ABSTRACT
There has been substantial evolution in the treatment of metastatic renal cell carcinoma with notable changes in the first-line setting. Currently, doublet combination therapy with either two immune checkpoint inhibitors or a combination of an immune checkpoint and tyrosine kinase inhibitor is considered the standard of care. The doublet combination therapies have demonstrated significantly improved clinical outcomes. A recently conducted trial (COSMIC-313) showed superior efficacy with a triplet combination of cabozantinib, nivolumab, and ipilimumab when compared to a placebo, nivolumab, and ipilimumab but at the cost of additional toxicity. Many other combination treatments, such as pembrolizumab plus lenvatinib plus belzutifan (NCT04976634), are being investigated, possibly leading to more options in the first-line setting in the future.
Introduction
The treatment paradigm of metastatic renal cell carcinoma (mRCC) has rapidly evolved in recent years with the emergence of immuno-oncology (IO) combination therapies. Currently, there are various IO treatment options in the first-line setting as the standard of care, including doublet immune checkpoint blockade (ICB) therapy or ICB with VEGF receptor inhibitor [IO-tyrosine kinase inhibitor (TKI)].Citation1 This has led to a significant improvement in clinical outcomes and disease prognosis. All approved combinations are superior to the historical standard of TKI monotherapy. However, there are no prospective studies directly comparing these combinations. The current practice of choosing one combination regimen over another is mainly driven by the physician’s discretion and the patient’s preference. Despite the advancements made so far, 5–20% of the patients have primary progressive disease (PD) as their best overall response, highlighting the unmet need for more effective first-line therapies for these patients.Citation2–5 In this review, we discuss the recent therapeutic advancements made in the first-line setting of mRCC, including the approved doublets and the recent publication of COSMIC-313, the first reported triplet therapy in mRCC.Citation6 Although there are multiple histologic subtypes of RCC, herein we review the data only in the context of clear cell mRCC, the most common variant of RCC.
Treatment update in metastatic clear renal cell carcinoma
The International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk is a prognostic model for clear cell mRCC that integrates two clinical and four laboratory criteria (). Based on these criteria, patients are stratified into three categories: favorable, intermediate, or poor risk. IMDC stratification strongly correlates with survival outcomes, specifically median overall survival in both IO/IO and TKI/IO combinations (OS).Citation7,Citation8 Landmark clinical trials design and many guidelines worldwide are based on the IMDC prognostic model to stratify patients and direct therapy.Citation3,Citation9–11
Table 1. International Metastatic Renal Cell Carcinoma Database Consortium criteria.
First-line systemic therapy options
The combination of a Programmed Cell Death-1 (PD-1/PD-L1) inhibitor with either cytotoxic T-lymphocyte antigen 4 (CTLA-4) or VEGFR-target inhibitor has improved OS in metastatic clear cell renal cell carcinoma (mccRCC).Citation3,Citation4,Citation12–14 Five phase III trials assessed these combinations in the first-line setting in the advanced mRCC and all were compared with sunitinib as the control arm ().
Table 2. Landmark phase III trials investigating the various IO combination therapies in the first-line setting for advanced RCC.
Combination therapy of two immune checkpoint inhibitors (IO/IO therapy)
The CheckMate-214 study
The CheckMate-214 study investigated the ipilimumab/nivolumab versus sunitinib in treatment-naïve patients with mccRCC.Citation2 Patients with IMDC favorable-, intermediate-, and poor-risk disease were enrolled (n = 1096). However, the co-primary endpoints of OS, progression-free survival (PFS), and objective response rate (ORR) were only assessed in the intermediate- and poor-risk patients (n = 847). Secondary endpoints included analysis of OS, PFS, and ORR in all enrolled patients. At the time of initial analysis, with a median follow-up of 25.2 months, ipilimumab/nivolumab showed OS (median not reached [NR] versus 26.0 months, HR, 0.63, p < .001), PFS (median 11.6 versus 8.4 months, HR 0.82, p = .03), and ORR (42.0% versus 27%, p < .001) versus sunitinib, respectively, in intermediate- or poor-risk patients (n = 425 versus 422). In the favorable-risk category (n = 249), no significant difference in terms of 18-month OS rate was reported (88% versus 93%, HR 1.45, p = .27) but ORR (29% versus 52%, p < .001) and PFS (median 15.3 versus 25.1, HR 2.18, p < .001) favored sunitinib over the ipilimumab/nivolumab combination. It is important to highlight that the study was not powered for the analysis of the favorable-risk population and the data were immature for these patients. The combination ipilimumab/nivolumab was relatively well tolerated, with fewer grade ≥3 AEs as compared to sunitinib alone (46% versus 63%). More treatment-related discontinuation (related to AEs) occurred in the ipilimumab/nivolumab group (22%) than in the sunitinib group (12%). Of patients who were randomized to ipilimumab/nivolumab arm and who had immune-mediated side effects, 35% received high-dose glucocorticoids (i.e., ≥40 mg of prednisone daily or equivalent).
