ABSTRACT
The occurrence of markedly accelerated tumor growth during immunotherapy is considered a new mode of progression called hyperprogressive disease (HPD) and its impact on pancreatic cancer (PC) patients receiving immunotherapy is unknown. In this study, we described and explored the incidence, prognosis and predictors of HPD in patients with advanced PC treated with programmed cell death-1 (PD-1) inhibitors. We retrospectively analyzed clinicopathological data from 104 patients with advanced pancreatic cancer who were treated with PD-1 inhibitors at our institution during 2015–2020 and identified 10 (9.6%) patients with HPD. Overall survival (OS) was significantly poorer in patients with HPD compared to patients with progressive disease (PD) (median OS: 5.6 vs. 3.6 months, p < .01). Clinicopathological factors associated with the occurrence of HPD included smoking, metastatic sites >2, liver metastasis, antibiotic therapy within 21 days before immunotherapy (Abx B21), hemoglobin (Hb) level <110 g/L, and PD-1 inhibitor treatment line >2. Subgroup analysis showed that high levels of CA19-9 at baseline were associated with the development of subsequent HPD (p = .024) and a worse prognosis (mOS:16.2 months vs. 6.1 months, p < .01). Our study demonstrated that HPD may occur in PC patients treated with PD-1 inhibitors and is associated with several clinicopathological characteristics and poor prognosis. The baseline tumor marker CA19-9 may be one of the early predictors of HPD development in PC patients receiving immunotherapy.
Introduction
Since its insidious location and early symptoms are not obvious, pancreatic cancer (PC) is generally identified at a late stage, resulting in a dismal 5-year survival rate of 3–15%.Citation1,Citation2 Therapeutic choices for advanced PC are scarce, and currently, FOLFIRINOX or gemcitabine-based chemotherapy is the dominant treatment option.Citation3 However, better treatment options remain to be explored due to the insensitivity of PC to chemotherapy and the lack of significant progress in targeted therapy in the large pancreatic cancer population.Citation4
In recent years, immunotherapy has become a hot topic in antitumor therapy, and immune checkpoint inhibitors (ICIs) have been used successfully to treat a range of malignancies, including non-small cell lung cancer,Citation5 melanoma,Citation6 head and neck squamous cell carcinoma,Citation7 and gastric cancer.Citation8 In PC, some promising studies have also been reported and a study in 2017 demonstrated a 100% disease control rate in patients with advanced PC treated with programmed cell death-1 (PD-1) inhibitors in combination with chemotherapy.Citation9 Another study showed that the use of immunotherapy in conjunction with chemotherapy resulted in longer OS than chemotherapy-only patients with advanced PC.Citation10 However, most of the promising clinical trials had failedCitation11 probably due to the specificity of the tumor immune microenvironment in PC compared to most tumorsCitation12 and more seriously, instead of tumors being controlled after immunotherapy, some patients have experienced accelerated progression, a new pattern of progression known as hyperprogressive disease (HPD), the incidence of which has ranged from 4% to 29% in the various studies to date.Citation13 HPD lacks a uniform definition and its predictive markers remain to be discovered. The occurrence of HPD during immunotherapy has previously been reported to significantly impair overall survival (OS) of patients,Citation14,Citation15 but the incidence and clinical significance of HPD in patients with advanced PC receiving immunotherapy has not been fully elucidated.
This study aimed to investigate the incidence of HPD in PC patients treated with PD-1 inhibitors and to describe its impact on prognosis; In addition, we explored clinicopathological characteristics linked to the development of HPD to identify potential predictors.
Materials and methods
Patients and clinicopathological data
Clinicopathological data were retrospectively collected from patients with advanced PC treated with PD-1 inhibitors (nivolumab/pembrolizumab/sintilimab) at the Chinese PLA General Hospital (Beijing, China) from September 2015 to September 2020. Inclusion criteria for this study: ① age 18 years and above; ② patients with clinically and pathologically confirmed locally advanced/advanced PC; ③ treated with PD-1 inhibitors; ④ available computed tomography (CT)/magnetic resonance imaging (MRI) images before and after immunotherapy; ⑤ an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 1; ⑥ presence of measurable lesions for evaluation by RECIST 1.1 criteria.
