ABSTRACT
Proactive HPV vaccination at age 9 better prevents infection and improves vaccine series completion. Because national organizations recommend starting the vaccine at different ages, we sought to understand the impact of these recommendation frames. In 2022, we surveyed 2,527 US clinical staff (45% physicians) who provide HPV vaccine for children. We randomized respondents to one of three frames based on HPV vaccine recommendations of national organizations or a no-recommendation control, and assessed willingness to recommend HPV vaccine for children ages 9–10. Respondents also reported perceived benefits of HPV vaccination at ages 9 or 12. Recommending HPV vaccination “at ages 11–12” led to lower willingness to vaccinate at ages 9–10 than control (37% vs. 54%, p < .05). Recommending vaccination “at ages 9–12” led to similar willingness as control. However, “starting at age 9” led to higher willingness than control (63% vs. 54%, p < .05). Results were similar across respondents’ training, specialty, or years in practice, or their clinic’s rurality or healthcare system membership. More common benefits of recommending at age 9 than 12 were avoiding the topic of sex (24% vs. 10%, OR = 2.78, 95%CI: 2.23, 3.48) and completing the vaccine series before age 13 (56% vs. 47%, OR = 1.44, 95%CI: 1.23, 1.68). Less common benefits for age 9 were having parents ready to talk about HPV vaccine and agreeing to vaccination (both p < .05). An effective way to encourage proactive HPV vaccination is to say that it starts at age 9. Aligning national recommendations to start at age 9 can promote timely vaccination.
Introduction
Persistent infection with human papillomavirus (HPV) causes six types of cancer (cervical, anal, penile, vaginal, vulvar, and oropharyngeal) and genital warts.Citation1–3 Many studies have demonstrated that HPV vaccines are effective and safe.Citation4 A 2020 cohort study of 1.6 million girls and women found that HPV vaccination was associated with an 88% lower risk of invasive cervical cancer,Citation5 and several other studies have demonstrated the vaccine’s association with decreased cancer incidence and mortality.Citation6,Citation7 Since the approval and introduction of HPV vaccines in the United States, coverage has steadily risen,Citation8 though it remains below the Healthy People 2030 target goal of 80% for adolescents ages 13–15.Citation9 In particular, HPV vaccine initiation between the ages of 9 and 12 remains low, meaning many children are not meeting the on-time schedule of series completion before age 13.Citation10
HPV vaccination is more effective at younger ages. Multiple studies have found that a two-dose vaccine series given to children younger than age 15 elicits a stronger immune response compared to a three-dose series given to older teens and adults.Citation11,Citation12 Quality improvement initiatives have been found to help increase HPV vaccination at ages 9–10,Citation13 which we term proactive vaccination. Starting the HPV vaccine series at age 9 may also increase the likelihood of completion by age 13.Citation14,Citation15 One study found that children who initiated the HPV vaccine series at ages 9–10 had 22 times the adjusted odds of on-time series completion by age 13 compared to children who initiated the series at ages 11–12.Citation16
National organizations recommending HPV vaccination use different recommendation framing for when to start routine vaccination. The US Centers for Disease Control and Prevention’s (CDC’s) Advisory Committee on Immunization Practices (ACIP) routinely recommends HPV vaccination at ages 11 or 12 but says that vaccination can be given at age 9.Citation17 Meanwhile, the American Academy of Pediatrics (AAP) recommends that HPV vaccination begin between the ages of 9 and 12, at “an age that the provider deems optimal for acceptance and completion of the vaccination series.”Citation18 Last, the National HPV Vaccination Roundtable (RT)—a US coalition to increase uptake of the vaccine—says that HPV vaccination should begin at age 9.Citation19 No prior studies that we are aware of have evaluated the impact of these different recommendation frames on willingness to recommend HPV vaccine at ages 9–10.
