ABSTRACT
Varicella is a highly contagious disease caused by the varicella zoster virus (VZV). While the disease is usually mild, severe complications can occur requiring costly hospitalization. A thorough understanding of the healthcare resource use (HCRU) and costs of varicella is needed to inform health-economic models of preventive strategies. A systematic literature review was carried out to retrieve relevant publications between 1999 and 2021, reporting HCRU and cost outcomes for varicella and its complications. Data were extracted and stratified according to pre-specified age groups and complication categories. Costs were re-based to a $US2020 footing using both purchasing power parity and the medical component of consumer price indexes. Data were summarized descriptively due to high heterogeneity in study design and outcome reporting. Forty-four publications fulfilled the inclusion and exclusion criteria of which 28 were conducted in Europe, 6 in Middle East and Asia, 5 in South America, 3 in North America, and 2 in multiple regions. Primary healthcare visits accounted for 30% to 85% of total direct costs. Hospitalization costs varied between $1,308 and $38,268 per episode depending on country, complication type, and length of stay, contributing between 2% and 60% to total direct costs. Indirect costs, mostly driven by workdays lost, accounted for approximately two-thirds of total costs due to varicella. The management of varicella and related complications can lead to substantial HCRU and costs for patients and the healthcare system. Additional research is needed to further characterize the varicella-associated economic burden and its broader impact from a societal standpoint.
Plain Language Summary
Varicella, also known as chickenpox, is a highly contagious infectious disease which affects mostly children. Indeed, >90% of children will have had chickenpox by the age of 12 years. The symptoms are usually mild, but in some cases, serious complications can occur such as pneumonia, bacterial superinfection of the skin and encephalitis. A clear understanding of the complications of chickenpox for patients and the healthcare system would be helpful so that countries can assess the true health and economic burden of the disease.
In this study, we have summarized existing published data from around the world. We have included studies that reported on the number of varicella cases, doctor visits, hospitalizations, and costs due to varicella and associated complications.
These data showed that varicella causes high costs to the healthcare system. Even though less than 1% of varicella patients need to be hospitalized, costs remain high because varicella is so common. Furthermore, if the number of workdays lost are counted as well, then varicella-related costs are even higher.
HIGHLIGHTS
Varicella and its complications lead to significant primary healthcare resource use because of its high incidence with each episode leading to 1 healthcare visit on average.
Varicella-related primary healthcare visits account for 30% to 80% of total direct costs.
Varicella-related hospitalizations contribute between 2% and 60% to total direct costs.
Varicella-related hospitalizations may occur both in children and adults, with costs ranging between $1,308 and $38,268 per episode depending on severity, complication type and length of stay.
Indirect costs are mostly driven by workdays lost and account for approximately two-thirds of total costs.
Introduction
Varicella, also referred to as chickenpox, is a very common infection caused by the varicella zoster virus (VZV). Patients with chickenpox typically develop a rash, which progresses from papules to itchy blisters and scabs over the course of several days. Other clinical symptoms, which may precede the rash by a few days, include fever, fatigue, headaches and loss of appetite. The disease is usually benign, lasting between 4 and 7 days; however, severe complications may occur. These can include respiratory complications (pneumonia), bacterial superinfection of the skin and soft tissue by bacteria, foremost by Group A Streptococcus, neurological complications (e.g., cerebellitis, encephalitis, cerebellar ataxia), dehydration and in rare cases systemic complications and death. Infants and children aged <1 year, pregnant women, adults and immunocompromised people are at highest risk to develop complications caused by VZV.Citation1
VZV is a highly contagious virus with an estimated reproduction rate (R0) ranging from 3 to 17, dependent upon age and social mixing patterns.Citation2,Citation3 In the absence of vaccination, more than 90% of children will be infected with VZV by the age of approximately 12 years.Citation4–6
Incidence rates and economic burden of varicella have been addressed in several systematic literature reviews;Citation4,Citation7–11 however, these reviews focused either only on incidence or a specific geographic region, while recent reviews adopting a global perspective with emphasis on complications and associated costs are missing. To characterize the economic burden of VZV infection, it is necessary to review existing knowledge on healthcare resource use (HCRU) and costs for the treatment of varicella and its complications across different populations and geographies. Hence, the objective of this systematic literature review (SLR) was to synthetize the evidence available on the HCRU, as well as direct and indirect costs of varicella disease and associated complications.