Demographic, clinical and pathological data were collected including age, gender, type of PD-1 inhibitor, history of smoking, history of surgery, degree of tumor tissue differentiation, number of metastatic sites, liver metastases, antibiotic therapy within 21 days before immunotherapy (Abx B21), baseline CA19-9 levels, hemoglobin (Hb) levels, absolute neutrophil counts, absolute lymphocyte counts and serum LDH levels, PD-L1 status, whether PD-1 inhibitors were used in combination with other drugs, and line of immunotherapy. This research was authorized by the Ethics Committee of Chinese PLA General Hospital and performed according to the principles of the Declaration of Helsinki.
Definition of tumor growth rate (TGR) and HPD
At least three CT/MRI scans were collected at baseline, as well as 6–8 weeks before and after. The radiological data was reviewed independently by two radiologists. TGR was derived by calculating the monthly percentage increase in tumor volume based on the longest measurable diameter of the target lesion in RECIST 1.1 and the Ferté team’s definition.Citation16
TGR was assessed before and after PD-1 inhibitors treatment. ΔTGR is the TGR at the time of treatment minus the TGR before treatment. The higher the ΔTGR, the faster the tumor grows after receiving immunotherapy. The HPD was defined as ΔTGR ≥ 100%.
Statistical analysis
The χ2 or Fisher’s exact test was used to test categorical variables. Cut-off values for baseline tumor markers were determined based on receiver operating characteristic (ROC) curve analysis. The Kaplan-Meier method was used to depict overall survival curves and the long-rank test was used to compare the survival curves. All tests were two-tailed and considered statistically significant if the p-value was less than 0.05. Statistical analyses were performed using SPSS version 26.
Results
Patient characteristics
A total of 104 patients with PC who received immunotherapy at Chinese PLA General Hospital from September 2015 to September 2020 were included in the characterization according to the inclusion criteria of this study (). Most patients were under 60 years old (66.3%), with a greater proportion of males (70.2%), and 91.3% received a combination of PD-1 inhibitors with other agents and the majority of patients received immunotherapy line ≤ 2 (93.3%), as shown in for specific baseline clinicopathological characteristics. According to the RECIST 1.1 evaluation, 22 (21.2%) patients were identified with progressive disease (PD) at the first efficacy assessment after immunotherapy, of whom 10 (9.6%) developed HPD after excluding pseudoprogression (see , Figure S1 and Table S1).
Figure 1. Flowchart of patient selection.
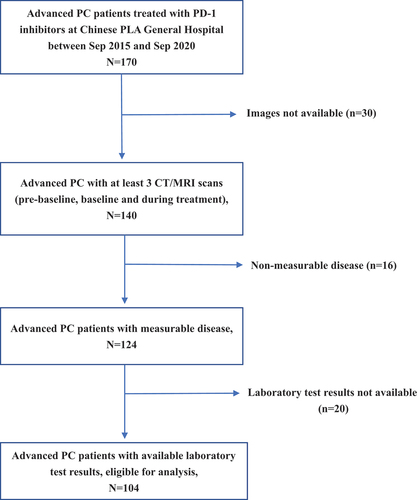
Figure 2. Case study of a patient with PC with HPD during treatment with a PD-1 inhibitor.
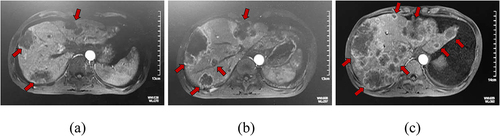
Table 1. Characteristics of patients with advanced PC.
