ABSTRACT
The proportion of the population vaccinated in Israel against COVID-19 in 2021 was significantly higher than that of the annual uptake rates of the influenza vaccine over time. Understanding the reasons behind the high vaccination rate may facilitate maintaining these rates if annual COVID-19 vaccination is required. Using a mixed-methods design, we characterized the individuals who were vaccinated and studied their attitudes toward vaccines and motivators for the COVID-19 vaccine. The first part was a cross-sectional study of adults aged 65 and over who were vaccinated against COVID-19. We stratified them according to their annual influenza vaccination patterns, and compared variables such as age, gender, health status, and timing of COVID-19 vaccination. The second part consisted of a questionnaire administered to a subsample of the above population, inquiring about vaccine hesitancy, motivators for vaccination, and intention to be vaccinated in the future. We found that motivating factors for COVID-19 vaccination are similar between those who regularly vaccinate against influenza and those who don’t. Internal motivators such as perceived vaccine effectiveness and the desire to protect others were stronger than external rewards or sanctions. High adherence to annual influenza vaccine recommendations was associated with earlier COVID-19 vaccine uptake. Respondents with lower adherence to influenza vaccines were more likely to demonstrate higher levels of vaccine hesitancy. These factors should be addressed in future vaccination campaigns.
Introduction
Pfizer’s COVID-19 vaccine (BNT126b2) was approved for use in Israel in December 2020, followed by a nationwide vaccination campaign. The campaign initially targeted individuals aged 65 and over, healthcare workers, and residents of long-term care facilities. The vaccine was gradually made available to younger age groups.Citation1 It was offered free of charge and granted vaccinees a “green pass” which allowed greater freedom of movement.
As of April 2021, 54% of the general population and 88% of individuals aged 50 and above had received two doses of the vaccine.Citation2
Following the emergence of new variants of the COVID-19 virus with high infection rates, a third ‘booster’ dose of the vaccine was approved in Israel in July 2021.Citation3 It was gradually offered to younger age groups, and finally to all individuals aged 12 and above.Citation4 Following this, studies found substantially lower rates of confirmed COVID-19 infection and severe illness in individuals who received the booster dose.Citation3,Citation4
Despite high acceptance of the first doses of the COVID-19 vaccine in Israel, vaccination rates decreased with each additional dose.Citation5 A recent review found that current or previous influenza vaccination affected COVID-19 vaccine acceptance,Citation6 suggesting these vaccines may share similarities in terms of motivation. Exploring the relationship between them may be beneficial to identify key factors that may facilitate future COVID-19 vaccine uptake.
Annual revaccination against influenza vaccines is recommended annually to manage infection rates and prevent severe illness. Due to frequent antigenic variation and waning immunity, mass revaccination against COVID-19 will be required.Citation7
The Israeli Ministry of Health (IMoH) recommends annual influenza vaccination for all populations over 6 months old, and vaccines are provided free of charge via health maintenance organizations. Individuals over 65, those with chronic diseases or the immunosuppressed, pregnant women and children under five are specifically targeted.Citation8 Despite vaccine availability, the influenza vaccination rate between 2014–2018 was only 20% in the general population.Citation9 A study conducted in Israel found that in individuals aged 65 and over, vaccination rates were higher than in the general population, and remained approximately 60% between 2004–2009. Female sex, lower socio-economic status, living in a rural setting, and immigration from the former Soviet Union were identified as factors associated with lower uptake of the influenza vaccine.Citation10
In order to improve or maintain vaccination rates, it is important to understand the motivators behind the decision to be vaccinated, An important concept related to this is ‘Vaccine hesitancy’ - a delay in acceptance or refusal of vaccination despite availability.Citation11 As undergoing vaccination for any disease requires three basic conditions: belief that the disease may have a significant negative impact, trust that the vaccine is safe and effective, and easy access, when addressing vaccine hesitancy, one must assess and address these three dimensions both at an individual and population level.Citation12
A study conducted in Israel found that 60% of people vaccinated with the COVID-19 vaccine had not received an influenza vaccine in the previous 5 years. Furthermore, only 13% had received an influenza vaccine in all five previous years.Citation13 A systematic review of influenza vaccine hesitancy found that prior vaccination against influenza can predict future acceptance of the same vaccine, and can even predict future acceptance of the anti-pneumococcal vaccine.Citation14
The objective of our study was to identify underlying factors that may support future uptake of COVID-19 boosters by comparing motivating factors between those regularly vaccinated against influenza and those who were not. We hypothesized that beliefs and values would play a bigger role in motivating those who typically received influenza vaccines compared with individuals who did not routinely vaccinate.