Methods
Relevant publications between 1999 and 2021 were identified through a comprehensive literature search which included biomedical electronic databases and gray literature, following methodology described by the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guidelines.Citation12
The following databases were searched via embase.com: Medline and Medline in Progress, Embase, the York Centre for Reviews and Dissemination database which includes the National Health System Economic Evaluation Database and health technology assessment databases. Poster presentations from the International Congress of Infectious Diseases and from the International Professional Society for Health Economics and Outcomes Research (ISPOR) were considered if presented between 2019 and 2021, assuming that any earlier study would have been published as full article thereafter. Selected sources of gray literature were analyzed, including the Cost-effectiveness Analysis registry, conference proceedings from the Global Summit on Vaccines and Immunology and the International Online Congress on Vaccines and Virology (published between 2019 and 2021), and reports from the European Centre for Disease Prevention and Control varicella vaccination working group and the Joint Committee on Vaccination and Immunization varicella vaccine sub-committee.
Inclusion and exclusion criteria are summarized in and the full Embase search is provided in (supplementary information [SI], Table S1). All studies in humans with varicella or break-through varicella (varicella infection occurring in vaccinated people) were eligible if they reported selected outcomes, i.e., HCRU and/or costs associated with varicella and its complications. For countries that have universal varicella vaccination in place, epidemiologic data were only considered for the pre-vaccination era. All study designs were acceptable except case studies, protocols, and conference abstracts containing insufficient information. SLRs were not included in this review but analyzed for citations that might fulfill inclusion and exclusion criteria.
Table 1. Inclusion/Exclusion criteria for publications.
Abstracts were screened by three authors independently. To align the screening process and reduce bias during abstract selection, a sample of 10% of abstracts were screened by all three authors and any disagreement was reconciled by the project leader. Duplicate and secondary publications were excluded.
The quality of studies was assessed using the mixed methods appraisal tool (MMAT)Citation13 for quantitative and qualitative studies and the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist in case of cost-effectiveness publications.Citation14
Information was extracted into a predefined excel template including information regarding the study (e.g., study design, year of publication, country, objectives), patient characteristics, results (e.g., complications, costs, HCRU). Results were extracted for predefined age groups, selecting the closest match among the following: <1 year, 1–5 years, 6–10 years, 11–24 years, 25–44 years and 45–65 years. Complications were grouped into different categories to facilitate aggregated analysis: at least one complication, cardiovascular, cerebellitis/ataxia, ear/nose/throat (ENT), encephalitis/myelitis, febrile convulsion/seizure, gastrointestinal (e.g., dehydration and diarrhea), hematological, lower respiratory tract infection, meningitis, musculoskeletal, neurological, ocular, renal, respiratory, skin, systemic, and other. The grouping of complications was based on complications reported by the centers for disease control and prevention (CDC) and common categories published in the literature.Citation15,Citation16
Costs were converted to US dollars ($US) using purchasing power parityCitation17 for the year costs were reported and inflated to $US2020 values using the medical care component of the consumer price index.Citation18
Results
The initial search yielded 2,000 matches from databases and grey literature. After elimination of duplicates, 1,451 entries were retained for screening of abstracts and titles. Among these records, 164 publications were eligible for full-text screening, but 13 publications could not be obtained as full text or did not contain data for selected outcomes and were excluded (SI Table S2). Thus, 151 full text publications were further analyzed ().
