ABSTRACT
Cases of thromboembolic events in 2021 flared up the discussion about the safety of Astra Zeneca’s AZD1222 vaccine. We hereby report three cases of pulmonary embolism (PE), one case of extended portal vein thrombosis, and one case of combined portal vein thrombosis and PE within 2 weeks after vaccination with the Astra Zeneca AZD1222 vaccine in a 60-year-old, a 50-year old, a 33-year-old, a 30-year old, and a 40-year-old male in that year. All patients were healthy before. In three patients, we observed thrombocytopenia and to some extent unusually low antibody levels for the Spike Protein (S-protein), while the other two had normal thrombocyte counts. Only one patient had anti-platelet factor 4 (PF4)-antibodies detectable as it has been described in the “heparin-induced thrombocytopenia (HIT)-like” disease of “vaccine-induced prothrombotic immune thrombocytopenia” (VIPIT) and we therefore assume that heterogeneous mechanisms led to PE. Therefore, we advise to collect and report more cases, in order to determine the age-related risks of vaccination balanced against the benefits of immunity to SARS-COV-2 for the AZD1222 vaccine in order to gain knowledge for the next pandemic.
Introduction
In 2021 several European countries had temporarily suspended vaccination with Astra Zeneca’s COVID-19 vaccine (AZD1222) for persons under 60 years because of the occurrence of more than 60 cases of thromboembolic events after AZD1222 vaccination until the middle of the year.Citation1 These include pulmonary embolism (PE), deep vein thrombosis (DVT), and sinus vein thrombosis (SVT).Citation2
SARS-COV-2 has been demonstrated to cause thromboembolism in more than 30% of cases, and thromboembolism is a major determinant of prognosis.Citation3 Thus, thromboembolism potentially caused by a vaccine is a serious concern and should receive extreme attention. Yet, vaccine induced, unlike virus-induced, thromboembolism appears to be an extremely rare phenomenon.
This concern slowed down a complex vaccination campaign, of a safe and tested agent, a central pillar against the SARS-COV-2 caused pandemic and in the race against potential game-changing mutations. In particular, the emergence of the SARS-COV-2 delta variant, which eluded the immune response generated by the first generation of Moderna and Biontech/Pfizer vaccines 6.8 times better than the wildtype virus, called into question the long-term efficiency of a single-agent vaccination campaign.Citation4 The data on AZD1222 had been rather positive: an early Oxford University publication pointed out that a single-shot vaccination significantly reduced the virus transmission.Citation5,Citation6 An efficacy of up to 76% was described (vs. 82% after a second vaccination after 12 weeks), which could also protect against the SARS-COV-2 variant B.1.17. Up until May 2021, following the vaccination of approximately half of the British population with AZD1222, there have been reports of around 30 fatal cases of thromboembolism.Citation7 According to the Paul Ehrlich Institute, overall vaccination-associated death is estimated around 0.002% (cumulative for all vaccines). Regarding AZD1222 vaccinations in Germany, 13 mainly female patients with thromboembolic events (mostly SVTs) were described, with four lethal occurrences after the administration of the first 1,000,000 doses. At that same time, 94 thrombotic events with 17 fatalities had been reported in the overall German vaccination campaign.Citation8,Citation9
Therefore, if causality can be proven, according to the latter source, the incidence of thromboembolic events can be estimated as 1:100 000, their overall lethality as 0.25 in 100,000 or 2.5 in 1,000,000. These data are highly controversial as independently of the vaccination, the incidence of DVT and PE is described with 120 and 60 cases per 100,000 inhabitants in Germany, respectively.Citation10 With an increasing number linked to more operations in a multimorbid and aging society, these numbers are expected to rise.Citation10
In comparison, additional thromboembolic complications occur in around 40–80:100,000 women that were prescribed hormonal contraception depending on the formulation.Citation11 Death by SVT in young patients is extremely rare, but of utmost importance because the age-related benefit and potential harm of the vaccination must be balanced against age-related threat by SARS-COV-2.