These data led to the approval of the ipilimumab/nivolumab combination in the IMDC intermediate- or poor-risk groups. This was the first combination therapy earning the U.S. Food and Drug Administration (FDA) approval for mccRCC, thus changing the standard of care.
Recently, an extended 5-year follow-up of the CheckMate-214 study was presented, which is the longest follow-up period for a phase III trial utilizing an immunotherapy-based combination in the first-line treatment setting of advanced RCC.Citation15 At a median follow-up of 67.7 months, ipilimumab/nivolumab continued to show durable clinical benefits: OS (median 55.7 versus 38.4 months, HR 0.72), PFS (median 12.3 versus 12.3 months, HR 0.86), and ORR (39.3% versus 32.4%) versus sunitinib, respectively, in intent-to-treat patients (N = 550 versus 546).
Combination therapy of an immune checkpoint inhibitor with a tyrosine kinase inhibitor (IO/TKI therapy)
The KEYNOTE-426 study
The KEYNOTE-426 study was the phase III randomized IO/TKI combination trial, investigating axitinib/pembrolizumab versus sunitinib in treatment-naïve patients. The primary endpoints were OS and PFS in patients with any IMDC risk classification. Patients (n = 861) were enrolled in this study with 432 patients in the axitinib/pembrolizumab cohort and 429 patients in the sunitinib cohort. This study led to the approval of the axitinib/pembrolizumab in patients across all three IMDC risk groups.Citation5 At the time of initial analysis, with median follow-up of 12.8 months, axitinib/pembrolizumab showed better 12-month OS rate (89.9% versus 78.3%, HR 0.53, p < .0001), PFS (median 15.1 versus 11.1 months, HR 0.69, p < .001), and ORR (59.3% versus 35.7%, p < .001) versus sunitinib, respectively. Grade ≥3 AEs of any cause were higher with axitinib/pembrolizumab than sunitinib (75.8% versus 70.6%). More treatment-related discontinuation (related to AEs) occurred in the axitinib/pembrolizumab group (either drug in 30.5% and both drugs in 10.7% patients) than in the sunitinib group (13.9%). A reduction in the dose of axitinib was observed in 20.3% of the patients.
Recently, an extended follow-up of KEYNOTE-426 study was presented, which was the longest follow-up for a phase III trial of an IO/TKI combination in the first-line advanced RCC treatment setting.Citation16 At a median follow-up of 42.8 months, axitinib/pembrolizumab continued to show durable clinical benefits: 42-month OS rate (57.5% versus 48.5%, HR 0.73, p < .001), 42-month PFS rate (25.1% versus 10.6%, HR 0.68, p < .0001), and ORR (60.4% versus 39.6%, p < .0001) versus sunitinib, respectively. The results of the extended follow-up confirmed the superior efficacy of axitinib/pembrolizumab over sunitinib with no new safety signals.
The CheckMate 9ER
The CheckMate 9ER investigated cabozantinib/nivolumab versus sunitinib in treatment-naïve patients.Citation3 This trial led to the approval of cabozantinib/nivolumab in patients across all three IMDC risk groups. The primary endpoint was PFS in all patients enrolled with any IMDC risk classification. A total number of 651 patients were recruited in this study, with 323 patients in the cabozantinib/nivolumab cohort and 328 patients in the sunitinib cohort. At the time of initial analysis, with median follow-up of 18.1 months, cabozantinib/nivolumab showed better 12-month OS rate (85.7% versus 75.6%, HR 0.60, p = .001), PFS (median 16.6 versus 8.3 months, HR 0.51, p < .001), and ORR (55.7% versus 27.1%; p < .001) versus sunitinib, respectively. Grade ≥3 AEs of any cause were higher with cabozantinib/nivolumab than sunitinib (75.3% versus 70.6%). Of patients randomized to cabozantinib/nivolumab arm, 19.1% received high-dose glucocorticoids (i.e., ≥40 mg of prednisone daily or equivalent) for the management of immune-mediated side effects. More treatment discontinuation due to AEs of any cause occurred in the cabozantinib/nivolumab group (19.7%) than in the sunitinib group (16.9%). A reduction in the dose of cabozantinib was observed in 56.3% of the patients. In an updated analysis, at 3 years follow-up, the median OS of patients treated with cabozantinib plus nivolumab was significantly improved, reaching 49.5 months compared to 35.5 months in the sunitinib arm. Notably, the responses to this treatment were enduring, with higher complete response rates compared to sunitinib, irrespective of the IMDC risk group. Importantly, no new safety signals emerged with additional follow-up in either arm. These results further support the utilization of nivolumab plus cabozantinib as a first-line treatment choice for patients with advanced RCC.Citation17
The CLEAR study
