ABSTRACT
The effectiveness of the vero cell inactivated vaccine (CoronaVac®) against severe acute respiratory infection (SARI) caused by SARS-CoV-2 in the real world was assessed. A matched test-negative case-control design was employed using the web-based national information system, as well as the hospitalization dataset in Sibu Hospital. Vaccine effectiveness was measured by conditional logistic regression with adjustment for gender, underlying comorbidity, smoking status, and education level. Between 15 March and 30 September 2021, 838 eligible SARI patients were identified from the hospitalization records. Vaccine effectiveness was 42.4% (95% confidence interval [CI]: −28.3 to 74.1) for partial vaccination (after receiving the first dose to 14 days after receiving the second dose), and 76.5% (95% CI: 45.6 to 89.8) for complete vaccination (at 15 days or more after receiving the second dose). This analysis indicated that two doses of CoronaVac® vaccine provided efficacious protection against SARI caused by SARS-CoV-2 in the short term. However, the duration of protection, and performance against new variants need to be studied continuously.
Introduction
As of January 2022, nine COVID-19 vaccines have been added to the World Health Organization (WHO) Emergency Use Listing, including two inactivated vaccines (BIBP-CorV and CoronaVac®) which are the most used vaccines in China and some low and middle-income countries (LMIC).Citation1 More than 2.7 billion doses of CoronaVac® COVID-19 vaccine have been delivered globally. Studies conducted worldwide demonstrated good safety profiles and promising efficacy of the CoronaVac® vaccine against symptomatic infection caused by ancestral strain in the short-term.Citation2,Citation3 However, studies of its performance characteristics in the real world, such as protection against different virus variants, protection in persons more than 60 years old and in those with comorbidities, as well as its effectiveness in relation to the time interval between the first and second dose, are still sparse, and thus are encouraged to be investigated by WHO.Citation4
The first three cases of COVID-19 in Malaysia were imported cases, which were confirmed on 25 January 2020.Citation5 As of 17 May 2022, there were 4.47 million confirmed cases of COVID-19, with 35,620 deaths reported. The major transmission of SARS-CoV-2 in the Sibu Division started on 2 January 2021.Citation6 Since then, Sibu (the capital of the Sibu Division in the central region of Sarawak, Malaysia) has experienced three waves of pandemic. The first wave broke out in January 2020, which lasted until June 2021, and was associated with lineage B.1.466.2;Citation6,Citation7 the second wave occurred between mid-August and mid-November of 2021 due to the Delta variant (lineages B.1.617.2); and the third wave started in mid-February and ended in mid-April 2022, which was linked to the Omicron variant[8]. Seven phases of the Movement Control Order (MCO) were implemented from 18 March to 31 December 2020Citation8 to contain the spread of the virus. Although the MCO was essential to slow down the transmission rate, it had strongly impacted society and economy, and thus has been gradually removed, along with the launch of a national vaccination campaign (Program Imunisasi COVID-19 Kebangsaan; PICK) which started on 24 February 2021. As of 15 September 2021, 54.6% of Malaysians have completed the primary vaccination. Of these, CoronaVac® and Comirnaty (BNT162b2) accounted for 26.5% and 23.0%, respectively.Citation9 In this study, we aim to assess the effectiveness of the CoronaVac® vaccine against severe acute respiratory infection (SARI) associated with laboratory-confirmed SARS-CoV-2 infection in the real world.