As of 2023, VIPIT can be confirmed either by specific anti-PF4 antibody test, heparin induced platelet aggregation (HIPA) test or serotonin release assay (SRA).Citation8 While these methods were not broadly available at the time of the suspected diagnosis, not all possible cases could be confirmed with this method. Therefore, in the following we try to identify other homogeneous criteria that we observed in these cases.
Methods
In the following, we try to draw conclusions about VIPIT by analyzing five very heterogeneous cases. At the same time, we ruled out VIPIT in 14 other patients that presented with similar symptoms using mainly radiologic and lab methods. They showed different clinical symptoms and presented with mild fever and discomfort as a physiological reaction after vaccination. It is important to emphasize that we willingly excluded all SVTs in our VIPIT cohort, because we wanted to focus on the subgroup of males where VIPIT presents differently. Diagnosis was based on CT scan and lab values, mainly high-sensitive Troponin-T (hsTNT), d-dimer values, CBC, SARS-COV-2 antibodies, and the specific VIPIT-Test in patients that had been vaccinated with AZD 1222 within the last month. The interquartile range for S-protein antibody was based on the AZD 1222 approval study and included IgG alone. Our lab method measured both IgG and IgM as we wanted to show that VIPIT is associated with a low antibody response. The nature of the thrombosis, including its localization and its extent, was analyzed. An active SARS-COV-2- infection was ruled out via PCR, and a HIT- II test and other probable causes for thrombocytopenia were ruled out. This study has approval from the Ethics section of the university in Ulm. Consent for this study could not be confirmed by all patients in time, as a part of them was unconscious while the critical lab values, diagnosis, and treatment were performed but consent was obtained retrospectively.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request as they include personal data of a rare disease and as long as they are not in infringement with German applicable law.
Observations
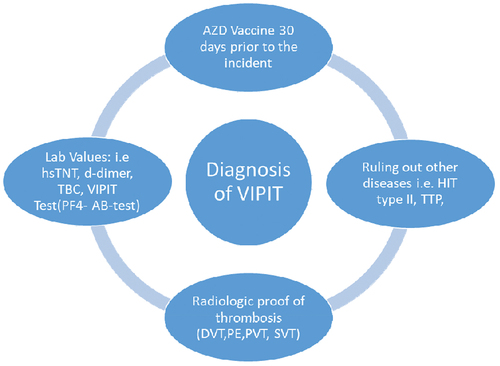
We describe a case series of thromboembolic complications following vaccination with AZD1222. shows our decisional matrix for the suspicion of VIPIT, when patients presented with clinical symptoms that could be associated with thrombosis (i.e. calf pain, shortness of breath, and abdominal pain). Patients who received the AstraZeneca’s AZD1222 vaccine within 30 days prior to admission and presented with suspicious lab values, particularly high d-dimer levels and low platelet (TBC) counts, were suspected to have a vaccine-associated thrombosis. As a result, they underwent radiologic diagnostic procedures to assess the presence and extent of the thrombotic condition. In addition, other diseases that were likely with these symptoms were ruled out.
Figure 1. Our decisional matrix for the diagnosis/suspicion of VIPIT, clinical data, i.e. suspicion of thrombosis after AZD 1222 vaccination was the main cause for undergoing this matrix.
A 60-year-old male patient who received the first dose of COVID-19 vaccine by Astra Zeneca 7 days prior to admission presented with worsening of his general state of health and dyspnea. He had been tested negative for COVID −19 via polymerase chain reaction via PCR.
His lab analysis showed a slightly elevated white blood cell count (WBC) of 11.4 G/L (<11 G/L), no lymphopenia, C-reactive protein (CRP) of 200 mg/L (normal <5 mg/L), elevated d-dimer of 1,8 mg/fibrinogen equivalent unit (FEU) (<0.5 mg/FEU), and normal high-sensitivity troponin T (hsTNT) level of 6 (<14 ng/L). Computed tomography revealed pulmonary embolism. Antibodies against PF4 were not detectable. The thrombocyte count was 208 G/L (>150 G/L), and the patient was discharged 4 days later on oral anticoagulation with rivaroxaban. Simplified pulmonary embolism severity index (sPESI)-Score was 0. DVT of the popliteal vein extending to the femoral vein could be identified as the cause of pulmonary embolism. Thrombus burden could be observed on the wall of the vein. Right ventricular function was measured with a tricuspid annular plane systolic excursion (TAPSE), which was 30 mm (≥20 mm).