To address this gap, we sought to understand the impact of national recommendation framing in an experiment with US providers and other clinical staff. We expected recommendations that started at age 9 to lead to higher willingness to recommend at ages 9–10. In a second experiment, we examined whether the perceived benefits of HPV vaccination differed by recommended starting age at vaccination.
Methods
Participants
Participants were clinical staff working in primary care clinics that provided HPV vaccination to children. Eligible clinical staff: 1) were certified to practice in the US; 2) practiced as a physician, physician assistant (PA), advanced practice nurse (APN) (including clinical nursing specialist and nurse practitioner), registered nurse (RN), licensed practical/vocational nurse (LPN/LVN), medical assistant (MA), or certified nursing assistant (CNA); 3) had a specialty of pediatrics, family medicine, or general medicine or were working in a clinic with one of these specialties; and 4) had a role in HPV vaccination for children ages 9 through 12 years (e.g., assessed the child’s vaccination status, let the parent know that the child is due for HPV vaccine, recommended the vaccine, addressed parents’ questions and concerns about the vaccine, or administered the vaccine).
WebMD Market Research generated a national US convenience sample of clinical staff through their Medscape Network, a website providing information, continuing education, and research participation opportunities to the medical community. About 60% of US physicians are members of their panel.Citation20 The company verifies the identity of physician and advanced practitioner members.
Sampling occurred in two phases. During the pre-recruitment phase, Medscape emailed their members about participating in an upcoming survey to establish eligibility. Eligible members then received a link to complete the online survey. Respondents who clicked the survey link began by completing a 4-item screener that ensured they met eligibility criteria—1,680 clinical staff were ineligible or did not complete the screener. To ensure diversity in clinical staff training, we set quotas for roughly equal numbers of pediatricians, family physicians and other physicians, PAs and APNs, RNs, and MAs. Because the company was unable to reach the quota for MA/CNAs, we moved those quotas to the other training categories. We aimed for 20% of our sample to practice in clinics located in rural counties as defined by US Department of Agriculture (USDA) Rural-Urban Continuum Codes (RUCC) 4–9. A total of 2,527 primary care providers and other clinical staff completed the survey, yielding a response rate of 57% (AAPOR Response Rate 3).Citation21
Procedures
The survey was in the field from May through July 2022. After providing informed consent, respondents completed the survey online. The company sent two reminders to potential respondents who indicated interest in participating during the pre-recruitment phase but who did not yet begin the survey. Upon completion, respondents received a $30–45 honorarium, depending on their clinical training. The University of North Carolina Institutional Review Board approved the study protocol.
The survey included two experiments regarding starting age at vaccination. For the recommendation frame experiment, we randomly assigned respondents to one of four HPV vaccination age recommendations shown below.
“The American Academy of Pediatrics recommends starting HPV vaccination at age 11 or 12, which supports series completion before HPV exposure risk increases.” This frame was meant to align with the current CDC recommendations.
“The American Academy of Pediatrics recommends starting HPV vaccination between age 9 and 12, which supports series completion before HPV exposure risk increases.” This frame was meant to align with the current AAP recommendations.
“The American Academy of Pediatrics recommends starting HPV vaccination at age 9, which supports series completion before HPV exposure risk increases.” This frame was meant to align with the current National HPV Vaccination RT recommendations.
No-recommendation control.
We attributed all the recommendations to the same organization to remove this potential confound in the experiment. We chose AAP because the organization is well respected in pediatric primary care.
For the benefits experiment, we randomly assigned respondents to consider the perceived benefits of recommending HPV vaccine at age 9 versus age 12.
Measures
To refine survey items for the main survey, we conducted 39 cognitive interviews with clinical staff. Cognitive interviewing assesses whether the meaning participants bring to survey items matches that intended by researchers. We updated the survey instructions and items iteratively, based on feedback from the cognitive interview participants.