Methods
Study design, setting and study population
A retrospective, test-negative case-control designCitation10 was used to assess the effectiveness of the CoronaVac® vaccine against SARI in Sibu with a population of 162,676. Health care services are provided majorly by Sibu Hospital, together with four public health clinics, several private clinics and two private medical specialist centers. During the COVID-19 pandemic, all SARI and influenza-like illnesses were investigated for COVID-19 at designated hospitals/clinics. Sibu Hospital, with a 750-bed capacity, was designated as the only hybrid hospital in the region to manage all suspected COVID-19 in-patients, including those who presented with SARI.Citation5
All medical records of SARI patients admitted to Sibu Hospital between 15 March and 30 September 2021 were retrieved if they were aged ≥18 years and resided in Sibu and Selangau districts (populations in both were served by Sibu Hospital). Exclusion criteria were foreigners, travelers from interstate or overseas, polymerase chain reaction (PCR) positive for SARS-CoV-2 more than ten days after the onset of current SARI, and within 90 days before the onset of SARI, and patients who received COVID-19 vaccines other than CoronaVac®. Clinical information at the time of presentation and during hospitalization was extracted from the medical records. Risk factors, including gender, smoking, comorbidities and education level, which may affect the risk of severe COVID-19 disease, were collected via telephone survey. An online consent form was used to obtain informed consent from SARI patients before the interview, and only those who agreed and signed the online consent form were interviewed. For those who passed away, informed consent and permission for an interview were obtained from the next of kin. Patients or families who refused or could not be contacted for any reason were excluded. All data were collected by research staff who were unaware of vaccination status and PCR results.
The WHO case definition for SARI was used:Citation11 patients hospitalized with an acute respiratory infection for more than 24 hours; and with a history of fever or a measured fever of ≥38°C; and with or without cough; and with symptom onset within the last ten days. A SARI patient caused by SARS-CoV-2 infection was defined as a SARI case with a positive reverse transcriptase (RT)-PCR testCitation12 that occurred within 14 days before to less than 72 hours after admission, ordered by clinicians to detect infection.
Data sources
Initially, nasopharyngeal swab specimens collected from suspected COVID-19 patients in Sibu were tested by the Sibu Hospital Clinical Research Centre Laboratory or contracted to other laboratories in West Malaysia or Sarawak’s capital, and later (from 15 June 2020) mainly by the Molecular Laboratory in Sibu Hospital, as well as other private laboratories. In Malaysia, it is mandatory to report all PCR test results to a web-based national information system (Sistem Informasi Makmal Kesihatan Awam, SIMKA, https://simka-result.moh.gov.my/result/), which clinicians could access with their username and password.Citation5 In this study, we traced the PCR results of recruited patients through the SIMKA.
The CoronaVac® COVID-19 vaccine was approved in Malaysia on 2 March 2021 and started to be administered in Sibu on 15 March 2021. A paper vaccination card was issued to the vaccinees. The local public health department kept an electronic record of the vaccines, consisting of demographic information, vaccination dates and vaccine brand. Without knowing the PCR results beforehand, vaccination records of enrolled patients in this study were ascertained with this database. The vaccination status of the enrolled patients was determined based on the hospital admission date. Patients were considered unvaccinated if they had not received any dose of the COVID-19 vaccines as of the admission date, and partial vaccination was defined as the interval between day 0 after receiving the first dose and day 14 after receiving the second dose. Complete vaccination was defined as the interval of 15 days or more after receiving the second dose, which was consistent with the CoronaVac® trial.Citation2,Citation3
Sample size and statistical analysis
Sample size was calculated to ensure adequate evaluation of the effectiveness against SARI caused by SARS-CoV-2 virus infection in a ratio of 1:1 for the test-positive and test-negative groups. We assumed, on average, 50% of the test-negative controls had received the COVID-19 vaccine at the time of admission during the study period. Therefore, effectiveness against SARI of 60% (odd ratio, OR = 0.40), with the lower boundary of 95% confidence interval (95% CI) of 20%, was expected. With a 5% level of significance (one-tailed), and 80% statistical power, a minimum of 337 SARI cases were required in this study. As the study period overlapped with the first and second wave of SARS-CoV-2 raging, there were far more test-positive (776 patients) than test-negative patients (62 patients) when reviewing the PCR test records. Therefore, in the final analysis, test-positive and test-negative patients were matched blinded to vaccination status using SAS programming by age group (18–34 years, 35–49 years, 50–59 years and ≥60 years) and workplace (work-at-home vs others) in a ratio of 4:1. Without changing other parameters, a post hoc calculation indicated that the statistical power was >95% under the actual measured vaccine effectiveness derived from 4:1 test-positive to test-negative matching.