This study was conducted in the midst of the booster COVID-19 vaccine campaign, and thus can provide real-time insight into the public stance on vaccine hesitancy in general, and specifically on vaccination against COVID-19. We described and compared the characteristics of three groups of people, who were vaccinated against COVID-19 but differ in the level of their adherence to influenza vaccination recommendations.
Methods
This study was conducted between July 2021 and February 2022 in Meuhedet, a Health Maintenance Organization providing healthcare to 1.3 million people throughout Israel. All medical records are computerized, and demographic, medical, and treatment data are stored in a central data warehouse.
The study consisted of two stages. The first was a cross-sectional study of all Meuhedet members aged 65 and above, who were vaccinated against COVID-19. The second was a survey conducted among a stratified random sample of the same population.
As influenza vaccination among adults is mainly focused on the older age group in the population, the study was conducted in this age group.
Ethical approval for the study was obtained from the Institutional Review Board (Number 02-21-06-21). As the data were de-identified during extraction, informed consent was not required.
Cross-sectional study
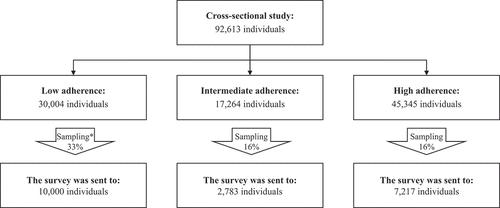
The analysis was based on medical data from the Meuhedet database, which were extracted on November 4, 2021. Members included in the analysis were those aged 65 and over in June 2021, who had been members continually between 2014–2021, and who had received two doses of Pfizer’s vaccine (BNT126b2) between January and March 2021. All participants included in the study were 58 or over at the start of the follow-up period in June 2014. A total of 92,613 participants met the inclusion criteria for the study.
Variables
We categorized the number of influenza vaccine doses that were received by each person between October 2014 and February 2019 into three categories: High adherence (HA) − 4–5 doses, Intermediate adherence (IA) − 2–3 doses, and Low adherence (LA) − 0–1 doses. Additionally, we extracted demographic information – age, sex, population, and ethnic sectors from Meuhedet’s database. The three primary ethnic sectors in the database are the general Jewish population, which makes up 47% of the total, followed by the ultra-orthodox Jewish population at 35%, and finally the Arab population at 18%.
We extracted prior diagnosis of one or more chronic conditions (cancer, diabetes mellitus, asthma, chronic obstructive pulmonary disease, congestive heart failure, cerebrovascular accident, dementia, ischemic heart disease, Parkinson’s disease, peripheral vascular disease) using their ICD-9 codes, as documented by the physician at a medical encounter, or from Meuhedet cancer and diabetes mellitus registries.
Survey
The survey was sent via text messages to a stratified random sample of 20,000 participants of the cross-sectional study population. We stratified by to influenza vaccination categories: 10000 from the low adherence (LA) category, 2783 from intermediate adherence (IA), and 7217 from high adherence (HA). The sample size was calculated based on the number of individuals in each of the groups that were extracted in the cross-sectional part of this study: a sample of 16% of the individuals in HA and IA groups, and a sample of 33% of the individuals in the LA group. The survey was sent to a larger sample of the LA group under the assumption that the response rate in this group would be lower than that of the other categories.
The IMoH began administering the third dose of the COVID-19 vaccine to at-risk populations during July 2021, and to the entire population by the end of August 2021. The survey was distributed in October 2021, following a widespread campaign to encourage vaccination, and responses were collected through November 2021.
shows a general overview of the study design.