For the recommendation frame experiment, the outcome was the age of children for whom the respondent would be willing to start routinely recommending HPV vaccine. Response options were ages 9–10, ages 11–12, ages 13–17, an older age, or not willing to routinely recommend the vaccine. We coded responses to be ages 9–10 (1) or older ages/not at all (0). For the benefits experiment, respondents answered the following question: “What advantages do you see of recommending HPV vaccine starting at age [9 or 12]? Check all that apply.” The response options were: 1) Parents are ready to talk about the vaccine; 2) Discussions with parents are brief; 3) Sex does not come up in discussions with parents; 4) Parents agree to vaccination; 5) Children get fewer vaccines during a visit; 6) Children complete the vaccine series before age 13; 7) Children receive protection before exposure to HPV; and 8) None of these. We coded options 1–7 as dichotomous variables (yes = 1, no = 0). An additional question assessed how many vaccines respondents would give to children ages 9–12 at a single visit, with the response options being one vaccine per visit, two vaccines per visit, three vaccines per visit, four vaccines per visit, or no limit.
Demographic questions included respondents’ medical training, their specialty area, the geographic location of their practice, and their role in HPV vaccination for children ages 9–12. Listed roles included assessing the child’s vaccination status, letting the parent know that the child is due for HPV vaccine, recommending the vaccine, addressing parents’ questions and concerns about the vaccine, and administering the vaccine. The survey also assessed respondents’ views about which staff have a role in HPV vaccination in their clinic, including physicians, PAs, APNs, nurses (RN, LPN, LVN), and CNAs or MAs.
Data analysis
Recommendation frame experiment
To examine the impact of the recommendation frames, we used logistic regression with frame as the predictor and with no-recommendation control as the referent group. The outcome was being willing to recommend HPV vaccine at ages 9–10 (yes, no). To assess potential moderators of the impact of frame, we added an interaction term in separate analyses for each candidate moderator. We examined five potential moderators: staff training (physician versus other), staff specialty (pediatrics versus other), staff years in practice (0–9 years versus 10 or more years), home clinic’s healthcare system membership (yes or no), and clinic rurality (rural primary/secondary versus non-rural location).
Perceived benefits by age experiment
To identify whether perceived benefits of HPV vaccination differed for ages 9 and 12, we used logistic regression with age as the predictor (age 12 was the referent group) and each of the benefits as outcomes in separate regressions. All analyses were conducted using SAS v9.4 (Cary, NC) with two-tailed tests and a critical alpha of .05.
Results
The sample included mostly women (72%), and about a third of respondents were people of color (34%) (). Our sample included clinical staff across five categories of training: physician (48%), physician assistant (8%), advanced practice nurse (16%), nurse (24%), and MA/CNA (4%). Nearly half of respondents worked in pediatrics (50%), with the remaining working in family or general medicine (50%), and 40% of respondents saw between 10–24 pediatric patients per week. Most respondents worked at clinics that held healthcare system membership (62%) and were located in non-rural settings (79%). Nearly half of respondents worked at group practices (50%), and practices typically included 2–5 providers each (39%).
Table 1. Participant and clinic characteristics (n = 2,527).
Almost all respondents (94%) had a role in assessing HPV vaccination status, indicating that a child is due for HPV vaccination (92%), recommending HPV vaccine (91%), and addressing concerns about the HPV vaccine (93%). Almost half (47%) said they administered HPV vaccine to patients. Around 6% of respondents said that one vaccine was their recommended limit per visit, 22% said two vaccines, 17% said three vaccines, and 11% said four vaccines. Less than half (43%) said that they had no limit to the number of vaccines they offer a child during a visit.
Recommendation frame experiment
The recommendation for HPV vaccination starting at ages 11–12 yielded the lowest willingness to vaccinate at ages 9–10 (37%), which was lower than the no-recommendation control (54%, p < .05) (). The recommendation for HPV vaccination starting at ages 9–12 yielded similar willingness (58%) to control (p =.19). The recommendation for HPV vaccination starting at age 9 yielded the highest willingness (63%), which was higher than the no-recommendation control (p < .05). The impact of recommendation frame did not differ across respondents’ training, specialty, or years in practice, or their clinic being a member of a healthcare system or rural (all moderation tests p > .05) ().