Following WHO guidance,Citation13 a range of factors might have influenced the estimate of vaccine effectiveness, such as the likelihood of accepting a vaccine or the risk of exposure to the SARS-CoV-2 virus. In this study, apart from the matching variables (age group and workplace), characteristics including gender, duration of vaccination, comorbidities, smoking and education levels were also collected, and severed as covariates. These characteristics of test-positive (cases) and test-negative (controls) groups were portrayed and compared by the standardized difference, and a standardized difference >10% indicated a negligible correlation.Citation14
Vaccine effectiveness was estimated as one minus the adjusted OR (×100%). The effectiveness provided by full and partial vaccination was measured in comparison with those non-vaccinated, respectively. A crude odds ratios of vaccination status, as well as other covariates was calculated with simple logistic regression in case patients as compared with controls, with associated 95% confidence intervals. Subsequently, a conditional logistic-regression models for the prespecified primary outcomes was constructed to calculate odds ratios of antecedent vaccination (fully or partially vaccinated vs. unvaccinated) adjusted by abovementioned covariates. To estimate a plausible range of effectiveness, sensitivity analyses were performed. Vaccine effectiveness was determined in different combinations comorbidities, smoking, and education levels with vaccination status and gender in the model.
All statistical analyses were conducted using the SAS software, version 9.4 (SAS Institute). Tests were two sided, with p < .05 considered as significant.
Results
Characteristics of participants
Between 15 March and 30 September 2021, 1,083 SARI cases were diagnosed and admitted to Sibu Hospital. Of these, 838 (77.4%) patients were eligible and successfully matched to the SIMKA database for their PCR test results and the local electronic database for vaccination status, respectively. Among the 838 eligible SARI patients, 835 (99.6%) patients first sought health care at Sibu Hospital; 776 (92.6%) were PCR positive, and 62 (7.4%) were negative (). Demographics, risk factors associated with SARS-CoV-2 and clinical characteristics of eligible and matched patients were compared (). Before matching, a significant difference between test-positive and test-negative SARI patients was observed on most variables except for vaccination information and gender. Compared to test-positive patients, test-negative patients were more likely to have underlying comorbidities, home-based, were smokers and were less educated elderlies (p < .05) (). By age group and workplace matching in a ratio of 4:1, 248 test-positive and 62 test-negative patients were included in the effectiveness analysis. After matching, a significant difference on comorbidities, and smoking between test-positive and test-negative patients remained (). All characteristics of those matched test-positives were similar to those eligible test-positives except for vaccination status, comorbidities and education levels. Notably, the matched test-positives were more likely to have comorbidities, were less educated, and fewer of them received the second dose () (p < .05). In addition, the mean time between the latest dose and admission was 24.9 days and 25.8 days for matched test-positive and test-negative patients, respectively (p > .05).
Figure 1. Summary of participants.
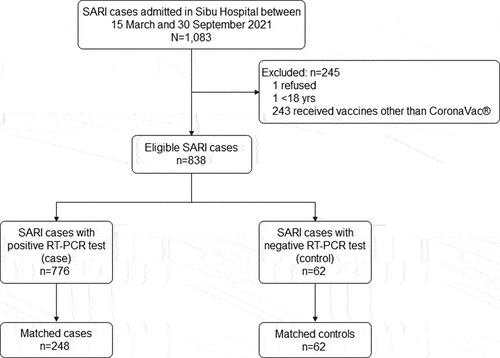
Table 1. Characteristics and vaccination status of eligible severe acute respiratory infection (SARI) cases and matched case-control pairs.
