S, Fischer A, Meurice L, Njoya IM, Gregoris M, Ndiaye B, Ghenassia A, Poujol I, Stahl JP, Antona D, et al. Waning immunity against mumps in vaccinated young adults, France 2013. Euro Surveill. 2016;21(10):30156. doi:10.2807/1560-7917.ES.2016.21.10.30156.
- Cardemil CV, Dahl RM, James L, Wannemuehler K, Gary HE, Shah M, Marin M, Riley J, Feikin DR, Patel M, et al. Effectiveness of a third dose of MMR vaccine for mumps outbreak control. N Engl J Med. 2017;377(10):947–56. doi:10.1056/NEJMoa1703309.
- Fields VS, Safi H, Waters C, Dillaha J, Capelle L, Riklon S, Wheeler JG, Haselow DT. Mumps in a highly vaccinated Marshallese community in Arkansas, USA: an outbreak report. Lancet Infect Dis. 2019;19(2):185–92. doi:10.1016/S1473-3099(18)30607-8.
- Westphal DW, Eastwood A, Levy A, Davies J, Huppatz C, Gilles M, Lyttle H, Williams SA, Dowse GK. A protracted mumps outbreak in Western Australia despite high vaccine coverage: a population-based surveillance study. Lancet Infect Dis. 2019;19(2):177–84. doi:10.1016/S1473-3099(18)30498-5.
- Perez Duque M, San-Bento A, Leon L, Custodio P, Esperanca MA, Albuquerque MJ, Nascimento M, Balasegaram S, Machado RS. Mumps outbreak among fully vaccinated school-age children and young adults, Portugal 2019/2020. Epidemiol Infect. 2021;149:e205. doi:10.1017/S0950268821002028.
- Hamami D, Cameron R, Pollock KG, Shankland C. Waning immunity is associated with periodic large outbreaks of mumps: a mathematical modeling study of Scottish data. Front Physiol. 2017;8:233. doi:10.3389/fphys.2017.00233.
- Kenny L, O’Kelly E, Connell J, De Gascun C, Hassan J. Mumps outbreaks in a highly vaccinated population: investigation of a neutralization titre against the current circulating wildtype genotype G5 mumps virus. J Clin Virol. 2016;74:8–12. doi:10.1016/j.jcv.2015.11.023.
- Yang L, Grenfell BT, Mina MJ. Waning immunity and re-emergence of measles and mumps in the vaccine era. Curr Opin Virol. 2020;40:48–54. doi:10.1016/j.coviro.2020.05.009.
- Cui A, Zhu Z, Hu Y, Deng X, Sun Z, Zhang Y, Mao N, Xu S, Fang X, Gao H, et al. Mumps epidemiology and mumps virus genotypes circulating in mainland China during 2013-2015. PLoS One. 2017;12(1):e0169561. doi:10.1371/journal.pone.0169561.
- Dong Y, Wang L, Burgner DP, Miller JE, Song Y, Ren X, Li Z, Xing Y, Ma J, Sawyer SM, et al. Infectious diseases in children and adolescents in China: analysis of national surveillance data from 2008 to 2017. BMJ. 2020;369:m1043. doi:10.1136/bmj.m1043.
- Fu X, Ge M, Xu W, Yu M, Ju J, Zhong Y, Huang H. Epidemiological features and sociodemographic factors associated with mumps in mainland China from 2004 to 2018. J Med Virol. 2022;94(10):4850–59. doi:10.1002/jmv.27955.
- Li YT, Luo XQ, Zhong XB. Seroprevalences of antibodies against pertussis, diphtheria, tetanus, measles, mumps and rubella: a cross-sectional study in children following vaccination procedure in Guangzhou, China. Vaccine. 2020;38(23):3960–67. doi:10.1016/j.vaccine.2020.03.056.