The second case is a 50-year-old male who received his first dose of AZD1222 15 days prior to admission presenting with worsening of his general state of health and progressive shortness of breath. Pulmonary embolism had already been confirmed prior to admission and an outpatient radiology practice. He also tested negative for COVID-19 by PCR test. His lab tests showed no evidence of infection, especially normal CBC and CRP levels. At that point, his antibody levels (both IgG and IgM) against the SARS-COV-2- spike protein were 7.82 U/mL. D-dimer levels were 1.86 mg/FEU, hsTNT was 5 ng/L, and thrombocytes were 159 G/L. No other lab abnormalities could be detected. With sPESI-Score being 0, he was prescribed oral anticoagulation with rivaroxaban and discharged on the same day.
The third case is a 33-year-old male patient who received the first dose of COVID-19 vaccine by Astra Zeneca 13 days prior to admission, presenting with fever, cough, dyspnea, hypertension (up to 170 mmHg systolic), and calf and chest pain. He had been tested negative for COVID −19 as well.
His lab results showed hsTNT of 500 ng/L, high d-dimer levels of over 60 mg/FEU and thrombopenia of 60 G/L. The remaining CBC was normal. In addition, his antibody levels were 31.8 U/mL and CRP was 60. He showed elevated levels of lactate dehydrogenase (LDH) with 304 U/L (normal <250 U/L). We confirmed pulmonary embolism with infarction pneumonia by computed tomography and found DVT of the posterior tibial vein extending to the superficial femoral vein as the cause of calf pain. TAPSE was normal with 28 mm. The sPESI Score was 0. The patient was discharged 6 days later on oral anticoagulation with apixaban.
We also report about a 32-year-old male that received his first dose of AZD1222 14 days prior to admission and presented in an outpatient clinic with lower abdominal pain that was first mistaken for appendicitis. In the intraoperative situs, a thrombosis of the portal vein was suspected. Because of the prior history with vaccination, the patient was anticoagulated, received human antibodies and was sent to us, where an emergency laparotomy was performed after confirmation via another CT scan. PE was excluded. A mechanical thrombectomy was performed. In this process, the remaining thrombotic material was lysated and aspirated. Because of necrosis, 1.5 m of small intestine had to be removed. The remaining thrombotic material and subsequent portal hypertension led to the implantation of a transjugular intrahepatic portosystemic shunt (TIPS). The initial CBC was deranged, RBC was 3,0 T/L, TBC was 31 G/L, WBC was 24, with a CRP of 273 mg/L. HsTNT peaked at 15 ng/L. LDH was elevated with 307 U/L. Bilirubin was 31,5 µmol/L. He tested negative for COVID-19. HIT-Test was negative, anti PF4- antibodies were not measured. The patient was discharged after 33 days, 24 of which he spent on intensive care. Upon discharge in the follow-up care, consolidated thrombotic material was still present in the portal system and the splenic vein, even though the patient was thoroughly anticoagulated.