The CLEAR Study was a three-arm trial (n = 1069) investigating pembrolizumab/lenvatinib (n = 355), everolimus/lenvatinib (n = 357), or sunitinib (n = 357) in treatment-naïve patients.Citation4 This trial led to the approval of pembrolizumab/lenvatinib in treatment-naïve patients across all three IMDC risk groups. At the time of initial analysis, with median follow-up of 26.6 months, pembrolizumab/lenvatinib showed a better OS (median was not reached in both arms, HR 0.66, p = .005) and prolonged PFS (median 23.9 versus 9.2 months, HR 0.39, p < .001) versus sunitinib, respectively. For everolimus/lenvatinib arm, PFS was prolonged (median 14.7 versus 9.2 months, HR 0.65, p < .001) but not OS (median was not reached, HR 1.15, p = .30) when compared to sunitinib. Grade ≥3 AEs of any cause occurred most frequently in the everolimus/lenvatinib group, followed by pembrolizumab/lenvatinib and then sunitinib (83.1%, 82.4%, and 71.8%, respectively). AEs of any grade-related treatment discontinuation occurred more in the pembrolizumab/lenvantinib group (37.2%) than in either the everolimus/pembrolizumab group (27%) or the sunitinib group (14.4%). In the lenvatinib/pembrolizumab group, 68.8% of patients experienced a dose reduction of lenvatinib. Also, pembrolizumab/lenvatinib showed significant improvement in the median time to first deterioration for physical functioning, dyspnea, appetite loss, and EuroQol 5 Dimension Visual Analog Scale (EQ-5D VAS) as compared to sunitinib.Citation18 Combining all these findings, the combinaton of lenvatinib/pembrolizumab but not everolimus/lenvatinib was approved for the first-line treatment setting for mRCC. Given the PFS advantage over everolimus, everolimus/lenvatinib combination is still approved in the salvage therapy mRCC setting.Citation19
JAVELIN Renal 101
JAVELIN Renal 101 was another phase III randomized IO/TKI trial, which investigated the avelumab/axitinib versus sunitinib in treatment-naïve mRCC patients.Citation20 The primary endpoints were PFS and OS in patients with PD-L1-positive tumors (defined as ≥1%) irrespective of IMDC risk classification. This trial led to the approval of avelumab/axitinib in patients across all three IMDC risk groups. At initial analysis for patients with PD-L1-positive tumors (n = 560), avelumab/axitinib showed prolonged PFS (median 13.8 versus 7.2 months, HR 0.61, p < .001) and ORR (55.2% versus 25%) versus sunitinib, respectively. However, OS was not significantly improved in the combination arm (HR 0.82, p = .38). Grade ≥3 AEs of any cause were similar in avelumab/axitinib group and sunitinib group (71.2% versus 71.65%). Eleven percentage of patients received high-dose glucocorticoids (≥40 mg total daily dose of prednisone or equivalent) for the management of immune-mediated AEs in the avelumab/axitinib group. AEs-related treatment discontinuation occurred in 7.6% and 13.4% of patients who received the combination treatment or sunitinib monotherapy, respectively. At least one reduction in the dose of axitinib was observed in 42.2% of the patients. In the extended follow-up, an ORR benefit was noted for avelumab/axitinib over sunitinib across all the IMDC risk strata. However, PFS and OS benefit was only noted among the IMDC intermediate- and poor-risk disease. The follow-up is still ongoing as the final OS is not achieved for the avelumab/axitinib arm.Citation9 This combination is FDA approved for the first-line treatment of patients with advanced RCC but the National Comprehensive Cancer Network (NCCN) panel categorizes this combination under “other recommended regimens” given the absence of OS advantage.
Selecting between the first-line IO combination therapies?
summarizes the landmark phase III trials that led to the approval of the various IO combination therapies in the first-line setting for mRCC. Except for the CheckMate 214 trial, these trials have investigated an ICI plus an anti-angiogenic agent. In the absence of head-to-head comparisons of the currently FDA-approved IO-based combination regimens, choosing a treatment option for a patient with advanced mRCC is based on multiple factors, and any of these regimens associated with an OS advantage can be considered appropriate in the first-line treatment setting. The lack of any predictive biomarker further poses a challenge in this context. Certain factors that can be considered includeCitation1 IMDC risk category,Citation2 sarcomatoid differentiation,Citation3 toxicity profile,Citation4 performance status,Citation5 intolerance or lack of affordability of oral medications,Citation6 cardiovascular comorbidities,Citation7 history of autoimmune disease,Citation8 anticipated patient compliance, andCitation9 availability of a clinical trial.