Vaccine effectiveness
In terms of the standardized differences of variables, vaccination status, gender, comorbidities, smoking and education levels were fixed, and their combinations were introduced into the logistic regression model in a proper sequence. Based on the final model, after adjusting for gender, comorbidities, smoking and education levels, for those people who only received one dose, or received the second dose within 14 days, the vaccine effectiveness was 42.4% (95% CI: −28.3 to 74.1). Whilst for those who completed the second dose for more than 14 days, the vaccine effectiveness was 76.5% (95% CI: 45.6 to 89.8%) (p < .05). There was no notable difference in vaccine effectiveness derived from unadjusted and adjusted analysis. However, patients who did not smoke and had no comorbidities were more likely to have COVID-19 (p < .05) (). Sensitivity analysis for complete vaccination was performed by adjusting the number of variables in the final analytical model, and the measured effectiveness was quite similar, which varied between 74.5% (95% CI: 42.9 to 88.6) and 78.2% (95% CI: 49.9 to 90.5) ().
Figure 2. Sensitivity analysis of vaccine effectiveness.
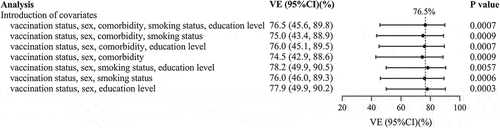
Table 2. Odds ratio (OR) of risk factors among severe acute respiratory infection (SARI) patients in Sibu, 15 March to 30 September 2021.
Discussion
This study demonstrated an efficacious protection of the CoronaVac® vaccines against SARI in Sibu, Malaysia. In an average duration of about 25 days after the latest injection, partial vaccination (mostly received a single dose) of the CoronaVac® protected against SARI for more than 40%, while complete vaccine (two doses injection >14 days) was about 75% effective. Moreover, about 40% of eligible patients included in the final analytic model were elderlies aged greater than 60 years; of these, about 70% of patients had more than one underlying comorbidities. The information generated from this study has addressed several knowledge gaps for the use of CoronaVac® in LMIC countries, including its performance concerning different variants, in elderly with comorbidities, and about time intervals between the first and second dose.Citation4
These findings are consistent with several other studies published. A nation-wide real-world prospective cohort study including 10.2 million participants over 16 years of age was conducted in Chile from 2 February to 1 May 2021 during the circulation of lineages P.1 (gamma variant) and B.1.1.7 (alpha variant). This study’s duration partially overlapped with our study. Among those people who received complete vaccination, the adjusted effectiveness against hospitalization was 88% (95% CI: 87 to 88%) in the entire population and 85% (95% CI: 84 to 86%) in the elderlies (>60 years).Citation15 In a recent preprint from Hong Kong, the vaccine’s effectiveness was assessed from 31 December 2021 to 8 March 2022, while the Omicron variant was dominant, using an ecological study design. There was no protection against mild/moderate diseases provided by two doses of CoronaVac® across all age groups. In contrast, high effectiveness (92% [95% CI: 88 to 94%]) against severe diseases was found in younger age groups. However, reduced effectiveness of 74% (95% CI: 68 to 79%) was measured in adults who were more than 60 years old.Citation16 During the outbreak with 153 cases caused by Delta variant in Guangzhou between May and June 2021, a protection of 70% (95% CI: 30 to 89%) against moderate infections (defined as cases with fever, respiratory symptoms and imaging characteristics of pneumonia) was measured, and CoronaVac® vaccine had accounted for 61% of the doses administrated in the study population.Citation17 Studies from Singapore and Malaysia estimated about 55% protection against severe disease, including hospitalization requiring oxygen supplementation and admission to the intensive care unit (ICU).Citation18,Citation19 The most likely explanation for the differences might be that there are some underlying differences in the exposures to vaccination and SARS-CoV-2 infection, such as study design and population, the vaccination status, the outcome definitions, and a mix of natural infections, etc. Therefore, real-world evidence should be interpreted with caution.