Figure 1. Impact of recommendation frame on willingness to recommend HPV vaccination for patient ages 9–10 years.
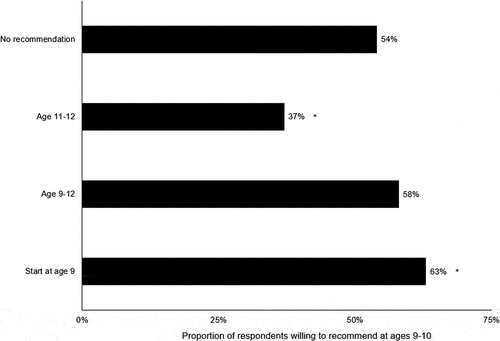
Table 2. Moderators of the impact of recommendation frame on willingness to recommend HPV vaccination for patient ages 9–10 years.
Perceived benefits by age experiment
Respondents said that “sex does not come up in discussions with parents” (24% vs. 10%, OR = 2.78, 95%CI: 2.23, 3.48) and that “children complete the vaccine series before age 13” (56% vs. 47%, OR = 1.44, 95%CI: 1.23, 1.68) were both benefits more often for age 9 than age 12 (). Respondents were less likely to identify several other options as perceived benefits for age 9 than age 12: parents being ready to talk about HPV vaccine (12% vs. 43%, OR = 0.18, 95%CI: 0.15, 0.22) and parents agreeing to vaccination (14% vs. 24%, OR = 0.53, 95%CI: 0.43, 0.64). No differences by age appeared for perceived benefits of keeping discussions with parents brief, children receiving fewer vaccines during a visit, and children receiving protection before exposure to HPV.
Table 3. Perceived benefits of HPV vaccination at ages 9 and 12.
Discussion
Proactive HPV vaccination starting at age 9 has emerged as a critical issue in ensuring high uptake of HPV vaccine.Citation22 In a large national study of clinical staff, the recommendation that framed HPV vaccination as starting at age 9 led to greater willingness to recommend the vaccine at ages 9–10. Furthermore, the strength of the age 9 recommendation was present across all subgroups that we examined in our analyses. Of the four recommendation conditions included in our experiment (including no recommendation at all), recommending HPV vaccination at ages 11–12 performed the worst. Comparing perceived benefits of proactive HPV vaccination, the analysis found that avoiding conversations about sex with parents and children completing the vaccine series before age 13 were benefits of starting younger. However, the analysis identified several other perceived benefits for starting at an older age, including parents being ready to talk about HPV vaccine and agreeing to vaccinate. We also found that over a quarter of providers and other clinical staff were unwilling to give more than two vaccine doses per visit, suggesting that recommending HPV vaccine alongside other routine childhood vaccines is a barrier in a meaningfully large number of clinics.