- Wang D, Nie T, Pan F, Wang Y, Wang J, Qin W. Loss of protective immunity of two-dose mumps-containing vaccine over time: concerns with the new strategy of the mumps immunization program in China. Hum Vaccin Immunother. 2021;17(7):2072–77. doi:10.1080/21645515.2020.1861877.
- Wang Q, Cheng X, Liu D, Chen C, Yao K. One single-center serological survey on measles, rubella and mumps antibody levels of people in Youyang, China. Hum Vaccin Immunother. 2021;17(11):4203–09. doi:10.1080/21645515.2021.1924522.
- Chinese-Ministry-of-Health. Diagnostic criteria for mump: WS. Infectious Disease Standards Committee of the Ministry of Health. Beijing, China: People’s Medical Publisher House; 2007. p. 270–007.
- Anderson RM, May RM. Vaccination and herd immunity to infectious diseases. Nature. 1985;318(6044):323–29. doi:10.1038/318323a0.
- Lang PO. Adverse effects of the herd immunity or when childhood vaccination becomes deleterious for the epidemiology of infectious diseases in adults. Geriatr Psychol Neuropsychiatr Vieil. 2011;9(1):11–19. doi:10.1684/pnv.2011.0260.
- Liu Y, Liu Z, Deng X, Hu Y, Wang Z, Lu P, Guo H, Sun X, Xu Y, Tang F, et al. Waning immunity of one-dose measles-mumps-rubella vaccine to mumps in children from kindergarten to early school age: a prospective study. Expert Rev Vaccines. 2018;17(5):445–52. doi:10.1080/14760584.2018.1445529.
- Davidkin I, Peltola H, Leinikki P, Valle M. Duration of rubella immunity induced by two-dose measles, mumps and rubella (MMR) vaccination. A 15-year follow-up in Finland. Vaccine. 2000;18(27):3106–12. doi:10.1016/s0264-410x(00)00139-0.
- Barskey AE, Schulte C, Rosen JB, Handschur EF, Rausch-Phung E, Doll MK, Cummings KP, Alleyne EO, High P, Lawler J, et al. Mumps outbreak in Orthodox Jewish communities in the United States. N Engl J Med. 2012;367(18):1704–13. doi:10.1056/NEJMoa1202865.
- Wohl S, Metsky HC, Schaffner SF, Piantadosi A, Burns M, Lewnard JA, Chak B, Krasilnikova LA, Siddle KJ, Matranga CB, et al. Combining genomics and epidemiology to track mumps virus transmission in the United States. PLoS Biol. 2020;18(2):e3000611. doi:10.1371/journal.pbio.3000611.
- Kaaijk P, Wijmenga-Monsuur AJ, van Houten MA, Veldhuijzen IK, Ten Hulscher HI, Kerkhof J, van der Klis FR, van Binnendijk RS. A third dose of measles-mumps-rubella vaccine to improve immunity against mumps in young adults. J Infect Dis. 2020;221(6):902–09. doi:10.1093/infdis/jiz188.
- Wodi AP, Murthy N, Bernstein H, McNally V, Cineas S, Ault K. Advisory committee on immunization practices recommended immunization schedule for children and adolescents aged 18 years or younger - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):234–37. doi:10.15585/mmwr.mm7107a2.
- Aasheim ET, Inns T, Trindall A, Emmett L, Brown KE, Williams CJ, Reacher M. Outbreak of mumps in a school setting, United Kingdom, 2013. Hum Vaccin Immunother. 2014;10(8):2446–49. doi:10.4161/hv.29484.
- May M, Rieder CA, Rowe RJ. Emergent lineages of mumps virus suggest the need for a polyvalent vaccine. Int J Infect Dis. 2018;66:1–4. doi:10.1016/j.ijid.2017.09.024.
- Dittrich S, Hahne S, van Lier A, Kohl R, Boot H, Koopmans M, van Binnendijk R. Assessment of serological evidence for mumps virus infection in vaccinated children. Vaccine. 2011;29(49):9271–75. doi:10.1016/j.vaccine.2011.09.072.