Clinicopathological factors associated with HPD
After treatment with PD-1 inhibitors, HPD was more common in PC patients with a history of smoking (p = .017), metastatic sites >2 (p = .01), liver metastases (p < .01), Abx B21 (p < .01), Hb levels <110 g/L and immunotherapy lines >2 (see ).
Table 2. Differences of patients’ characteristics between the non-HPD group and the HPD group.
Survival data
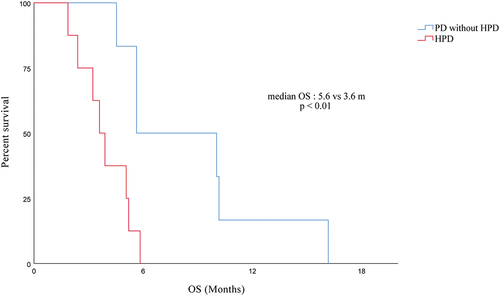
In this study, individuals with advanced PC who received immunotherapy had a median overall survival (mOS) of 11.67 months. Patients with HPD had a significantly worse OS than patients with PD without HPD (mOS, 3.6 m [95% CI, 2.7–4.5 m] vs. 5.6 m [95% CI, 1.2–10.0 m]; p < .01) according to survival analysis (see ).
Association of HPD with tumor marker CA19-9
It was found that high levels of CA19-9 at baseline may be associated with the development of HPD in PC patients treated with PD-1 inhibitors. According to the ROC curve (p = .024), the area under the curve (AUC) was 0.729, and when the baseline CA19-9 level cutoff value was taken as 1279 U/ml, the Youden index was maximum at this time, with a sensitivity of 66.7% and specificity of 87.1% (see and ). We can speculate that patients are less likely to develop HPD after immunotherapy when baseline CA19-9 ≤ 1279 U/ml, while the occurrence of subsequent HPD should be alerted when baseline CA19-9 > 1279 U/ml.
Figure 4. ROC curves of baseline CA19-9 predicting the occurrence of HPD during treatment with PD-1 inhibitors in patients with advanced PC.
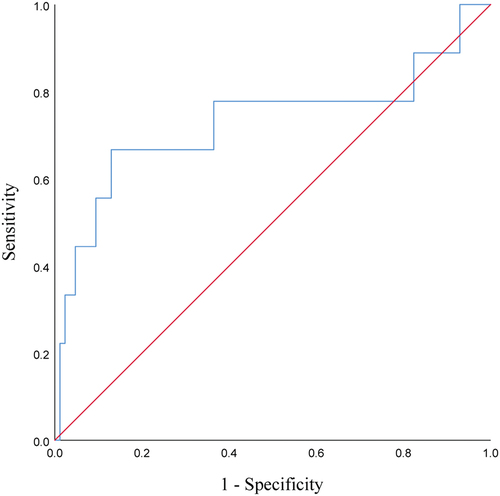
Table 3. Data related to ROC analysis of baseline CA19-9 predicted HPD.
In addition, the magnitude of change in CA19-9 within the first month after immunotherapy was predictive of HPD, with a cutoff value of 56.31% ( and ).
Figure 5. ROC curves of the first month’s increase in CA19-9 predicting the occurrence of HPD during treatment with PD-1 inhibitors in patients with advanced PC.
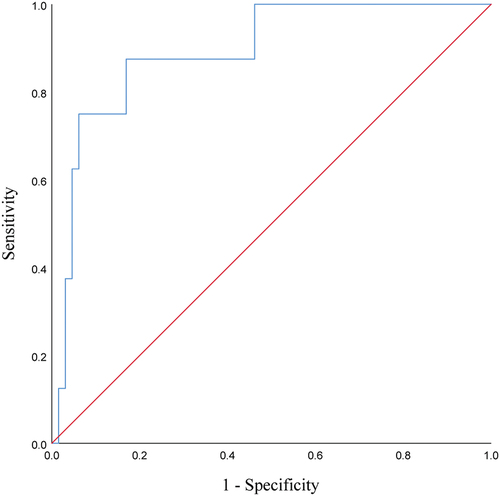
Table 4. Data associated with the ROC analysis of the first month’s increase in CA19-9 predicted HPD.