The questionnaire
The study questionnaire was comprised of three sections:
Section one was based on an adapted version of the Vaccine Hesitancy Scale (VHS) published by Luyten et. al. in 2019.Citation15 The questionnaire, originally in English, was translated into Hebrew and then translated back into English by two independent translators to ensure accuracy and content validity. Questionnaire terminology was adapted to the Israeli population.
Section two asked about respondents’ intention to receive further COVID-19 and influenza vaccines, followed by questions regarding the factors that influenced respondents’ decision to receive an additional COVID-19 vaccine. These included the effectiveness of the vaccine in preventing disease, the desire to protect the health of people close to you, and external motivators such as benefits for vaccinees and workplace pressure.
Section three was comprised of several demographic questions, including age, sex, education, and a health self-assessment question (a 5-point Likert scale).
The survey questionnaire is presented in the Appendix.
Statistical methods
We reported categorical variables as frequencies and percentages. Non-normally distributed continuous variables were reported as medians and interquartile ranges (IQR). The Likert scale responses that described the degree of agreement with statements in the questionnaire were re-distributed into two categories: agree (agree and strongly agree) and disagree (unsure, disagree, strongly disagree). We defined a ‘hesitant’ dichotomic score for section one questions: for positively phrased questions (e.g., ‘Vaccines are important for my health’) we considered the answer as hesitant if it strongly disagreed, disagreed, or unsure, while for negatively phrased questions (e.g., ‘I am concerned about side effects of vaccines’) we defined a hesitant answer as strongly agree and agree.Citation15
We used the chi-square test to compare proportions, and the Mann-Whitney test to compare medians. All statistical tests were two-sided and p < .05 was considered statistically significant. The analyses for this study were generated using © 2016 The R Foundation software (v 4.1.0).
Data analysis was conducted using de-identified data.
Results
Cross-sectional study
The population consisted of 92,613 members of MHS aged 65 and over, who had received at least two doses of Pfizer COVID-19 vaccine (BNT162b2) at the time of the study. The population was divided into three groups, according to Influenza vaccine history in the previous five years: low adherence (LA) (n = 30,004), intermediate adherence (IA) (n = 17,264), and high adherence (HA) (n = 45,345). Over 50% of all groups were female, however, the proportion of males in the IA and HA categories was slightly higher than in the LA category. The HA group was comprised of older individuals with higher proportions of chronic conditions compared to the LA group, which consisted of younger individuals with less chronic conditions. The characteristics of the three groups are described in .
Table 1. Demographic and clinical characteristics of the cross-sectional study population.
COVID-19 vaccine uptake
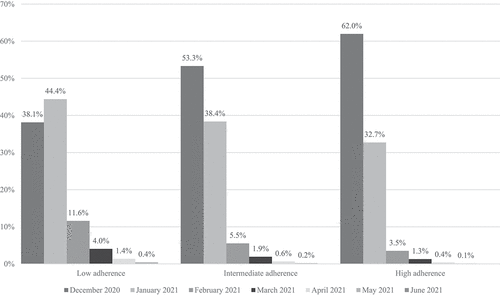
COVID-19 vaccines were offered to all members aged 65 and over starting December 2020. In the study population, 52.6% were vaccinated in December 2020, an additional 37.5% were vaccinated in January 2021 and the remaining 9.8% were vaccinated later in 2021. There was a statistically significant difference between the three groups in the timing of the first vaccine (). The proportion of members vaccinated in December 2020 was highest in the HA group (61.9%), followed by the IA group (53.3%), and lowest in the LA group (38.1%. p < .001).
Survey
The number of respondents to the survey was 1,263 (overall response rate 6.3%). Of these, 384 were in the LA group, 191 in the IA group, and 688 in the HA group (per group response rate 3.8%, 6.8%, and 9.5% respectively. P < .001).