Based on the long-term follow-up for ipilimumab/nivolumab, this strategy appears to provide a more favorable long-term quality of life compared to a TKI base strategy and is typically well tolerated, especially after the completion of the induction period with ipilimumab.Citation14,Citation21 Additionally, the combination of immune therapy appears to provide long-term remission even after treatment discontinuation in some patients. However, the long-term remission rate is relatively low and appears to mostly be limited to patients who achieve a deep radiographic response such as a complete response or a very good partial response early on after starting the treatment.Citation22 Two notable challenges with this treatment strategy include 1) the higher observed rate of autoimmune toxicities which are unpredictable in onset and 2) the relatively high primary progressive disease rate of 20%, which makes this regimen a sub-optimal choice in patients with rapidly progressive disease or those with high symptom burden. Finally, this combination is only approved for patients with IMDC intermediate- or poor-risk disease.Citation14 Based on three phase II studies, it is not recommended to use a response-based approach to ipilimumab/nivolumab due to the low response rates in patients treated with salvage ipilimumab/nivolumab after the initial first-line nivolumab monotherapy.Citation23–26
Notably, the ORR is very high with IO/TKI combinations. That means very few patients experience primary progressive disease as the best response to therapy, which makes IO/TKI therapy a more attractive approach for patients whose cancer is growing rapidly or who have high symptom burden from their disease. Compared to an IO/IO regimen, the IO/TKI regimes are also more suitable treatment for most patients with preexisting autoimmune diseases.Citation24 IO/TKI combinations are also appropriate for all IMDC risk groups. Long-term TKI toxicity can be problematic as evidenced by a larger proportion of patients with dose modifications (either drug discontinuation or dose reduction) with TKI-based combinations compared with IO/IO therapy in the Checkmate-214 clinical trial. Due to payment structure differences in some countries the direct patient costs can also be higher with TKI-based combinations.
For patients with favorable IMDC risk, the choice of the first-line therapy is still an area of controversy. The studies were not powered to evaluate survival outcomes in patients specifically with IMDC good-risk disease. The subgroup analysis for these three IO/TKI studies consistently shows a PFS benefit but no OS advantage over sunitinib alone. Patients with favorable-risk disease may have a more angiogenic environment, which could partially explain why tyrosine kinase inhibitor therapy may be the primary factor in achieving a survival benefit and no survival benefit was associated with the addition of IO agent.Citation27 However, specific tumor histologies or gene expression levels could indicate a more inflammatory environment, even among the favorable-risk group. For instance, the presence of sarcomatoid features have been associated with a reduced frequency of PBRM1 mutations, frequent CDKN2A/B alterations, and increased PD-L1 expression, all of which have been associated with low angiogenesis and enhanced cell-cycle activity.Citation27 These findings suggest that the tumor biology in the IMDC favorable-risk patients may be heterogeneous, indicating that there might exist a subgroup of favorable-risk tumors with biology that has an inflammatory microenvironment rather than angiogenic microenvironment. As a result, combining dual therapy may be more beneficial than sunitinib monotherapy.
Although there are no head-to-head comparisons, one recently published retrospective study has compared the FDA-approved IO combination regimens in the real-world setting.Citation28 Using a de-identified database, Zarrabi et al. showed similar 24-month median OS between treatment groups: axitinib/pembrolizumab or ipilimumab/nivolumab. However, for favorable IMDC risk group, axitinib/pembrolizumab treatment arm had a better OS compared to ipilimumab/nivolumab.Citation28
Recently reported trials
COSMIC-313 trial
At the 2022 European Society for Medical Oncology (ESMO) Annual Congress, Choueiri et al. presented the results of the global, double-blind, randomized phase III COSMIC-313 trial, investigating ipilimumab/nivolumab/cabozantinib compared to ipilimumab/nivolumab for mccRCC.Citation26 This is the first trial to compare the efficacy and safety of triplet therapy and also the first trial to have a modern combination therapy as a control instead of sunitinib. The study enrolled 855 treatment-naïve patients with IMDC intermediate- or poor-risk mccRCC. Patients were assigned to receive either triplet therapy (ipilimumab/nivolumab/cabozantinib) or doublet therapy (ipilimumab/nivolumab). The primary endpoint was PFS assessed by blinded independent radiology review in the first 550 randomized patients (PITT population). The secondary endpoint was OS in all randomized patients (ITT population); ORR and safety were additional secondary endpoints.
At a prespecified interim analysis, the study met its primary endpoint favoring triplet therapy over doublet (HR 0.73, p = .013). The median PFS was not yet achieved in the triplet therapy group compared to a PFS of 11.3 months in the doublet therapy group. ORR was better for patients receiving the triplet therapy (43% versus 36%). Patients receiving triplet therapy had higher grade ≥3 toxicities (73% versus 41%) and more treatment discontinuations (12% versus 5%) than the doublet therapy control group. In a recently presented subgroup analysis at the American Society of Clinical Oncology Genito-Urinary Symposium 2023, the triplet therapy was reported to be associated with improved PFS in intermediate-risk patients with an HR of 0.63 (95% CI, 0.47–0.85). However, no statistically significant benefit in PFS (HR 1.04, 95% CI 0.65–1.69) was observed in poor-risk patients.Citation29 At this stage, the data on OS are immature. Additionally, follow-up is expected until the key secondary endpoint of OS is mature. Triplet therapy is not yet approved by any regulatory body worldwide.