Subgroup analysis of baseline CA19-9 levels and prognosis
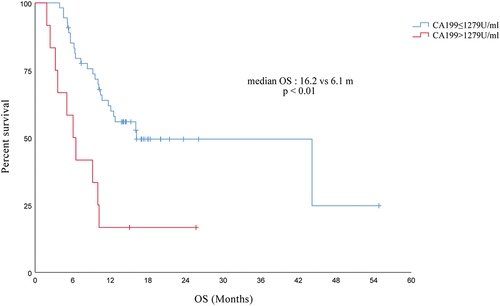
Based on baseline CA19-9 levels, patients with advanced PC receiving PD-1 inhibitors were divided into two groups, a high-level group (CA19-9 > 1279 U/ml) and a low-level group (CA19-9 ≤ 1279 U/ml), and the OS was shorter in the high-level group than that in the low-level group (16.2 vs 6.1 months, p < .01), as derived from survival analysis (see ).
Discussion
In this study, the development of HPD was observed in 9.6% (10/104) of patients with advanced PC treated with PD-1 inhibitors. patients with PC who developed HPD after PD-1 blockade had a poor prognosis with an mOS of 3.6 months. We found that HPD was associated with smoking history, metastatic site >2, liver metastases, Abx B21, Hb level and line of immunotherapy. Some studies have explored the occurrence of HPD after immunotherapy in a variety of malignancies, such as lung cancer,Citation17 gastric cancer,Citation18 sarcoma,Citation19 and head and neck squamous carcinoma.Citation20
To our knowledge, there were no previous studies specifically exploring the development of HPD in PC patients induced by PD-1 inhibitors, our present study is the first to particularly explore the incidence and clinical implications of HPD in patients with advanced PC treated with PD-1 inhibitors. Furthermore, we are the first to show a link between pre-immunotherapy antibiotic use, Hb levels, baseline CA19-9 levels, and the development of HPD in advanced PC patients treated with PD-1 inhibitors.
The exact definition of HPD is still up in the air. Based on RECIST 1.1, Matos’ team defined HPD as an increase of ≥10 mm in measurable lesions plus a total increase of ≥ 40% in all target lesions within 8 weeks after immunotherapy compared to baseline, or an increase of 20% and new lesions in at least 2 organs.Citation21 HPD was defined by Champiat et al. as a patient with PD as assessed by RECIST 1.1 criteria and a TGR greater than double the pre-treatment level while receiving immunotherapy.Citation22 In 2018, the TGR of more than 50% per month was used to define HPD in a study by Ferrara’s team,Citation15 but subsequent studies by Kas and Ferrara et al. further explored the criteria for identifying HPD and found that the optimal threshold for TGR to diagnose HPD is greater than 100%.Citation23 Similar to the definition of HPD mentioned in the studies of Kas and Ferrara and other scholars, our study performed TGR calculations for patients assessed as PD according to RECIST 1.1 and defined ΔTGR ≥100% as HPD since we thought TGR calculated based on tumor volume was stricter than TGR calculated based on tumor diameter. For example, a tumor that increased in volume by 50% per month before immunotherapy was assessed as HPD only if the tumor increased by at least 150% per month during immunotherapy.