The last case is a 40-year-old male patient who received his first dose of AZD1222 7 days prior to admission and presented with abdominal pain that started after excessive alcohol consumption. He had no history of any known liver condition. He reported macrohematuria. The computed tomography performed in an outpatient clinic revealed massive PE with thrombus burden also compromising basal sections of the pulmonary arteries, as well as thrombosis of the portal vein extending into the mesenteric veins with ascites and splenomegaly. TAPSE was 25 mm. Upon presentation he had a sPESI Score of 1. At that point, kidney function was normal, hsTNT was 3 ng/L, CRP was 153.5 mg/L, bilirubin was 60 (normal range: [2–21] µmol/L), LDH was elevated as it was 423 (<250 U/L). CBC was abnormal: Initially, RBC was normal (4,5T/L), WBC was 18.5 G/L (with normal lymphocyte count), and there was severe thrombocytopenia, with thrombocyte count of 7 G/L. Lactate was slightly elevated with 2.4 mmol/L at presentation. Von Willebrand factor (vWF) activity was elevated. S-protein-antibodies in response to the vaccination were detectable but at a very low level of 0.72 U/mL (normal after vaccination >0.8 U/mL). He tested negative for COVID-19 by PCR. Anti-PF4-antibodies were detectable. The HIT Test was negative. His condition deteriorated rapidly and within 5 hours, he developed hypotension, requiring noradrenaline to support circulation. The patient required invasive ventilation, he developed acute renal failure, hsTNT was 216 ng/L, and lactate levels rose to 10 mmol/L and subsequently peaked at 100 mmol/L. He received immunoglobulin (Gamunex® as well as Pentaglobin®). In addition, he was administered Dexamethasone and transferred to the intensive care unit. The patient underwent an operative thrombectomy of portal vein branches. Following a complicated course, the patient was discharged from the hospital after 2 months in good condition.
According to all patients, the worsening of their condition started with the vaccination against COVID-19. A conclusive work-up was conducted to investigate the origin of thrombosis. We identified DVT in two patients as the origin of embolism, but we could not find any procoagulatory condition such as cancer, recent surgery, or immobilization prior to admission to explain thrombosis. There were no findings in abdominal sonography, except for the 40-year-old patient who had ascites and portal vein thrombosis. Ascites was not described in his initial CT scan.
While the 60-year-old and 50-year-old males both had d-dimer values of around 1.8 (<0.5) mg/FEU a negative hsTNT and no pathological CBC, the younger patients had more abnormalities ().
Table 1. Overview over important lab values of the five patients, based on IgG alone (7), measured values based on IgG and IgM.Citation12.
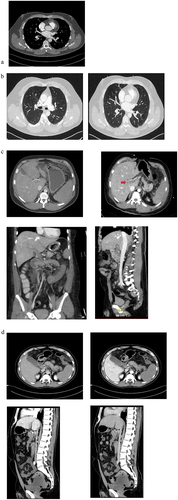
As depicts it, the first three scans reveal the presence of pulmonary emboli in patients 1 and 3. While there is a central component to the emboli, they are also distributed diffusely, extending into the peripheral pulmonary arteries. The emboli are not confined to a specific side and therefore diffuse. The same applies for mesenterial vein thrombosis, emboli are diffuse and non-localized, extending over the whole mesenteric vein and reaching into the spleen.
Figure 2. FCT scans of patients with suspected or confirmed VIPIT: The first scan (a) shows the pulmonary embolism in patient 1, the next two scans (b) shows the pulmonary embolism inpatient 3 and its diffuse pattern. The third scan series (c) show the mesenterial thrombosis in patient 4, and the last four scans (d) shows the mesenterial thrombosis and its extent in patient 5, with a positive anti PF4-test. They both show an extensive portal vein thrombosis (red arrow), best seen in (c) when comparing jugular and mesenterial vein contrast and best seen in d, in the sagittal view, when comparing arterial and venous phase. Both patients (c and d) show a extensive diffuse mesenteric thrombosis originating from the complete thrombosis of the splenic vein, indicating a possible immunologic origin with potential thrombotic chain reaction.