Figure 1. (a) PRISMA flow chart for the global search and B) geographical distribution.
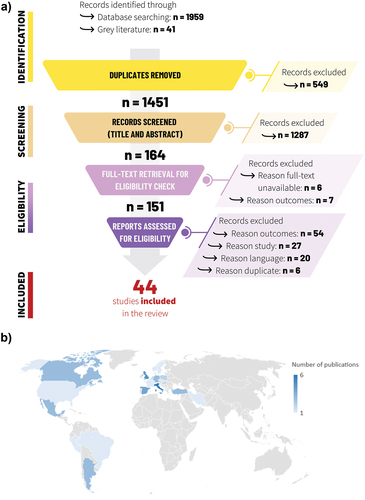
After analysis of the full text, 44 studies were included consisting of 19 observational/retrospective studies, 21 cost-effectiveness studies and 4 studies with a mixed design (i.e., internet questionnaire, surveys, pooled analysis of several studies, health cost analysis). The selected studies were conducted in 22 countries, most of them in Europe (n = 28), followed by Asia and Middle East (n = 6), South America (n = 5) and North America (n = 3) (). Two studies reported data from both American and European countries. Among observational studies and studies with mixed design, 14 focused on the pediatric population. Cost-effectiveness analyses were in general conducted over a time horizon that considered both children and adults [SI Table S3]. Ten studies provided information regarding the health status of the population, i.e., immunocompetent vs immunocompromised, but only two studies analyzed data separately for these populations [SI Table S3].Citation15,Citation21 Thirty-six publications presented direct costs and 26 studies included indirect cost estimates. Details regarding study design, population and outcomes are provided in [SI Table S3].
All quantitative and qualitative clinical and/or observational studies achieved a MMAT score > 60% [SI Table S4]. All cost-effectiveness studies (n = 21) were included since their quality was deemed acceptable after evaluation with the CHEERS checklist.
Primary care
Healthcare resource use
Twenty-six publications reported the frequency and/or costs of primary care visits (general practitioners [GPs], pediatricians, and outpatient visits) (). Average number of primary care visits per varicella episode ranged between 0.04 and 2.2 visits.
Table 2. Number of GP/Primary care visits and costs per varicella case.
There were large variations across countries in primary care seeking behavior for varicella; the proportion of patients having a primary care visit ranged between 40% to close to 100%.Citation27,Citation28,Citation35,Citation41,Citation46,Citation47 Only a few studies analyzed primary care visits by age group. In an economic analysis for the United States (US), the proportion of outpatient visits were highest in infants aged <1 year (78.8%) and adults aged >30–39 years (97.6%).Citation48 Age-specific data for Canada suggested that the proportion of patients with varicella having a GP visit were age-dependent with highest rates observed in infants and children aged <2 years (48–57%) and adults aged ≥25 years (≥90%).Citation49 In a postal survey carried out in Canada, the proportion of patients visiting a GP was slightly higher in adults ≥18 years (62.5%) compared with children and adolescents aged 5–17 years (43.2% GP visits and 10.3% pediatrician visits) and children <5 years of age (32.8% GP visits and 19.7% pediatrician visit).Citation27
Eight publications included information regarding the frequency of complications encountered in an outpatient setting.Citation21,Citation27,Citation28,Citation31,Citation35,Citation37,Citation39,Citation50 The proportion of outpatients requiring hospitalization or experiencing complications varied between 0.6% and 14.8%. Three studies compared complication rates between outpatients and inpatients: between 6.7% and 14.7% of outpatients had complications compared with 82.7% to 92.6% of inpatients.Citation31,Citation37,Citation50
Direct costs
Costs associated with primary care visits ranged between $4 (Brazil) and $148 (United Kingdom [UK]), with most countries reporting average costs <$50 (). Lowest GP costs were reported for studies conducted in South America and Eastern Europe. There were five studies reporting GP costs >$50, all of them being conducted in Europe.Citation21,Citation32,Citation38,Citation46,Citation51 Cost per outpatient visit did not change as a function of age group.Citation6,Citation25,Citation41
Secondary care
Healthcare resource use
Seventeen publications reported the proportion of varicella cases leading to hospitalization and 11 publications reported the hospitalization rate per 100,000 people (). In general, <1% of patients with varicella required hospitalization. Age-specific data showed that the risk of being hospitalized was highest in infants (<1 year) and adults. Hospitalization patterns were similar across countries and regions.