Due to the high degree of malignancy in advanced pancreatic cancer, it is more difficult to control tumor progression using a single immunotherapy. Therefore, when using PD-1 inhibitors, it often needs to be combined with other therapies. The majority of patients in our study received a combination of a PD-1 inhibitor with chemotherapy or targeted therapies, accounting for 91.3% (). But no statistically significant difference was found between the combination therapy and PD-1 inhibitor alone in the correlation analysis for hyperprogressive disease (), which is in line with the findings of Ferrara’s team.Citation15 Previous research has found that cigarette smoke can induce stem cell characteristics in PC cells via PAF1, making PC cells more aggressiveCitation24 and can affect the prognosis of PC patients on antitumor therapy,Citation25 while our study found that smoking history of PC patients may be associated with the development of HPD after their treatment with PD-1 inhibitors. In addition, we also found that the number of metastatic sites and liver metastases were also associated with the development of HPD, and these findings were consistent with those reported in previous study.Citation15 The liver is the most common metastatic organ in advanced pancreatic cancer and the percentage of patients with liver metastases in our study was up to 43.4%, A probable reason for the development of HPD induced by liver metastases is the ability of liver metastases to siphon activated CD8+T cells from the systemic circulation causing apoptosis and inducing peripheral immune tolerance in the host, which leads to acquired immunotherapy resistance.Citation26 In the context of immunosuppressive cancer therapy, treating or preventing infections is critical to maintaining patient health, such as neutropenic fever, which is an acute condition requiring rapid administration of broad-spectrum antibiotics.Citation27,Citation28 In addition, patients with advanced cancer are often accompanied by various bacterial infections due to low immunity, leading to frequent use of antibiotics.Citation29 Several studies suggested that antibiotic use may adversely affect immunotherapy prognosis,Citation30,Citation31 and in our study, the likelihood of HPD occurrence was higher in patients with advanced PC who had received Abx B21, perhaps because antibiotic use disrupted intestinal microecologyCitation32 and affected the antitumor effect of PD-1 inhibitors,Citation33 thus leading to a poor prognosis. We also explored the impact of baseline Hb levels on the development of immunotherapy HPD and found that patients with anemia (Hb <110 g/L) were more likely to develop HPD, possibly because anemia-induced hypoxia reduced their sensitivity to anticancer therapy further contributing to tumor progression,Citation34 and it had been previously demonstrated that baseline Hb levels adversely affected the prognosis of immunotherapy.Citation35 In addition, Some studies suggested high dNLR predicts poor immunotherapy efficacy.Citation36 In our study, patients with advanced pancreatic cancer treated with a PD-1 inhibitor with dNLR > 3 similarly had a higher rate of HPD than those with dNLR ≤ 3 (4/36 vs. 6/65). But the difference was not statistically significant which may be related to the limited number of cases. The later the immunotherapy line, which means that the patient has received several other therapies before, such as FOLFIRINOX (5-Fluorouracil, Folinic acid, Irinotecan, and Oxaliplatin), Gem-nabP (nab-paclitaxel and gemcitabine), targeted therapy, radiation therapy and local radiofrequency ablation treatment of liver metastases, and the patient’s physical fitness and tumor burden was worse, which could explain why PD-1 inhibitor treatment lines (which were greater than 2) were linked to the development of HPD.
Several studies have reported that HPD can significantly impair patient prognosis and lead to decreased OS.Citation15,Citation37 This was reconfirmed in our study, where patients with HPD had significantly shorter OS compared to those who developed PD only (3.6 vs 5.6 m), highlighting the importance of finding predictive biomarkers. CA19-9 is a well-recognized characteristic tumor marker for PC patients, but its predictive significance for HPD in immunotherapy is unknown. Therefore, we explored the relationship between baseline CA19-9 levels and the development of HPD after immunotherapy in patients with advanced PC, and found that baseline CA19-9 levels were predictive of HPD. If the pre-treatment CA19-9 level > 1279 U/ml, it required vigilance for the occurrence of subsequent HPD, whereas when the CA19-9 level ≤1279 U/ml, PC patients receiving immunotherapy were relatively safe and less likely to develop HPD. We further performed a survival analysis related to baseline CA19-9 levels, and showed that high baseline CA19-9 levels were linked to a shorter overall survival time.