Discussion
Our cases illustrate the heterogeneity of the findings and hence raise further questions about the causality and uniformity of thromboembolism caused by AZD1222. A publication by Greinacher and coworkers at the University of Greifswald, Germany, associated thrombosis in COVID-19 vaccines with a heparin-induced thrombocytopenia (HIT)-like disease and puzzles about the role of HIT in potential vaccine-induced thrombosis.Citation13,Citation14 In HIT, antibodies target a complex of PF4 and heparin and stimulate a clotting reaction with thrombocytes. This leads to potentially lethal blood clots. The publication linked a lethal outcome in SVT to very high antibody levels against PF4 and labeled this new entity “vaccine-induced prothrombotic immune thrombocytopenia” or “VIPIT” as it is not triggered by heparin.Citation7
According to the German Society of Hemostasiology, any potential thromboembolic event linked to a vaccine that takes place at least 5 days after vaccination should be investigated and HIT-tested, as long as it differs from a normal vaccination reaction.Citation8 As advised, we ruled out a type-II-HIT-reaction as well as an active COVID infection in all patients at baseline or in the follow- up. Nevertheless, a “HIT-like-reaction” as observed in VIPIT could be shown in the last case, which tested positive for these specific antibodies. Since he supposedly reacted the most against the vaccine, we confirm the hypothesis of Greinacher et al. that the level of specific anti-PF4-antibodies rises with the severity of the disease, as observed in lethal SVT cases. We would like to clarify that this test is designed to identify individuals with severe illness and is not widely accessible. Consequently, during the time of our cases, it was not suitable or readily available for screening purposes. The same limitations apply to additional tests such as HIPA or SRA. Although these tests have the potential to detect VIPIT (Vaccine-Induced Prothrombotic Immune Thrombocytopenia) patients who test negative for anti-PF4 antibodies, they were also not widely accessible during the incidents mentioned. Therefore, in most cases, we cannot definitively establish a causal relationship between vaccination and thrombosis, but can only hypothesize it.
In addition, we cannot determine the mechanism leading to high PF4 levels. Since it is available in platelets and a high turnout in platelets might cause specific platelets protein levels to rise, it might just be a surrogate value.
We cannot rule out another cause or pathomechanism involved in the other four patients. The pathogenesis involved might still be similar to the etiology of the SVT due to “VIPIT” but we believe there is a different mechanism to be involved as it only targeted men. It has yet to be determined but as thrombosis seems to be coming from the spleen, an immunologic cause is very probable.
We speculate that the heterogeneity of the patients’ lab results may be linked to the immune response to the SARS-COV-2-vaccine. In the following, we would like to discuss the younger patient’s findings (cases 3–5). On day 14, IgG levels against S-Protein usually are around 137 units/mL (IQR 43.7–186 units/mL) in studies with healthy probands.Citation5,Citation15 We noticed respective levels of 7 and 33 units/mL in the patients for (IgG and IgM) and thus have lower IgG levels in these patients in response to the COVID-19-spike protein (S-protein) antigen after a single immunization shot. This could, for example, be explained by weak humoral immune response or a cross reactivity of said antibodies leading to high “consumption.” A cross-reaction resembling molecular mimicry could cause an antibody binding to antigens involved in clotting. A possible target could be the ADAM system. COVID-19 is known to be able to worsen preexisting ITPs. ITP is a rare, mostly idiopathic disease associated with high activity/levels of von Willebrand factor because of the altered activity of matrix metalloproteinases, especially ADAMTS13.Citation16 As the spike protein, whose production is partly induced by the vaccine, is known to activate ADAM 17 (a disintegrin and metalloproteinase 17), belonging to the same protein family as ADAMTS13, this might be a target/system to investigate.
Indeed, in our third patient, we could observe an overregulated coagulation with higher vW- factor-collagen and factor VIII activity that consumes thrombocytes. Anticoagulation quickly reversed these mechanisms, as thrombocytes regenerated 3 days after the initiation of low-molecular-weight heparin (Enoxaparin).
In our specific cases, no adverse reaction was observed after the application of heparin for anticoagulation.