PIVOT 09
PIVOT 09 was a phase III open-label study (n = 623 underwent 1:1 randomization) which investigated the bempegaldesleukin/nivolumab versus a TKI of the investigator’s choice (sunitinib or cabozantinib) in a treatment-naïve patient with mccRCC.Citation30 Bempegaldesleukin is a pegylated interleukin (IL)-2 prodrug, which is designed to bind IL-2 receptor βγ complex preferentially located on the cell surface of natural killer cells and CD8+ T cells.Citation31 The primary endpoints of this study were OS and ORR. At a median follow-up of 15.5 months, the ORR was 23% versus 30.6% in patients receiving bempegaldesleukin/nivolumab (n = 310) versus a TKI, respectively (sunitinib n = 221; cabozantinib n = 85). Median OS was not significantly different in IMDC intermediate- or poor-risk patients between the bempegaldesleukin/nivolumab versus TKI group (29.0 months versus NR, HR 0.82, p = .19). The trial was stopped due to lack of benefit in the primary endpoint.
Looking to the future: ongoing phase III trials of IO combination therapy in advanced RCC
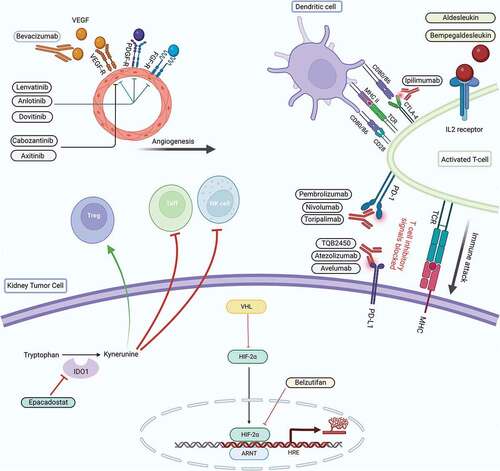
The pharmacological therapies for the management of newly diagnosed mRCC are rapidly evolving. There are multiple phase III trials underway, many interrogating novel combinations with new targets in order to improve survival outcomes or toxicity as compared with existing agents ().Citation33
Figure 1. Mechanism of action of the newer drugs currently being investigated in advanced RCC.Citation32.
PEDIGREE
PEDIGREE is an adaptive, randomized, multicenter phase III trial with unique study design that provides the treating physician an opportunity to tailor the treatment based on the interim response to the induction therapy with ipilimumab/nivolumab.Citation34 At a 3-month assessment, patients achieving CR continue with nivolumab monotherapy, and those with disease progression switch therapy to cabozantinib. Patients with non-CR/PD status are randomized to either a combination of nivolumab/cabozantinib arm or nivolumab monotherapy (the current standard of care of these patients). The primary objective of this trial is a 3-year OS for the patients who are randomized to nivolumab/cabozantinib or nivolumab. This study is expected to provide a critical insight on the optimal sequencing of currently available combination regimens and to decide the next best line of treatment following the first-line IO/IO combination therapy.
CA209-8Y8
CA209-8Y8 is an ongoing phase III clinical trial investigating ipilimumab/nivolumab versus nivolumab monotherapy in treatment-naïve patients with IMDC intermediate- or poor-risk mRCC (NCT03873402). This study will provide evidence for the the efficacy of adding ipilimumab to nivolumab in the front-line setting.
TQB2450-III-07 (NCT04523272)
TQB2450-III-07 (NCT04523272) is a phase III study currently underway to investigate the efficacy and safety of the combination of anlotinib (a TKI) plus TQB2450 (a PD-L 1 inhibitor) versus sunitinib in the treatment-naïve patients with advanced RCC. This novel combination has been recently reported to be safe, with promising efficacy in phase Ib trial conducted on pretreated advanced biliary tract cancers.Citation35
JS001–036-III-RCC (NCT04394975)
JS001–036-III-RCC (NCT04394975) is a randomized, open-label, controlled, phase III trial to compare the efficacy and safety of a combination of toripalimab (a PD-1 inhibitor)/axitinib versus sunitinib monotherapy as a first-line therapy in treatment-naïve patients with advanced ccRCC. This combination therapy has previously shown promising results in the salvage therapy in mRCC setting: an ORR of 31.6% and a PFS of 11.0 months in IMDC intermediate-risk group and 7.8 months in IMDC poor-risk group, with a tolerable safety profile.Citation36
MK-6482-012 (NCT04736706
Like COSMIC 313 trial, this open-label, randomized phase III study is investigating a triplet therapy regimens in treatment-naïve patients with advanced ccRCC. This study aims to compare two triplet therapies with doublet therapy (pembrolizumab/lenvatinib). The two triplet arms constituteCitation1 pembrolizumab plus lenvatinib plus belzutifan [a Hypoxia-Inducible Factor (HIF) antagonist] andCitation2 pembrolizumab plus lenvantinib plus quavonlimab (CTLA-4 antagonist). This trial will test the safety and efficacy of the HIF and CTLA-4 triplets when compared to doublet regimen.