Interestingly, we found that smoking and having underlying comorbidities were associated with a lower risk of contracting SARI. In contrast, these variables were identified as factors associated with an increased SARS-CoV-2 infection.Citation13 Several population-level, even national-level studies about the impact of smoking on SARI had been carried out in the United States,Citation20,Citation21 United Kingdom,Citation22 Italy,Citation23 as well as Malaysia,Citation24,Citation25 and they had yielded similar results. These studies suggested possible explanations such as the fact that smokers may be more likely to be tested due to chronic cough; smoking may reduce the nasopharyngeal viral load, thus decreasing PCR test sensitivity.Citation20,Citation22 However, we suggest that the pronounced difference in some aspects between the eligible test-positive and test-negative groups might be a plausible explanation in our study. Compared to the test-negative group, the test-positive group was characterized as younger, better educated, fewer smokers and had fewer comorbidities, and more of them were working on site, a factor that might have increased the chance of being exposed to SARS-CoV-2. On the other hand, though the test-negatives in our study were mostly at home and less exposed due to smoking-induced lung disorders and underlying comorbidities, they might require frequent hospital visits, thus increasing the chance of being tested for COVID-19. The differences in exposures to SARS-CoV-2 hidden behind the discrepancy of age distribution between the two groups were reflected in the age-related covariates, such as smoking and education level, which led to a spurious association. To rule out the potential confounders, a matched logistic regression model was implemented for the vaccine effectiveness analysis.
This study has some limitations. First, as abovementioned, test-negative patients were matched with test-positive patients by age group and workplace in a ratio of 1:4, and thus a selection bias might have been introduced here. However, differences were detected in comorbidities and education levels between matched test-positives patients and all eligible test-positives patients, and these two variables were adjusted along with other potential confounders. Secondly, as part of the response measures designed and implemented by the Ministry of Health Malaysia, all suspected COVID-19 cases were mandatory to be hospitalized or quarantined in various designated low-risk centers,Citation5 and patients with COVID-19 who needed hospitalized care in Sibu and Selangau districts were admitted to Sibu Hospital. Less severe cases who were otherwise well enough might also be admitted and misclassified as SARI cases during the pandemic, and this misclassification would attenuate the vaccine effects. Bearing the limitations in mind, the results generated should be interpreted with caution. Lastly, we did not include mortality cases that were brought in dead to the hospital in our study due to technical difficulty in collecting the essential clinical information. We believe some of these brought in dead cases might have been due to COVID-19.
In conclusion, our study indicated that two doses of CoronaVac® inactivated vaccine was efficacious in preventing SARI caused by SARS-CoV-2 in Sibu, Malaysia, in the short term after completing primary vaccination. However, the long-term protection conferred by a 2-dose regimen, incremental effectiveness induced by either homologous or heterologous boosting, and performance against new variants, need to be studied continuously.
Author contributions
XYW, THT, SMY, YYQ, JSYL, JLX, JC and NFL conceptualized and designed the study, collected, analyzed and interpreted the data. THT is the lead of the study in Malaysia. SMY, JSYL, NFL, RYHS, MZQY, NJL, DWSC and AZT collected the clinical data while JWL, CNM, WWL, NT and MRS contributed the data collected. XYW also wrote the first draft of the manuscript and the draft was critically revised by TTH, JSFYL, SMY, JHT, NT, JC and MRS. MASLN provided statistical design of the protocol and critically reviewed the statistical analysis. There was no honorarium, grant, or another form of payment given to anyone to produce the manuscript. All authors critically revised, read and approved the final manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Ethical approval statement
This study was conducted in Sibu Hospital with ethical approvals from the Medical Research and Ethics Committee of the Ministry of Health Malaysia, and the Institutional Review Board, the Institutions of Biomedical Sciences, Fudan University, China. This study was conducted according to the Declaration of Helsinki, and reported in compliance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Supplemental Material
Download PDF (467.2 KB)Acknowledgement
We thank the Director General of Health Malaysia for his permission to publish this study. In addition, we thank the medical officers, nurses, health inspectors, research officers and assistant medical officers from Sibu Hospital and various health clinics in the Sibu division for their invaluable help with patient management, sample processing and documentation. We also thank Dr Amir Ghadaffi, Chieh-Ran Toh and Caleb Cheng-Yi Wong for helping to collect some of the clinical data, and Rache Wong with her team for helping to design a data management algorithm. Furthermore, we appreciate the willingness of the Sarawak State Health Department, Sibu Specialist Medical Centre, SEGi University and Rejang Medical Centre to share their data with us. Our special gratitude to Dr Swee-Sing Chan, Dr Betty Leh-Sieng Lee, Dr Deburra Ngadan, Dr Norraliza Binti Md Zain and Kuek-Sen Chong for their assistance in accessing the patient’s registry. We appreciate Dr Zhi-Wei Jiang and Yong-Ji Wang of Beijing KeyTech Statistical Consulting Co) for their dedicated support in statistical analysis and Dr Wong See Chang for his valuable advice during this study. In particular, we thank Dr Firdausi Qadri of the International Centre for Diarrhoeal Disease Research, Bangladesh, for sharing her protocol with us.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2167438.