Of interest, we also found that the changes of CA19-9 within the first month after immunotherapy in patients with advanced pancreatic cancer can predict the occurrence of HPD. This finding has significant clinical value. Because imaging is usually performed 6–8 weeks after the patient has received treatment, the tumor marker CA19-9 is monitored every time a patient is admitted to the hospital for every cycle of treatment, showing that changes in CA19-9 can predict HPD earlier than imaging.
There are several limitations to this study. Firstly, due to the nature of a single-center study and the fact that all patients were Asian subjects from the same Chinese institution, the sample size was limited and possible confounding and selection bias could not be avoided. In addition, since our study is retrospective, it is impossible to deeply explore the mechanism of HPD. At present, the possible mechanisms of HPD after PD-1 inhibitors treatment include the increase of Treg cells, depletion of CD8+T cells, polarization of affected immunosuppressed cell subsets, dysfunctional inflammation, activation of protooncogene signal pathway/gene mutation.Citation14 In future studies, high-throughput sequencing of tumor and blood samples from HPD patients before and after treatment could help to elucidate the mechanisms behind this pattern and its causal relationship to treatment.
In conclusion, HPD caused a shorter OS and a considerably worse prognosis in patients with advanced PC treated with immunotherapy. The development of HPD was associated with certain clinicopathological features and baseline CA19-9 levels were predictive of HPD. As a result, further studies are needed to elucidate the molecular mechanisms of HPD and its biomarkers.
Abbreviations
Antibiotic therapy within 21 days before immunotherapy (Abx B21); area under the curve (AUC); computed tomography (CT); derived neutrophil-to-lymphocyte ratio (dNLR); eastern cooperative oncology group performance status (ECOG PS); hemoglobin (Hb); hyperprogressive disease (HPD); immune checkpoint inhibitors (ICIs); lactate dehydrogenase (LDH); magnetic resonance imaging (MRI); median overall survival (mOS); overall survival (OS); pancreatic cancer (PC); programmed cell death-1 (PD-1); programmed death ligand-1(PD-L1); progressive disease (PD); receiver operating characteristic (ROC); tumor growth rate (TGR).
Supplemental Material
Download TIFF Image (160.9 KB)Supplemental Material
Download MS Word (119.5 KB)Supplemental Material
Download MS Word (16.4 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2252692.
Additional information
Funding
References
- Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TM, Myklebust T, Tervonen H, Thursfield V, Ransom D, Shack L, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20(11):1493–9. doi:10.1016/s1470-2045(19)30456-5.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492.
- Manji GA, Olive KP, Saenger YM, Oberstein P. Current and emerging therapies in metastatic pancreatic cancer. Clin Cancer Res. 2017;23(7):1670–8. doi:10.1158/1078-0432.Ccr-16-2319.
- Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet (London, England). 2020;395(10242):2008–20. doi:10.1016/s0140-6736(20)30974-0.
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus chemotherapy for PD-L1–Positive non–small-Cell lung cancer. N Engl J Med. 2016;375(19):1823–33. doi:10.1056/NEJMoa1606774.
- Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390(10105):1853–62. doi:10.1016/s0140-6736(17)31601-x.
- Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet (London, England). 2019;393(10167):156–67. doi:10.1016/s0140-6736(18)31999-8.
- Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al. Safety and efficacy of Pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. doi:10.1001/jamaoncol.2018.0013.
- Weiss GJ, Waypa J, Blaydorn L, Coats J, McGahey K, Sangal A, Niu J, Lynch CA, Farley JH, Khemka V. A phase Ib study of pembrolizumab plus chemotherapy in patients with advanced cancer (PembroPlus). Br J Cancer. 2017;117(1):33–40. doi:10.1038/bjc.2017.145.
- Padrón LJ, Maurer DM, O’Hara MH, O’Reilly EM, Wolff RA, Wainberg ZA, Ko AH, Fisher G, Rahma O, Lyman JP, et al. Sotigalimab and/or nivolumab with chemotherapy in first-line metastatic pancreatic cancer: clinical and immunologic analyses from the randomized phase 2 PRINCE trial. Nat Med. 2022;28(6):1167–77. doi:10.1038/s41591-022-01829-9.