The language we used in the recommendation to emphasize starting HPV vaccination at ages 11–12 reflects what is currently used by CDC, though it is important to acknowledge that they also include additional mitigating language in their recommendation: They routinely recommend “HPV vaccination at age 11 or 12 years; vaccination can be given starting at age 9 years.”Citation17 Our experience with providers and other clinical staff indicates that they often do not remember the additional caveat, only the “big picture” approach. The results of our experiment suggest that it may be productive for CDC to more fully align their recommendation with that of the National HPV Vaccination Roundtable. Such a recommendation would state that “HPV vaccination begins at age 9” and encourage routine recommendation at this age. A unified position on this topic is important given strong evidence showing that provider recommendations increase vaccine uptake,Citation23,Citation24 and the critical role of providers and other clinical staff in ensuring high HPV vaccine coverage among children and adolescents.Citation25–29
Our work has implications for practice in changing the way that clinical staff communicate with the parents or caregivers of children ages 9–12. Saying that HPV vaccination starts at age 9 in national recommendations is an effective way to encourage all clinical staff, including physicians, to recommend HPV vaccine at ages 9–10. Previous research indicates a need for interventions to encourage timely HPV vaccination.Citation30 While about one fifth of clinical staff already recommend HPV vaccine starting at this age, more than half of those who are not yet doing so are willing to begin recommending earlier.Citation31 Clinical staff should adopt this practice in order to ensure higher HPV vaccine series completion by age 13Citation32 and should also consider implementing communication training to effectively do so.Citation33,Citation34
Our study had several strengths. First, the recommendation frame experiment served as a straightforward approach to determine how recommendation framing language impacts clinical staff willingness to recommend HPV vaccine at ages 9–10. The experimental design allowed us to make strong inferences about the impact of the age language used in the recommendations. Moreover, the study had a national sample of clinical staff with diverse training and a relatively high response rate. Our study had several limitations as well. When considering whether or not clinical staff would actually recommend the vaccine at age 9, we do not yet know how this experiment would work in the more naturalistic environment of clinical practice compared to the survey context. While our study used a national sample, the respondent pool included a small number of MAs and CNAs. Our sample was also skewed toward clinical staff who practice in urban or suburban settings. Last, we relied on single-item measures, which may understate the associations described in our results due to potentially unreliable measurement.
Looking ahead, coordination between key national stakeholders is critical for improving HPV vaccine uptake and series completion, an issue noted in the US Vaccines National Strategic Plan 2021–2025.Citation35 Better aligning national organizations’ recommendations can promote HPV vaccination at a younger age and help to increase timely uptake of the vaccine. Future research could explore HPV vaccine recommendation framing and perceived benefits across ages among parents, caregivers, and other stakeholders.
Disclosure statement
Brewer has served as a paid consultant for Merck, CDC, and WHO. Other authors declared no potential conflicts.
Additional information
Funding
References
- Cheng L, Wang Y, Du J. Human papillomavirus vaccines: an updated review. Vaccines. 2020;8:391. doi:10.3390/vaccines8030391. PMID: 32708759.
- Jørgensen L, Gøtzsche PC, Jefferson T. Benefits and harms of the human papillomavirus (HPV) vaccines: systematic review with meta-analyses of trial data from clinical study reports. Syst Rev. 2020;9:43. doi:10.1186/s13643-019-0983-y. PMID: 32106879.
- Senkomago V, Henley SJ, Thomas CC, Mix JM, Markowitz LE, Saraiya M. Human Papillomavirus–Attributable Cancers — United States, 2012–2016. MMWR Morb Mortal Wkly Rep. 2019;68:724–7. doi:10.15585/mmwr.mm6833a3. PMID: 31437140.
- Phillips A, Patel C, Pillsbury A, Brotherton J, Macartney K. Safety of human papillomavirus vaccines: an updated review. Drug Saf. 2018;41(4):329–46. doi:10.1007/s40264-017-0625-z. PMID: 29280070.
- Lei J, Ploner A, Elfström KM, Wang J, Roth A, Fang F, Sundström K, Dillner J, Sparén P. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340–48. doi:10.1056/NEJMoa1917338. PMID: 32997908.
- Falcaro M, Castañon A, Ndlela B, Checchi M, Soldan K, Lopez-Bernal J, Elliss-Brookes L, Sasieni P. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet. 2021;398:2084–92. doi:10.1016/S0140-6736(21)02178-4. PMID: 34741816.
- Tabibi T, Barnes JM, Shah A, Osazuwa-Peters N, Johnson KJ, Brown DS. Human papillomavirus vaccination and trends in cervical cancer incidence and mortality in the US. JAMA Pediatr. 2022;176:313–16. doi:10.1001/jamapediatrics.2021.4807. PMID: 34842903.