KEYNOTE-679/ECHO-302 (NCT03260894)
KEYNOTE-679/ECHO-302 (NCT03260894) is a randomized, phase III study to evaluate the efficacy and safety of combination of pembrolizumab/epacadostat [Indoleamine 2,3-dioxygenase 1 inhibitor (IDO1) antagonist] versus standard of care (sunitinib or pazopanib) as first-line treatment for locally advanced or mRCC. As of now, the significance of IDO1 inhibition in combination with anti-PD-1 therapy in cancer is unclear. It is important to note that this combination therapy (ECHO-301/KEYNOTE-252) was not found to be efficacious in patients with unresectable or metastatic melanoma.Citation37
Conclusion
Currently, treatment of advanced or metastatic renal cell carcinoma involves targeting the most relevant molecular pathways implicated in cancer initiation and progression. Results of the ongoing phase III trials are expected to provide important breakthroughs. In addition, there still exists an unmet need for the predictive biomarkers to guide the best initial choice of therapy and to optimize the sequential use of available therapeutic agents. The future holds a strong possibility to witness more immune combinations that hopefully will allow us to achieve long-term remission, and even cure more patients with metastatic RCC.
Abbreviations
RCC: Renal cell carcinoma
mRCC: Metastatic RCC
ICIs: Immune checkpoint inhibitors
PD1: Programmed death receptor 1
PD-L1; PD-ligand 1
PR Partial response
CR: Complete response
PD: Disease progression
SD: Stable disease
RECIST: Response evaluation criteria in solid tumors
PFS: Progression-free survival
OS: Overall survival
ORR: Overall response rate
Disclosure statement
N.A: Consultancy to Astellas, Astra Zeneca, Aveo, Bayer, Bristol Myers Squibb, Calithera, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Gilead, Janssen, Merck, MEI Pharma, Nektar, Novartis, Pfizer, Pharmacyclics, and Seattle Genetics. Research funding to Neeraj Agarwal's institution: Arnivas, Astellas, Astra Zeneca, Bavarian Nordic , Bayer, Bristol Myers Squibb, Calithera, Celldex, Clovis, Crispr, Eisai, Eli Lilly, EMD Serono, Exelixis, Genentech, Gilead, Glaxo Smith Kline, Immunomedics, Janssen, Lava, Medivation, Merck, Nektar, Neoleukin, New Link Genetics, Novartis, Oric, Pfizer, Prometheus, Rexahn, Roche, Sanofi, Seattle Genetics, Takeda, and Tracon.
B.L.M is a paid consultant/advisor to Abbvie, Pfizer, AVEO oncology, Janssen, Astellas, Bristol-Myers Squibb, Clovis, Tempus, Merck, Exelixis, Bayer Oncology and Peloton Therapeutics; Huntsman Cancer Institute has received research funding from Exelixis (Inst), Bavarian-Nordic (Inst), Clovis (Inst), Genentech (Inst) and Bristol-Myers Squibb (Inst) on his behalf.
Additional information
Funding
References
- Navani V, Ernst M, Wells JC, Yuasa T, Takemura K, Donskov F, Basappa NS, Schmidt A, Pal SK, Meza L, et al. Imaging response to contemporary immuno-oncology combination therapies in patients with metastatic renal cell carcinoma. JAMA Netw Open. 2022;5(6):e2216379. doi:10.1001/jamanetworkopen.2022.16379.
- Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–8. doi:10.1056/NEJMoa1712126.
- Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, Oyervides Juárez VM, Hsieh JJ, Basso U, Shah AY, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–41. doi:10.1056/NEJMoa2026982.
- Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, Grünwald V, Hutson TE, Kopyltsov E, Méndez-Vidal MJ, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–300. doi:10.1056/NEJMoa2035716.
- Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–27. doi:10.1056/NEJMoa1816714.
- Montironi R, Cimadamore A. Tumors of the urinary system and male genital organs: 2022 World Health Organization classification and multidisciplinarity. Eur Urol. 2022;82(5):483–6. doi:10.1016/j.eururo.2022.07.032.
- Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, Mackenzie M, Wood L, Donskov F, Tan M-H, et al. External validation and comparison with other models of the international metastatic renal-cell carcinoma database consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141–8. doi:10.1016/S1470-2045(12)70559-4.
- Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor–targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–9. doi:10.1200/JCO.2008.21.4809.