- Bockorny B, Grossman JE, Hidalgo M. Facts and hopes in immunotherapy of pancreatic cancer. Clin Cancer Res. 2022;28(21):4606–17. doi:10.1158/1078-0432.Ccr-21-3452.
- Li X, Gulati M, Larson AC, Solheim JC, Jain M, Kumar S, Batra SK. Immune checkpoint blockade in pancreatic cancer: trudging through the immune desert. Semin Cancer Biol. 2022;86(Pt 2):14–27. doi:10.1016/j.semcancer.2022.08.009.
- André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, et al. Pembrolizumab in microsatellite-Instability–High advanced colorectal cancer. N Engl J Med. 2020;383(23):2207–18. doi:10.1056/NEJMoa2017699.
- Champiat S, Ferrara R, Massard C, Besse B, Marabelle A, Soria JC, Ferté C. Hyperprogressive disease: recognizing a novel pattern to improve patient management. Nat Rev Clin Oncol. 2018;15(12):748–62. doi:10.1038/s41571-018-0111-2.
- Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, Mazieres J, Zalcman G, Brosseau S, Le Moulec S, et al. Hyperprogressive disease in patients with advanced non–small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4(11):1543–52. doi:10.1001/jamaoncol.2018.3676.
- Ferté C, Fernandez M, Hollebecque A, Koscielny S, Levy A, Massard C, Balheda R, Bot B, Gomez-Roca C, Dromain C, et al. Tumor growth rate is an early indicator of antitumor drug activity in phase I clinical trials. Clin Cancer Res. 2014;20(1):246–52. doi:10.1158/1078-0432.Ccr-13-2098.
- Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, Mazieres J, Zalcman G, Brosseau S, Le Moulec S, et al. Comparison of fast-progression, hyperprogressive disease, and early deaths in advanced non–small-Cell lung cancer treated with PD-1/PD-L1 inhibitors or chemotherapy. JCO Precis Oncol. 2020;4(4):829–40. doi:10.1200/po.20.00021.
- Sasaki A, Nakamura Y, Mishima S, Kawazoe A, Kuboki Y, Bando H, Kojima T, Doi T, Ohtsu A, Yoshino T, et al. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer. 2019;22(4):793–802. doi:10.1007/s10120-018-00922-8.
- Klemen ND, Hwang S, Bradic M, Rosenbaum E, Dickson MA, Gounder MM, Kelly CM, Keohan ML, Movva S, Thornton KA, et al. Long-term follow-up and patterns of response, progression, and hyperprogression in patients after PD-1 blockade in advanced Sarcoma. Clin Cancer Res. 2022;28(5):939–47. doi:10.1158/1078-0432.Ccr-21-3445.
- Saâda-Bouzid E, Defaucheux C, Karabajakian A, Coloma VP, Servois V, Paoletti X, Even C, Fayette J, Guigay J, Loirat D, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28(7):1605–11. doi:10.1093/annonc/mdx178.
- Matos I, Martin-Liberal J, García-Ruiz A, Hierro C, Ochoa de Olza M, Viaplana C, Azaro A, Vieito M, Braña I, Mur G, et al. Capturing hyperprogressive disease with immune-checkpoint inhibitors using RECIST 1.1 criteria. Clin Cancer Res. 2020;26(8):1846–55. doi:10.1158/1078-0432.Ccr-19-2226.
- Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A, Soria JC, et al. Hyperprogressive disease is a New pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920–8. doi:10.1158/1078-0432.Ccr-16-1741.
- Kas B, Talbot H, Ferrara R, Richard C, Lamarque JP, Pitre-Champagnat S, Planchard D, Balleyguier C, Besse B, Mezquita L, et al. Clarification of definitions of hyperprogressive disease during immunotherapy for non–small cell lung cancer. JAMA Oncol. 2020;6(7):1039–46. doi:10.1001/jamaoncol.2020.1634.