- Pingali C, Yankey D, Elam-Evans LD, Markowitz LE, Williams CL, Fredua B, McNamara LA, Stokley S, Singleton JA. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:1183–90. doi:10.15585/mmwr.mm7035a1. PMID: 34473682.
- US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy people 2030: Increase the proportion of adolescents who get recommended doses of the HPV vaccine — IID‑08. Washington (DC). Office of Disease Prevention and Health Promotion, Office of the Assistant Secretary for Health, Office of the Secretary, US Department of Health and Human Services; 2020 Aug 18 [accessed 2022 Aug 18]. https://health.gov/healthypeople/objectives-and-data/browse-objectives/vaccination/increase-proportion-adolescents-who-get-recommended-doses-hpv-vaccine-iid-08.
- Chido-Amajuoyi OG, Talluri R, Wonodi C, Shete S. Trends in HPV vaccination initiation and completion within ages 9-12 years: 2008-2018. Pediatrics. 2021;147(6):e2020012765. doi:10.1542/peds.2020-012765. PMID: 33941585.
- Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, Sauvageau C, Scheifele DW, Kollmann TR, Halperin SA, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309(17):1793–802. doi:10.1001/jama.2013.1625. PMID: 23632723.
- Iversen OE, Miranda MJ, Ulied A, Soerdal T, Lazarus E, Chokephaibulkit K, Block SL, Skrivanek A, Nur Azurah AG, Fong SM, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411–21. doi:10.1001/jama.2016.17615. PMID: 27893068.
- Goleman MJ, Dolce M, Morack J. Quality improvement initiative to improve human papillomavirus vaccine initiation at 9 years of age. Acad Pediatr. 2018;18(7):769–75. doi:10.1016/j.acap.2018.05.005. PMID: 29842924.
- Biancarelli DL, Drainoni ML, Perkins RB. Provider experience recommending HPV vaccination before age 11 years. J Pediatr. 2020;217:92–97. doi:10.1016/j.jpeds.2019.10.025. PMID: 31757474.
- Perkins RB, Legler A, Jansen E, Bernstein J, Pierre-Joseph N, Eun TJ, Biancarelli DL, Schuch TJ, Leschly K, Fenton ATHR, et al. Improving HPV vaccination rates: a stepped-wedge randomized trial. Pediatrics. 2020;146(1):e20192737. doi:10.1542/peds.2019-2737. PMID: 32540986.
- St Sauver JL, Rutten LJF, Ebbert JO, Jacobson DJ, McGree ME, Jacobson RM. Younger age at initiation of the human papillomavirus (HPV) vaccination series is associated with higher rates of on-time completion. Prev Med. 2016;89:327–33. doi:10.1016/j.ypmed.2016.02.039. PMID: 26930513.
- Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2019;68:698–702. doi:http://dx.doi.org/10.15585/mmwr.mm6832a3. PMID: 31415491.
- O’Leary ST, Nyquist AC Why AAP recommends initiating HPV vaccination as early as age 9. American Academy of Pediatrics (AAP) News; 2019 Oct 4 [accessed 2022 Oct 4]. https://publications.aap.org/aapnews/news/14942/Why-AAP-recommends-initiating-HPV-vaccination-as.
- National HPV Vaccination Roundtable. HPV vaccination starts at age 9. National HPV Vaccination Roundtable; 2021 [accessed 2022 Oct 3]. https://hpvroundtable.org/hpv-vaccination-starts-at-9/.
- Medscape Market research. WebMD Medscape; 2019 [accessed 2022 Oct 18]. https://www.medscape.com/sites/public/marketresearch.
- The American Association for Public Opinion Research (AAPOR). Standard definitions: Final dispositions of case codes and outcome rates for surveys. 9th ed. AAPOR; 2016 [accessed 2022 Oct 3]. https://www.aapor.org/aapor_main/media/publications/standard-definitions20169theditionfinal.pdf.