- Haanen JBAG, Larkin J, Choueiri TK, Albiges L, Rini BI, Atkins MB, Schmidinger M, Penkov K, Thomaidou D, Wang J, et al. Efficacy of avelumab + axitinib (A + Ax) versus sunitinib (S) by IMDC risk group in advanced renal cell carcinoma (aRCC): extended follow-up results from JAVELIN Renal 101. J Clin Oncol. 2021;39(15_suppl):4574. doi:10.1200/JCO.2021.39.15_suppl.4574.
- Choueiri TK, Halabi S, Sanford BL, Hahn O, Michaelson MD, Walsh MK, Feldman DR, Olencki T, Picus J, Small EJ, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J Clin Oncol. 2016;35(6):591–7. doi:10.1200/JCO.2016.70.7398.
- Graham J, Heng DYC, Brugarolas J, Vaishampayan U. Personalized management of advanced kidney cancer. Am Soc Clin Oncol Educ Book. 2018;38:330–41. doi:10.1200/EDBK_201215.
- Powles T, Plimack ER, Soulières D, Waddell T, Stus V, Gafanov R, Nosov D, Pouliot F, Melichar B, Vynnychenko I, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563–73. doi:10.1016/S1470-2045(20)30436-8.
- Choueiri TK, Motzer RJ, Rini BI, Haanen J, Campbell MT, Venugopal B, Kollmannsberger C, Gravis-Mescam G, Uemura M, Lee JL, et al. Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol. 2020;31(8):1030–9. doi:10.1016/j.annonc.2020.04.010.
- Motzer RJ, Rini BI, McDermott DF, Arén Frontera O, Hammers HJ, Carducci MA, Salman P, Escudier B, Beuselinck B, Amin A, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20(10):1370–85. doi:10.1016/S1470-2045(19)30413-9.
- Motzer RJ, McDermott DF, Escudier B, Burotto M, Choueiri TK, Hammers HJ, Barthélémy P, Plimack ER, Porta C, George S, et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer. 2022;128(11):2085–97. doi:10.1002/cncr.34180.
- Rini BI, Plimack ER, Stus V, Waddell T, Gafanov R, Pouliot F, Nosov D, Melichar B, Soulieres D, Borchiellini D, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma (ccRCC): results from 42-month follow-up of KEYNOTE-426. J Clin Oncol. 2021;39(15_suppl):4500. doi:10.1200/JCO.2021.39.15_suppl.4500.
- Burotto M, Powles T, Escudier B, Apolo A, Bourlon MT, Shah A, Suárez C, Porta C, Barrios CH, Richardet M, et al. Nivolumab plus cabozantinib vs sunitinib for first-line treatment of advanced renal cell carcinoma (aRCC): 3-year follow-up from the phase 3 CheckMate 9ER trial. J Clin Oncol. 2023;41(6_suppl):Abstract 603. doi:10.1200/JCO.2023.41.6_suppl.603.
- Motzer RJ, Porta C, Alekseev B, Rha SY, Choueiri TK, Mendez-Vidal MJ, Hong S-H, Kapoor A, Goh JC, Eto M, et al. Health-related quality-of-life (HRQoL) analysis from the phase 3 CLEAR trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO) or everolimus (EVE) versus sunitinib (SUN) for patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol. 2021;39(15_suppl):4502. doi:10.1200/JCO.2021.39.15_suppl.4502.
- Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, Jassem J, Zolnierek J, Maroto JP, Mellado B, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16(15):1473–82. doi:10.1016/S1470-2045(15)00290-9.
- Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, Uemura M, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–15. doi:10.1056/NEJMoa1816047.
- Cella D, Grünwald V, Escudier B, Hammers HJ, George S, Nathan P, Grimm M-O, Rini BI, Doan J, Ivanescu C, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncol. 2019;20(2):297–310. doi:10.1016/S1470-2045(18)30778-2.
- Suárez C, Choueiri TK, Burotto M, Powles T, Bourlon MT, Shah AY, Tomita Y, Bedke J, Zhang J, Simsek B, et al. Association between depth of response (DepOR) and clinical outcomes: exploratory analysis in patients with previously untreated advanced renal cell carcinoma (aRCC) in CheckMate 9ER. J Clin Oncol. 2022;40(16_suppl):4501. doi:10.1200/JCO.2022.40.16_suppl.4501.
- Atkins MB, Jegede OA, Haas NB, McDermott DF, Bilen MA, Stein M, Sosman JA, Alter R, Plimack ER, Ornstein M, et al. Phase II study of nivolumab and salvage nivolumab/ipilimumab in treatment-naive patients with advanced clear cell renal cell carcinoma (HCRN GU16-260-Cohort A). J Clin Oncol. 2022;40(25):2913–23. doi:10.1200/JCO.21.02938.