- Nimmakayala RK, Seshacharyulu P, Lakshmanan I, Rachagani S, Chugh S, Karmakar S, Rauth S, Vengoji R, Atri P, Talmon GA, et al. Cigarette smoke induces stem cell features of pancreatic cancer cells via PAF1. Gastroenterology. 2018;155(3):892–908.e896. doi:10.1053/j.gastro.2018.05.041.
- Vienot A, Beinse G, Louvet C, de Mestier L, Meurisse A, Fein F, Heyd B, Cleau D, d’Engremont C, Dupont-Gossart AC, et al. Overall survival prediction and usefulness of second-line chemotherapy in advanced pancreatic adenocarcinoma. J Natl Cancer Inst. 2017;109(10). doi:10.1093/jnci/djx037.
- Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, Rizvi SM, Qin A, Waninger JJ, Lang X, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27(1):152–64. doi:10.1038/s41591-020-1131-x.
- Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56–93. doi:10.1093/cid/cir073.
- Schimpff S, Satterlee W, Young VM, Serpick A. Empiric therapy with carbenicillin and gentamicin for febrile patients with cancer and granulocytopenia. N Engl J Med. 1971;284(19):1061–5. doi:10.1056/nejm197105132841904.
- Galloway-Peña JR, Jenq RR, Shelburne SA. Can consideration of the microbiome improve antimicrobial utilization and treatment outcomes in the oncology patient? Clin Cancer Res. 2017;23(13):3263–8. doi:10.1158/1078-0432.Ccr-16-3173.
- Ochi N, Ichihara E, Takigawa N, Harada D, Inoue K, Shibayama T, Hosokawa S, Kishino D, Harita S, Oda N, et al. The effects of antibiotics on the efficacy of immune checkpoint inhibitors in patients with non–small-cell lung cancer differ based on PD-L1 expression. Eur J Cancer (Oxford, England: 1990). 2021;149:73–81. doi:10.1016/j.ejca.2021.02.040.
- Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, Brock C, Power D, Hatcher O, Falconer A, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5(12):1774–8. doi:10.1001/jamaoncol.2019.2785.
- Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007;1(1):56–66. doi:10.1038/ismej.2007.3.
- Poizeau F, Kerbrat S, Balusson F, Tattevin P, Revest M, Cattoir V, Luque-Paz D, Lesimple T, Pracht M, Dinulescu M, et al. The Association between antibiotic use and outcome among metastatic melanoma patients receiving immunotherapy. JNCI. 2022;114(5):686–94. doi:10.1093/jnci/djac019.
- Bosco MC, D’Orazi G, Del Bufalo D. Targeting hypoxia in tumor: a new promising therapeutic strategy. J Exp Clin Cancer Res. 2020;39(1):8. doi:10.1186/s13046-019-1517-0.
- Chen Y, Wen S, Xia J, Du X, Wu Y, Pan B, Zhu W, Shen B. Association of dynamic changes in peripheral blood indexes with response to PD-1 inhibitor-based combination therapy and survival among patients with advanced non-small cell lung cancer. Front Immunol. 2021;12:672271. doi:10.3389/fimmu.2021.672271.
- Sui Q, Zhang X, Chen C, Tang J, Yu J, Li W, Han K, Jiang W, Liao L, Kong L, et al. Inflammation promotes resistance to immune checkpoint inhibitors in high microsatellite instability colorectal cancer. Nat Commun. 2022;13(1):7316. doi:10.1038/s41467-022-35096-6.
- Kim CG, Kim KH, Pyo KH, Xin CF, Hong MH, Ahn BC, Kim Y, Choi SJ, Yoon HI, Lee JG, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol. 2019;30(7):1104–13. doi:10.1093/annonc/mdz123.