Additional information
Funding
References
- World Health Organization. WHO COVID-19 Dashboard. Geneva: World Health Organization; 2020.
- Fadlyana E, Rusmil K, Tarigan R, Rahmadi AR, Prodjosoewojo S, Sofiatin Y, Khrisna CV, Sari RM, Setyaningsih L, Surachman F, et al. A phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18–59 years: an interim analysis in Indonesia. Vaccine. 2021;39:(44):6520–7. doi:10.1016/j.vaccine.2021.09.052.
- Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, Köse Ş, Erdinç FŞ, Akalın EH, Tabak ÖF, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:(10296)213–22. doi:10.1016/S0140-6736(21)01429-X.
- World Health Organization. Geneva: 2022 Mar 15 [accessed 2022 May 17]. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-Sinovac-CoronaVac-2021.1.
- Hashim JH, Adman MA, Hashim Z, Mohd Radi MF, Kwan SC. COVID-19 epidemic in Malaysia: epidemic progression, challenges, and response. Front Public Health. 2021;9:560592. doi:10.3389/fpubh.2021.560592.
- Sam IC, Chong YM, Abdullah A, JYL F, Hasan MS, Jamaluddin FH, Kamarulzaman A, Lim KK, Mohd nor MA, Pang YK, et al. Changing predominant SARS-CoV-2 lineages drives successive COVID-19 waves in Malaysia, February 2020 to March 2021. J Med Virol. 2021;94(3):1146–53. doi:10.1002/jmv.27441.
- Perera D. Sarawak COVID-19 genomic surveillance statement. 2021.
- Muhamad Khair NK, Lee KE, Mokhtar M. Community-based monitoring in the new normal: a strategy for tackling the COVID-19 pandemic in Malaysia. Int J Environ Res Public Health. 2021;18(13):6712. doi:10.3390/ijerph18136712.
- Jayasundara P, Peariasamy KM, Law KB, Abd Rahim KNK, Lee SW, Ghazali IMM, Abayawardana M, Le L-V, Khalaf RKS, Razali K, et al. Sustaining effective COVID-19 control in Malaysia through large-scale vaccination. Epidemics. 2021;37:100517. doi:10.1016/j.epidem.2021.100517.
- Patel MK, Bergeri I, Bresee JS, Cowling BJ, Crowcroft NS, Fahmy K, Hirve S, Kang G, Katz MA, Lanata CF, et al. Evaluation of post-introduction COVID-19 vaccine effectiveness: summary of interim guidance of the World Health Organization. Vaccine. 2021;39(30):4013–24. doi:10.1016/j.vaccine.2021.05.099.
- World Health Organization. WHO surveillance case definitions for ILI and SARI. Geneva: WHO [Internet]; 2018.