- Saslow D, Darville-Sanders G, Turpin C, Hora L National HPV vaccination roundtable age 9 call to action letter. National HPV Vaccination Roundtable; 2022 Aug 15 [accessed 2022 Oct 24]. https://hpvroundtable.org/wp-content/uploads/2022/08/HPVRT-Age-9-Call-to-Action-Letter_Final-2022.pdf.
- Brewer NT, Chapman GB, Rothman AJ, Leask J, Kempe A. Increasing vaccination: putting psychological science into action. Psychol Sci Public Interest. 2017;18(3):149–207. doi:10.1177/1529100618760521. PMID: 29611455.
- Brewer NT. What works to increase vaccination uptake. Acad Pediatr. 2021;21(4S):S9–16. doi:10.1016/j.acap.2021.01.017. PMID: 33958099.
- Brewer NT, Fazekas KI. Predictors of HPV vaccine acceptability: a theory-informed, systematic review. Prev Med. 2007;45(2–3):107–14. doi:10.1016/j.ypmed.2007.05.013. PMID: 17628649.
- Donahue KL, Hendrix KS, Sturm LA, Zimet GD. Human papillomavirus vaccine initiation among 9-13-Year-Olds in the United States. Prev Med Rep. 2015;2:892–98. doi:10.1016/j.pmedr.2015.10.003. PMID: 26594616.
- Mavundza EJ, Iwu-Jaja CJ, Wiyeh AB, Gausi B, Abdullahi LH, Halle-Ekane G, Wiysonge CS. A systematic review of interventions to improve HPV vaccination coverage. Vaccines. 2021;9(7):687. doi:10.3390/vaccines9070687. PMID: 34201421.
- Oh NL, Biddell CB, Rhodes BE, Brewer NT. Provider communication and HPV vaccine uptake: a meta-analysis and systematic review. Prev Med. 2021;148:106554. doi:10.1016/j.ypmed.2021.106554. PMID: 33857561.
- Zheng L, Wu J, Zheng M. Barriers to and facilitators of human papillomavirus vaccination among people aged 9 to 26 years: a systematic review. Sex Transm Dis. 2021;48(12):e255–62. doi:10.1097/OLQ.0000000000001407. PMID: 33783412.
- Henrikson NB, Tuzzio L, Gilkey MB, McRee AL. “You’re never really off time”: healthcare providers’ interpretations of optimal timing for HPV vaccination. Prev Med Rep. 2016;4:94–97. doi:10.1016/j.pmedr.2016.05.002. PMID: 27413667.
- Kong WY, Huang Q, Thompson P, Grabert BK, Brewer NT, Gilkey MB. Recommending human papillomavirus vaccination at age 9: a national survey of primary care professionals. Acad Pediatr. 2022;22:573–80. doi:10.1016/j.acap.2022.01.008. PMID: 35081470.
- Bednarczyk RA, Ellingson MK, Omer SB. Human papillomavirus vaccination before 13 and 15 years of age: analysis of national immunization survey teen data. J Infect Dis. 2019 Jul 31;220(5):730–34. 10.1093/infdis/jiy682. PMID: 30657920.
- Brewer NT, Hall ME, Malo TL, Gilkey MB, Quinn B, Lathren C. Announcements versus conversations to improve HPV vaccination coverage: a randomized trial. Pediatrics. 2017;139:1, e20161764. doi:10.1542/peds.2016-1764. PMID: 27940512.
- Shah PD, Calo WA, Gilkey MB, Boynton MH, Alton Dailey S, Todd KG, Robichaud MO, Margolis MA, Brewer NT. Questions and concerns about HPV vaccine: a communication experiment. Pediatrics. 2019;143(2):e20181872. doi:10.1542/peds.2018-1872. PMID: 30670584.
- US Department of Health and Human Services. Vaccines National Strategic Plan 2021–2025. Washington (DC): US Department of Health and Human Services; 2021 Jan 19 [accessed 2022 Dec 23]. https://www.hhs.gov/sites/default/files/HHS-Vaccines-Report.pdf