As of now, no direct link or pathway could be established between COVID-19 induced metalloproteinases such as ADAM 17 and coagulation even though this could be the underlying mechanism. The current hypothesis links vessel hyperinflammation with thrombosis.Citation17 More specifically, because of their high expression of ACE-2, a crucial structure for the Spike (S-) protein seems to be endothelial cellsCitation18,Citation19 and pericytes.Citation20 Their apoptosis/loss, which has also been described in inflammatory disease and sepsis, leads to wall thickening and capillary leak/dysfunction.Citation20,Citation21 Virus induced S- protein production therefore not only leads to the described syncytia formation in uninfected cells but also damages vessel regulation.Citation22
Further analysis will reveal the implications of the plasmatic coagulation system in the pathogenesis of this disease. Therefore, even though we do suppose there is more than coincidence between the vaccine and PE in the 33-year-old patient, we attribute the complication to him maybe being generally more prone to thrombosis. Even though the vaccine has been tested and declared safe to use for patients with a known history of thrombosis, it is likely that they remain at a higher risk in certain yet unidentified cases.Citation8
In 2017, a similar question was raised to observe the incidence of thromboembolic events in patients after influenza vaccination. The authors concluded that no correlation could be found.Citation23 As for now, we also have to consider that the known side effects (like fatigue, headache, or fever) cause immobility in patients, which consequently may serve as a cause for embolism. Such studies indicate the willingness to cautiously and transparently study possible side effects of vaccines in general. This way vaccination in general was linked in very few cases (around 1.6 in 1 million) to Guillain-Barre syndrome.Citation24 As for now, this has not been shown for AZD 1222 or any other COVID-vaccine.Citation25 Therefore, through thorough observation by treating physicians, we may unravel possible correlation or even causation of thromboembolism and other side effects of COVID-19 vaccines.
Considering the low projected incidence of possible vaccine-linked severe complications, the short observation period and the lack of data available to differentiate, causation or coincidence remains elusive. That PF4-antibodies were not detectable in three of the four patients may be explained by either an alternative non PF4-antibody-mediated mechanism of thromboembolism or VIPIT with PF4-antibodies below the detection threshold. Finally, in the PF4 antibody negative cases it cannot be excluded that thromboembolism occurred coincidentally, but temporal association with AZD 1222 vaccination makes a causal relationship appear likely. In these cases, the utilization of HIPA or SRA can provide valuable insights for differentiation. We strongly recommend employing these tests to obtain further information that can help address the question of whether VIPIT frequently presents as anti-PF4-negative and goes undetected or if most instances of embolism during the vaccination period were merely coincidental.
Author contributions
All aforementioned authors revised the article critically in their specific field of expertise (internal medicine, clinical chemistry, intensive care, and emergency medicine) as the diagnosis (or ruling out) and treatment of VIPIT is a very complex and an interdisciplinary process. All authors participated in diagnosing or ruling out VIPIT in complicated cases and in the treatment of the patients. In addition, they all approved the current version to be published and ensure the accuracy of the data in their area of expertise. In particular, the following states supplementary contributions:
Sascha d’Almeida: Author, Data Analysis, and Interpretation Research and comparison to existing VIPIT papers, lab value algorithm
Sinisa Markovic: Second Supervisor and Corrector, Idea to screen Patients for VIPIT after first PE
Patrick Hermann: Intensive Care Treatment of Patient and Identification of VIPIT in Patient 4
Hendrik Bracht: Head of Emergency medicine department, exclusion of most non-VIPIT Patients
Johannes Peifer: Identification of Patient 1 and 2 as potential VIPIT Patients, ruling out of VIPIT in emergency patients
Thomas J. Ettrich: Identification of the mesenteric thrombosis in Patient 5 and establishment interdisciplinary algorithm for the treatment of VIPIT with involvement of the portal vein
Armin Imhof: Head of Angiology Department Identification of Thrombosis in VIPIT-PEs establishment interdisciplinary algorithm for the treatment of VIPIT with involvement of the portal vein
Shaoxia Zhou: Lab Analysis that helped identify VIPIT, importance of differentiating PF4 antibody and HIT II Test
Manfred Weiss: Intensive Care Treatment and Identification of VIPIT in Patient 5, improvement of the treatment of VIPIT patients with involvement of the portal vein
Andreas Viardot: Analysis to link VIPIT with hematologic diseases, comparison to existing hematological thrombotic phenomena
Wolfgang Rottbauer: Head of Cardiology, final approval of the version to be published, coordination of the interdisciplinary effort to describe, categorize, and treat VIPIT with PE and portal vein thrombosis
Tillman Dahme: First Supervisor and Corrector, coordination of the interdisciplinary effort to describe, categorize, and treat VIPIT with PE and portal vein thrombosis
Acknowledgments
Thanks to Meinrad Beer Head of the department of radiology for supporting this project.