The survey respondents had a higher proportion of males compared with the cross-sectional study: 55% vs 46% (9.7% of survey respondents did not report their gender). Over one-third of respondents (36.7%) were younger than 70 years old, 27.8% were aged 70–74, 26% were over 75, and 9.5% did not report their age. A large majority of respondents (79.1%) reported having tertiary education (college or university), and only 11.6% reported having primary or secondary education, and 9.3% did not report education status. The demographic characteristics (age, gender, and education) by vaccine group are described in .
Table 2. Demographic characteristics by influenza vaccine group in the survey as reported by the respondents.
As can be seen in , there was no difference between vaccination groups in terms of gender or education level. However, the age distribution was significantly different between the three groups, with the HA group having a higher percentage of respondents aged 75 and over (33.7%), and the LA group having a higher percentage of those aged under 70 (50.5%, p < .001), similarly to the age distribution of the cross-sectional study.
Aggregate level of hesitancy
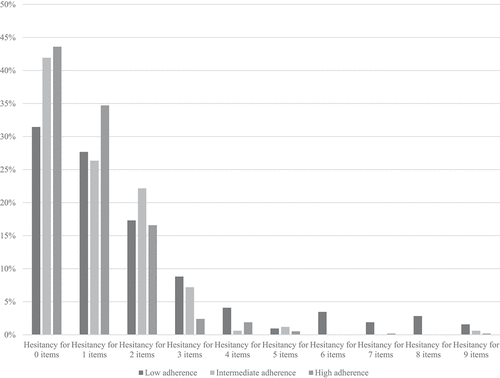
Of the 1071 respondents who fully completed section one, 318 respondents were in the LA group (29.7%), 167 in the IA group (15.6%), and 585 in the HA group (54.6%). provides a summary of the aggregate levels of hesitancy in all 9 categories of question 1 for each vaccine group individually. Responders who had never had an Influenza vaccine were significantly more likely to express vaccine hesitancy than those who had intermittent or annual vaccines.
Intent of COVID-19 and influenza vaccine uptake
We found a significant difference between the groups regarding uptake of the influenza vaccine for the study season (winter 2021–2022): 62.1% of respondents in the LA group either intended to be vaccinated or had already been vaccinated, while 94.9% and 97.2% intended to or had already been vaccinated in the IA and HA groups accordingly (p < .001).
However, regarding a COVID-19 vaccine booster, the proportion of respondents either vaccinated or intending to be vaccinated was higher in all groups − 96.1% in the LA group, 98.9% in the IA group, and 99.2% in the HA group (p = .00107).
Motivation for COVID-19 vaccine
Over 80% of respondents from all groups reported that the effectiveness of the vaccine in preventing disease and the desire to protect others were incentives for COVID-19 vaccination. However, respondents who were partially or fully vaccinated against influenza were significantly more likely to report that the effectiveness of the vaccine in preventing disease (90% vs. 82%) and the desire to protect others (87% vs. 80%) as incentives compared with the LA group ().
Table 3. Motivation for COVID-19 booster vaccine.
When asked whether benefits offered to vaccinees or pressure at work affected their decision to get vaccinated, a much lower proportion in all three groups agreed, with no statistically significant difference between the three ().
For all groups, 76.4% of respondents reported being concerned that someone close to them would contract the COVID-19 virus. Of these, 62% stated that this concern was a factor they considered regarding the COVID-19 vaccine. No differences were observed between the groups (p = .1287).
Discussion
This study aimed to identify characteristics that may be associated with potential adherence to routine COVID-19 vaccination using a cross-sectional database study and a questionnaire among people aged 65 and over who were vaccinated against COVID-19.
During the COVID-19 vaccination period there were several interventions in Israel to encourage acceptance of the vaccine. These included a media campaign, restriction of individuals who were not vaccinated (Green Pass) and workplace pressure. Other factors which may have influenced the population were perceived disease severity and risk to self and others, and belief in the vaccine and in the health system.