Table 3. Hospitalization rate (%) by age group.
Fifteen publications analyzed the relative proportion of different complications (or no complication) among hospitalized patients, length of stay (LoS) and/or complication-specific costs (). Average LoS in patients without complication ranged between 2.1 and 4.7 days;Citation15,Citation35 variability in average LoS was larger in patients with complications, varying from 1.1 days for patients with febrile convulsions to 48 days for patients with encephalitis or cerebral vasculitis associated with severe sequelae.Citation15,Citation58
Table 4. Hospitalizations, length of stay and costs by specific complication type.
Direct costs
Varicella-based hospitalization costs per episode or per day were reported in 36 studies. Average hospitalization costs per episode varied between approximately $190 and $38,000 while costs per inpatient day ranged between approximately $48 and $1,700 (SI Table S5). When looking at hospitalization costs by complication, the average costs per hospitalization episode ranged between $1,300 and $38,000 depending on the type of complication and average LoS (). One case of multiorgan failure and death was excluded from this analysis since considered an outlier: the patient spent 32 days in the intensive care unit, leading to a total cost of approximately $130,000. For most types of complications, both LoS and cost data were right skewed with few patients requiring prolonged periods of hospitalization. Uncomplicated cases were less costly compared with complicated varicella.Citation15,Citation21,Citation48 Costs were in general lower in younger age groups compared with adults.Citation52
Direct costs contributions
Eleven publications reported the relative contribution of different cost items to total direct costs, revealing differences in the contribution of primary and secondary healthcare to total costs and variations in health seeking behavior between countries (SI Figure S1). Zhou et al. [2005] found that in the US 47.9% and 52.1% of total direct costs due to varicella were generated by hospitalizations and ambulatory visits.Citation55 In a public health impact study in Germany, Banz et al. [2004] found that contributions from ambulatory and inpatient care were similar (21.3% vs 21.7%, respectively) from a payer’s perspective.Citation30 In this study, the payer’s perspective also included indirect costs (57.0%) for reimbursements related to workdays lost effectuated by the healthcare system to parents. In Argentina, 77.4% of total direct costs were generated by visits to hospital outpatient clinics, while visits to the doctor’s office and hospitalizations only accounted for 5.4% and 3.3% of total direct costs.Citation22 In Poland, GP visits accounted for the majority (85.4%) of total direct costs, while hospitalizations accounted for 2.3%.Citation37 Other major contributors were emergency rooms (ER) visits (5.5%) and prescription medication (4.3%). In Spain, primary healthcare visits accounted for 30.2% of total direct costs, while ER visits contributed 16.1% and prescription drugs 39.2% to total direct costs.Citation39 Hospitalization costs were not reported in this study. In another economic evaluation in Spain, total direct cost contributors were analyzed by age groups (≤14 years, >14 years).Citation40 Medical consultations accounted for 50.3% and 38.4% of direct costs in patients aged ≤14 years and >14 years, respectively, while hospitalizations accounted for 12.7% and 24.8%. In both age groups, prescription medications contributed markedly to total direct costs with 33.9% and 34.9%, respectively. In a cost-effectiveness model for Canada, physician visits and prescriptions accounted for 33.9% of total direct costs, while hospitalizations contributed 66.1% to total direct costs.Citation26 In Italy, outpatient and inpatient costs accounted for 51.3% and 44.1% of total