- World Health Organization. WHO COVID-19 Case definition: Updated in Public health surveillance for COVID-19. Geneva: World Health Organization; 2022 Jul 2 [accessed 2021 Jan 20]. https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2022.1.
- World Health Organization. Evaluation of COVID-19 vaccine effectiveness: interim guidance. Geneva: World Health Organization; 2021.
- Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat - Simul Comput. 2009;38(6):1228–34. doi:10.1080/03610910902859574.
- Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, Pizarro A, Acevedo J, Leo K, Leon F, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):875–84. doi:10.1056/NEJMoa2107715.
- McMenamin ME, Nealon J, Lin Y, Wong JY, Cheung JK, Lau EHY, Wu P, Leung G, Cowling BJ. Vaccine effectiveness of two and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong. medRxiv 2022:2022.03.22.22272769. doi:10.2139/ssrn.4064649.
- Li XN, Huang Y, Wang W, Jing QL, Zhang CH, Qin PZ, Guan W-J, Gan L, Li Y-L, Liu W-H, et al. Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: a test-negative case–control real-world study. Emerg Microbes Infect. 2021;10:(1):1751–59. doi:10.1080/22221751.2021.1969291.
- Premikha M, Chiew CJ, Wei WE, Leo YS, Ong B, Lye DC, Lee VJ, Tan KB. Comparative effectiveness of mRNA and inactivated whole virus vaccines against COVID-19 infection and severe disease in Singapore. Clin Infect Dis. 2022;75(8):1442–45. doi:10.1093/cid/ciac288.
- Suah JL, Husin M, Tok PSK, Tng BH, Thevananthan T, Low EV, Appannan MR, Muhamad Zin F, Mohd Zin S, Yahaya H, et al. Waning COVID-19 vaccine effectiveness for BNT162b2 and CoronaVac in Malaysia: an observational study. Int J Infect Dis. 2022;119:69–76. doi:10.1016/j.ijid.2022.03.028.
- Fan VS, Dominitz JA, Eastment MC, Locke ER, Green P, Berry K, O’Hare AM, Shah JA, Crothers K, Ioannou GN. Risk factors for testing positive for severe acute respiratory syndrome coronavirus 2 in a National United States healthcare system. Clin Infect Dis. 2021;73(9):e3085–94. doi:10.1093/cid/ciaa1624.
- Petrilli CM, Jones SA, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. Bmj. 2020;369:m1966. doi:10.1136/bmj.m1966.
- de Lusignan S, Dorward J, Correa A, Jones N, Akinyemi O, Amirthalingam G, de Lusignan S, Andrews N, Byford R, Dabrera G, et al. Risk factors for SARS-CoV-2 among patients in the Oxford royal college of general practitioners research and surveillance centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20(9):1034–42. doi:10.1016/S1473-3099(20)30371-6.
- Prinelli F, Bianchi F, Drago G, Ruggieri S, Sojic A, Jesuthasan N, Molinaro S, Bastiani L, Maggi S, Noale M, et al. Association between smoking and SARS-CoV-2 infection: cross-sectional study of the EPICOVID19 internet-based survey. JMIR Public Health Surveill. 2021;7(4):e27091. doi:10.2196/27091.
- Ismail N, Hassan N, Abd Hamid MHN, Yusoff UN, Khamal NR, Omar MA, Wong XC, Pathmanathan MD, Mohd Zin S, Muhammad Zin F, et al. Association of smoking and severity of COVID-19 infection among 5,889 patients in Malaysia: a multi-center observational study. Int J Infect Dis. 2022;116:189–96. doi:10.1016/j.ijid.2022.01.011.
- Sim BLH, Chidambaram SK, Wong XC, Pathmanathan MD, Peariasamy KM, Hor CP, Chua HJ, Goh PP. Clinical characteristics and risk factors for severe COVID-19 infections in Malaysia: a nationwide observational study. Lancet Reg Health West Pac. 2020;4:100055. doi:10.1016/j.lanwpc.2020.100055.