The most important finding of this study is that both those who adhere to influenza vaccine recommendations and those who do not share similar motivating factors for getting a COVID-19 booster vaccine. We hypothesized that individuals who adhere to routine vaccination are more likely to be motivated by internal motivators (belief in the vaccine, for example) while those who do not adhere are more likely to be motivated by external influences, such as the Green Pass. Among our study participants, significantly more respondents with a history of adherence to influenza vaccination replied that effectiveness of the vaccine and the desire to protect others were their main incentives for COVID-19 vaccination, yet 80% of those with no history of influenza vaccination also replied that these factors influenced their decision.
In contrast, benefits offered to vaccinees or restrictions on the non-vaccinated, scored lower among all respondents, with no significant difference between the groups. It is important to note that the survey was distributed following an intense IMoH campaign to encourage vaccination. This campaign consisted of a “green pass”, which restricted access to cultural, social and sports events along with restaurants, hotels, and gyms, allowing only those who had received a third vaccine or had recently recovered from COVID-19 to participate. The pass also provided an exemption from compulsory quarantine for those who were exposed to infected individuals. A recent publication identified the potential of a ‘green pass’ as an incentive for COVID-19 vaccination but pointed out that it might not suffice as lack of trust in the vaccine may negate these advantages.Citation15 The finding that even among people with higher vaccine hesitancy scores, the protective effect of the vaccine was a stronger motivator than benefits offered to vaccinees, stressed the importance of addressing issues related to trust and confidence in the vaccine and in the health system rather than relying on external motivators. Additionally, we found that people with strong adherence to annual influenza vaccine recommendations were significantly more likely to be vaccinated earlier against COVID-19 and had lower aggregate levels of hesitancy.
We found no studies that compared actual COVID-19 vaccination with influenza vaccine uptake. Several studies conducted before the distribution of the COVID-19 vaccines found that a positive attitude toward influenza vaccination (intention to be vaccinated or actual vaccination) was associated with intention to receive the COVID-19 vaccine when it is available.Citation16,Citation17 Earlier uptake of the COVID-19 vaccine among those who were regularly vaccinated against influenza in our study () may indicate a more positive attitude toward vaccines in general and a higher level of trust in the health system. This assumption is supported by our findings in the survey () that even among those vaccinated against COVID-19, a history of influenza vaccination was strongly associated with lower aggregate levels of hesitancy.
Despite a small difference between those who were routinely vaccinated against influenza and those who were not, the majority of respondents reported that they had either received a third COVID-19 vaccine or intended to do so.
Two recent studies addressed fluctuating attitudes toward the vaccine over time. A study conducted in the US between March-August 2020 found increasing resistance among the publicCitation18 while a British study conducted between February 2020-March 2021 among older adults found a decrease in mistrust of the vaccine, but increasing concerns regarding future side effects.Citation19 This study which was undertaken later shows less resistance to future vaccination.
Study limitations and strengths
This study was conducted among Meuhedet members aged 65 and over. We selected this age group due to the relatively high levels of both COVID-19 and influenza vaccination uptake as well as higher morbidity and mortality risk. The ability to extrapolate to other age groups may be limited.
Another limitation is the low response rate to the survey which was stratified to include a higher representation of low influenza adherent patients. Despite this limitation, 1263 participants responded, of these 1071 surveys were answered in full. As in many questionnaire-based studies, this study may be influenced by a selection bias. In this study individuals with a higher education level and higher influenza vaccine adherence were over-represented, which may have influenced findings related to vaccine hesitancy. However, despite these limitations it offers a unique insight into patients’ thinking in real-time, as it was undertaken at the height of the pandemic during the rollout of the third vaccination dose.
Conclusions
The COVID-19 pandemic and subsequent vaccine rollout provided a unique opportunity to better understand public reactions to new vaccines and adherence to vaccine recommendations over time. Our findings showed that the strongest motivators for vaccine uptake were the perceived effectiveness of the vaccine and the desire to protect others. Accordingly, we believe that future campaigns to encourage vaccination should target increasing trust and belief in the benefits of vaccination.
Ethical approval
This study was approved by the Meuhedet Institutional Review Board (Number 02-21-06-21).
Supplemental Material
Download MS Word (17.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2265170.
Additional information
Funding
References
- Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–8. doi:10.1016/S0140-6736(21)00947-8.