Special thanks to Philipp Mohr and Benedikt Haggenmüller from the department of radiology for the demonstration of pathology and emphasizing the thrombosis of the splenic vein.
Disclosure statement
We would like to emphasize that no cited author or contributor has a conflict of interest. There are no known competing financial interests or relationships, and the interest is purely scientific. In addition, our research does not infringe the Helsinki declaration of medical research of the WMA of 1975.
Additional information
Funding
References
- Wise J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ. 2021 Mar 11;372:n699. doi:10.1136/bmj.n699. PMID: 33707182.
- Fischer L. Spektrum der Wissenschaft. AstraZeneca-Impfstoff: Immunreaktion löst Thrombosen im Hirn aus. Heidelberg (Germany); 2021 Mar 19 [accessed 2021 May 1]. https://www.spektrum.de/news/astrazeneca-impfstoff-immunreaktion-loest-thrombosen-im-hirn-aus/1849132.
- Rico-Mesa JS, Rosas D, Ahmadian-Tehrani A, White A, Anderson AS, Chilton R. The role of anticoagulation in COVID-19-Induced hypercoagulability. Curr Cardiol Rep. 2020;22(7):53. doi:10.1007/s11886-020-01328-8. PMID: 32556892; PMCID: PMC7298694.
- Edara VV, Lai L, Sahoo MK, Floyd K, Sibai M, Solis D, Flowers MW, Hussaini L, Ciric CR, Bechnack S, et al. Infection and vaccine-induced neutralizing antibody responses to the SARS-CoV-2 B.1.617.1 variant. J Virol. 2021;95(17):e0131321. doi:10.1128/JVI.01313-21. PMID: 34013272; PMCID: PMC8132229.
- Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–7. doi:10.1016/S0140-6736(21)00432-3. Epub 2021 Feb 19. Erratum in: Lancet, 397(10277), 880. PMID: 33617777; PMCID: PMC7894131.
- Ewer KJ, Barrett JR, Belij-Rammerstorfer S, Sharpe H, Makinson R, Morter R, Flaxman A, Wright D, Bellamy D, Bittaye M, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med. 2021;27(2):270–8. doi:10.1038/s41591-020-01194-5. Epub 2020 Dec 17. PMID: 33335323.
- Mueller B. The U.K. reports more blood-clotting cases in people who received the AstraZeneca shot. New York Times. 2021 Apr 2 [accessed 2021 May 1]. https://www.nytimes.com/2021/04/02/world/uk-astrazeneca-blood-clots.html.
- Oldenburg J, Klamroth R, Langer F, Pötzsch B, Greinacher A. Gesellschaft für Thrombose- und Hämostaseforschung, Aktualisierte Stellungnahme der GTH nach dem Beschluss der EMA, die Impfungen mit dem AstraZeneca COVID-19 Vakzin fortzusetzen; Recommendations of the German Society of Thrombosis and Hemostasiology. Cologne (North Rhine Westphalia/Germany); 2021 Apr 1 [accessed 2021 May 1]. https://gth-online.org/wp-content/uploads/2021/04/GTH-Stellungnahme-AstraZeneca_4-1-2021.pdf.
- Paul-Ehrlich-Institut, Bundesinstitut für Impfstoffe und biomedizinische Arzneimittel. Sicherheit von COVID-19 Impfstoffen. Langen (Hesse/Germany); 2021 Mar [accessed 2021 May 1]. https://www.pei.de/DE/newsroom/dossier/coronavirus/coronavirus-inhalt.html?cms_pos=5.
- Augart J. Inzidenz und Altersverteilung der Lungenembolie in Deutschland auf Basis der DRG-Statistik [ Doctoral thesis]. Germany: Department of Angiology, University of Duisburg-Essen; 2012.
- Joint Statement of the German. Austrian and Swiss societies of Gynecology: S3-Leitlinie Hormonelle Empfängnisverhütung; 2020 Jan 22 [accessed 2021 May 1]. https://www.awmf.org/uploads/tx_szleitlinien/015-015l_S3__Hormonelle_Empfaengnisverhuetung_2020-02.pdf.