- McKay RR, McGregor BA, Xie W, Braun DA, Wei X, Kyriakopoulos CE, Zakharia Y, Maughan BL, Rose TL, Stadler WM, et al. Optimized management of nivolumab and ipilimumab in advanced renal cell carcinoma: a response-based phase II study (OMNIVORE). J Clin Oncol. 2020;38(36):4240–8. doi:10.1200/JCO.20.02295.
- Grimm MO, Schmidinger M, Duran Martinez I, Schinzari G, Esteban E, Schmitz M, Schumacher U, Baretton G, Barthelemy P, Melichar B, et al. LBA57 - tailored immunotherapy approach with nivolumab in advanced renal cell carcinoma (TITAN-RCC). Annal Oncol. 2019;30:v892. doi:10.1093/annonc/mdz394.051.
- Msaouel P. Less is more? First impressions from COSMIC-313. Cancer Invest. 2022;41:1–6. doi:10.1080/07357907.2022.2136681.
- Motzer RJ, Banchereau R, Hamidi H, Powles T, McDermott D, Atkins MB, Escudier B, Liu L-F, Leng N, Abbas AR, et al. Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade. Cancer Cell. 2020;38(6):803–17.e4. doi:10.1016/j.ccell.2020.10.011.
- Zarrabi KK, Handorf E, Miron B, Zibelman MR, Anari F, Ghatalia P, Plimack ER, Geynisman DM. Comparative effectiveness of front-line ipilimumab and nivolumab or axitinib and pembrolizumab in metastatic clear cell renal cell carcinoma. Oncologist. 2022;28(2):157–64. doi:10.1093/oncolo/oyac195.
- Powles T, Motzer R, Albiges L, Suárez C, Schutz F, Heng D, Chevreau C, Kanesvaran R, Gurney H, Wang F, et al. Outcomes by IMDC risk in the COSMIC-313 phase 3 trial evaluating cabozantinib (C) plus nivolumab (N) and ipilimumab (I) in first-line advanced RCC (aRCC) of IMDC intermediate or poor risk. J Clin Oncol. 2023;41(6_suppl):Abstract 605. doi:10.1200/JCO.2023.41.6_suppl.605.
- Tannir NM, Agarwal N, Pal SK, Cho DC, Formiga M, Guo J, George DJ, Tagliaferri MA, Singel SM, O’Keeffe BA, et al. PIVOT-09: a phase III randomized open-label study of bempegaldesleukin (NKTR-214) plus nivolumab versus sunitinib or cabozantinib (investigator’s choice) in patients with previously untreated advanced renal cell carcinoma (RCC). J Clin Oncol. 2020;38(6_suppl):TPS763. doi:10.1200/JCO.2020.38.6_suppl.TPS763.
- Tannir NM, Cho DC, Diab A, Sznol M, Bilen MA, Balar AV, Grignani G, Puente E, Tang L, Chien D, et al. Bempegaldesleukin plus nivolumab in first-line renal cell carcinoma: results from the PIVOT-02 study. J ImmunoTher Cancer. 2022;10(4):e004419. doi:10.1136/jitc-2021-004419.
- BioRender: BioRender.com. 2022. https://app.biorender.com/biorender-templates.
- Dell’Atti L, Bianchi N, Aguiari G. New therapeutic interventions for kidney carcinoma: looking to the future. Cancers (Basel). 2022;14(15):3616. doi:10.3390/cancers14153616.
- Zhang T, Ballman KV, Choudhury AD, Chen RC, Watt C, Wen Y, Shergill A, Zemla TJ, Emamekhoo H, Vaishampayan UN, et al. PDIGREE: an adaptive phase III trial of PD-inhibitor nivolumab and ipilimumab (IPI-NIVO) with VEGF TKI cabozantinib (CABO) in metastatic untreated renal cell cancer (Alliance A031704). J Clin Oncol. 2021;39(6_suppl):TPS366. doi:10.1200/JCO.2021.39.6_suppl.TPS366.
- Zhou J, Sun Y, Zhang W, Yuan J, Peng Z, Wang W, Gong J, Yang L, Cao Y, Zhao H, et al. Phase Ib study of anlotinib combined with TQB2450 in pretreated advanced biliary tract cancer and biomarker analysis. Hepatology. 2022;77(1):65–76. doi:10.1002/hep.32548.
- Huang J, Shi G, Wang Y, Wang P, Zhang J, Kong W, Huang Y, Wang S, Xue W. Second-line treatment with axitinib plus toripalimab in metastatic renal cell carcinoma: a retrospective multicenter study. Future Oncol. 2022;18(12):1461–71. doi:10.2217/fon-2021-1267.
- Long GV, Dummer R, Hamid O, Gajewski TF, Caglevic C, Dalle S, Arance A, Carlino MS, Grob J-J, Kim TM, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 2019;20(8):1083–97. doi:10.1016/S1470-2045(19)30274-8.