- Leshem E, Wilder-Smith A. COVID-19 vaccine impact in Israel and a way out of the pandemic. Lancet. 2021;397(10287):1783–5. doi:10.1016/S0140-6736(21)01018-7.
- Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, Mizrahi B, Alroy-Preis S, Ash N, Milo R, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–400. doi:10.1056/NEJMoa2114255.
- Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Kohane IS, Reis BY, Balicer RD. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093–100. doi:10.1016/S0140-6736(21)02249-2.
- COVID-19 data dashboard [Internet]. Israel Ministry of Health. [ accessed 2021 Mar 19]. https://datadashboard.health.gov.il/COVID-19/general.
- Joshi A, Kaur M, Kaur R, Grover A, Nash D, El-Mohandes A. Predictors of COVID-19 vaccine acceptance, intention, and hesitancy: a scoping review. Front Public Heal. 2021;9(August). doi:10.3389/fpubh.2021.698111.
- Monto AS. The future of SARS-CoV-2 vaccination — lessons from influenza. N Engl J Med. 2021;385(20):1825–7. doi:10.1056/NEJMp2113403.
- MOH. Influenza | Ministry of health [Internet]. Ministry of Health; 2009. https://www.moh.gov.sg/content/moh_web/home/diseases_and_conditions/i/influenza.html.
- Shasha D, Valinsky L, Hershkowitz Sikron F, Glatman-Freedman A, Mandelboim M, Toledano A, Paran Y, Ben-Ami R, Goldman D. Quadrivalent versus trivalent influenza vaccine: clinical outcomes in two influenza seasons, historical cohort study. Clin Microbiol Infect [Internet]. 2020;26(1):101–6. doi:10.1016/j.cmi.2019.05.003.
- Schwartz AW, Clarfield AM, Doucette JT, Valinsky L, Karpati T, Landrigan PJ, Sternberg SA. Disparities in pneumococcal and influenza immunization among older adults in Israel: a cross-sectional analysis of socio-demographic barriers to vaccination. Prev Med. 2013;56(5):337–40. doi:10.1016/j.ypmed.2013.01.019.
- MacDonald NE SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–4. doi:10.1016/j.vaccine.2015.04.036.
- Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines [Internet]. 2021;9(2):160. doi:10.3390/vaccines9020160.
- Amit S, Regev-Yochay G, Afek A, Kreiss Y, Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397(10277):875–7. doi:10.1016/S0140-6736(21)00448-7.
- Schmid P, Rauber D, Betsch C, Lidolt G, Denker ML. Barriers of influenza vaccination intention and behavior - a systematic review of influenza vaccine hesitancy, 2005-2016. PLoS One. 2017;12:2005–16. doi:10.1371/journal.pone.0170550.
- Luyten J, Bruyneel L, van Hoek AJ. Assessing vaccine hesitancy in the UK population using a generalized vaccine hesitancy survey instrument. Vaccine [Internet]. 2019;37(18):2494–501. doi:10.1016/j.vaccine.2019.03.041.
- Graupensperger S, Abdallah DA, Lee CM. Social norms and vaccine uptake: college students’ COVID vaccination intentions, attitudes, and estimated peer norms and comparisons with influenza vaccine. Vaccine [Internet]. 2021;39(15):2060–7. doi:10.1016/j.vaccine.2021.03.018.
- Malik AA, McFadden SAM, Elharake J, Omer SB. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine [Internet]. 2020;26:100495. doi:10.1016/j.eclinm.2020.100495.
- Fridman A, Gershon R, Gneezy A. COVID-19 and vaccine hesitancy: a longitudinal study. PLoS One [Internet]. 2021;16(4 April):1–12. doi:10.1371/journal.pone.0250123.
- Gallant AJ, Brown Nicholls LA, Rasmussen S, Cogan N, Young D, Williams L. Changes in attitudes to vaccination as a result of the COVID-19 pandemic: a longitudinal study of older adults in the UK. PLoS One [Internet]. 2021;16(12):1–11. doi:http://dx.doi.org/10.1371/journal.pone.0261844.