- Beavis KG, Matushek S, Abeleda PF, Bethel C, Hunt C, Gillen S, Moran A, Tesic V. Evaluation of the EUROIMMUN anti-SARS-COV-2 ELISA Assay for detection of IgA and IgG antibodies. J Clin Virol. 2020;129:104468. Published online 2020 May 23. doi:10.1016/j.jcv.2020.104468.
- Brodard J, Kremer Hovinga JA, Fontana P, Studt JD, Gruel Y, Greinacher A. COVID-19 patients often show high-titer non-platelet-activating anti-PF4/heparin IgG antibodies. J Thromb Haemost. 2021;19(5):1294–8. Published online ahead of print, Feb 7, 2021. doi:10.1111/jth.15262. PMID: 33550713.
- Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021 June 3;384(22):2092–101. doi:10.1056/NEJMoa2104840. Epub 2021 Apr 9. PMID: 33835769; PMCID: PMC8095372.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi:10.1016/S0140-6736(20)32661-1. Epub 2020 Dec 8. Erratum in: The Lancet, 397(10269), 98. PMID: 33306989; PMCID: PMC7723445.
- Sukumar S, Lämmle B, Cataland SR. Thrombotic thrombocytopenic purpura: pathophysiology, diagnosis, and management. J Clin Med. 2021;10(3):536. doi:10.3390/jcm10030536. PMID: 33540569; PMCID: PMC7867179.
- Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–8. doi:10.1056/NEJMoa2015432. Epub 2020 May 21. PMID: 32437596; PMCID: PMC7412750.
- Siddiqi HK, Libby P, Ridker PM. COVID-19 - a vascular disease. Trends Cardiovasc Med. 2021;31(1):1–5. doi:10.1016/j.tcm.2020.10.005.
- Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–7. doi:10.1002/path.1570. PMID: 15141377; PMCID: PMC7167720.
- Cardot-Leccia N, Hubiche T, Dellamonica J, Burel-Vandenbos F, Passeron T. Pericyte alteration sheds light on micro-vasculopathy in COVID-19 infection. Intensive Care Med. 2020;46(9):1777–8. doi:10.1007/s00134-020-06147-7.
- Abdel Rahman F, d’Almeida S, Zhang T, Asadi M, Bozoglu T, Bongiovanni D, von Scheidt M, Dietzel S, Schwedhelm E, Hinkel R, et al. Sphingosine-1-phosphate attenuates lipopolysaccharide-induced pericyte loss via activation of Rho-A and MRTF-A. Thromb Haemostasis. 2021;121(3):341–50. doi:10.1055/s-0040-1716844. Epub 2020 Oct 4. PMID: 33011963.
- Theuerkauf SA, Michels A, Riechert V, Maier TJ, Flory E, Cichutek K, Buchholz CJ. Quantitative assays reveal cell fusion at minimal levels of SARS-CoV-2 spike protein and fusion from without. iScience. 2021;24(3):102170. doi:10.1016/j.isci.2021.102170. Epub 2021 Feb 9. PMID: 33585805; PMCID: PMC7871100.
- Vickers ER, McClure DL, Naleway AL, Jacobsen SJ, Klein NP, Glanz JM, Weintraub ES, Belongia EA. Risk of venous thromboembolism following influenza vaccination in adults aged 50 years and older in the vaccine Safety Datalink. Vaccine. 2017;35(43):5872–7. doi:10.1016/j.vaccine.2017.08.086. Epub 2017 Sep 6. PMID: 28888342; PMCID: PMC6508529.
- Destefano F, Offit PA, Fisher A. Vaccine safety. In: Plotkin A Orenstein WA, editors. Plotkin’s vaccines; 2018. pp. 1584–600.e10. doi:10.1016/B978-0-323-35761-6.00082-1. Epub 2017 Jul 17. PMCID: PMC7173515.
- Keddie S, Pakpoor J, Mousele C, Pipis M, Machado PM, Foster M, Record CJ, Keh RYS, Fehmi J, Paterson RW, et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain. 2020;144(2):682–93. doi:10.1093/brain/awaa433.