direct costs, respectively.Citation32 The remaining costs were due to encephalitis sequelae, analyzed separately by the authors. In a cost evaluation study in Poland, 85.4% of varicella-related direct costs were attributable to GP visits, while hospitalization and prescription medications accounted for 2.3% and 4.3% of total direct costs.Citation37 On the other hand, prescription medications were the major contributor (57.6%) to total direct costs in a Hungarian cost evaluation study; GP visits (17.0%), visits to outpatient clinics (12.6%) and hospitalizations (3.5%) contributed less to total costs.Citation31 Five cost-effectiveness studies included long-term care costs due to sequelae, but only three studies reported cost estimates. Annual costs related to institutional care of people with long-term disability varied between approximately $4,000 (Brazil) and $155,000 (US).Citation25,Citation48 Hsu et al. reported a cost related to long-term sequelae of approximately $494,000 for Taiwan.Citation45
Indirect costs
The most important contributor to indirect costs were workdays lost either due to parents taking time off to care for their sick child or adults themselves having varicella disease (SI Table S6). In preschool children, whether parents took days off work depended on the type of childcare solution: 41% to 51% of parents reported workdays lost if the child attended a childcare center while no workdays were lost with alternative care solutions (SI Table S6).Citation25,Citation35,Citation46 Overall, for children, the average number of workdays lost by the parent varied between 0.27 and 8.8 days/varicella case. In adults, a mean number of 2.5 to 5.0 workdays were lost for uncomplicated varicella cases vs 4.2 to 11.7 days for complicated cases.Citation26,Citation41 Several studies adopted a wider perspective, including also costs incurred by premature death or long-term sequelae, transportation costs and costs related to loss in leisure time.Citation6,Citation25,Citation32,Citation39,Citation45,Citation48
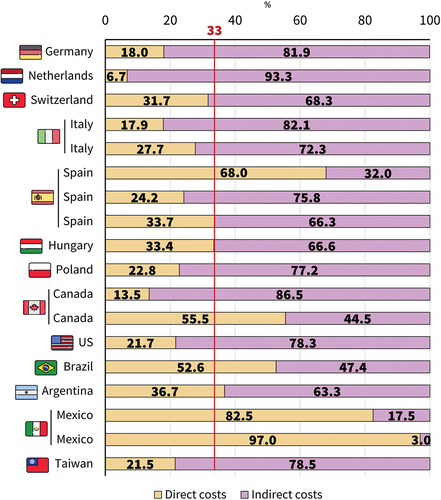
Despite marked variation between studies and/or countries, indirect costs account for approximately two-thirds of overall costs in most studies ().
Discussion
We investigated the data available on HCRU and costs related to varicella and its complications. Forty-four studies were included; 19 observational studies, 4 studies with a mixed design and 21 cost-effectiveness studies. Studies covered different geographic regions, i.e., Europe, North and South America, Middle East and Asia ().
Results of this SLR highlight the burden of varicella to the healthcare system, with the highest HCRU occurring in the primary care setting. Despite the relatively modest cost of primary healthcare encounters (<$50 per visit), these visits contributed between 30% and 85% to overall costs due to the high incidence of varicella. There were marked variations in primary care seeking behavior across countries and regions. While in Canada and the Netherlands, ≥40% of people with varicella had no primary healthcare visits, surveillance data from France, Italy, and Turkey suggested that >90% of cases lead to at least 1 primary healthcare encounter. Similar variations in primary care requirements were found in a previous SLR limited to Europe: between 18% and 100% of varicella cases led to a primary healthcare visit depending on country and age-group.Citation7 These findings may reflect cultural differences in health seeking behavior.Citation63 For example, qualitative research in Norway suggests that Northern European behaviors are more parsimonious in the use of diagnostic tests and prescriptions compared with their Southern counterparts.Citation64
Structural differences in healthcare systems, such as ease of access and copayments, could also influence health seeking behavior.Citation65,Citation66 However, comprehensive data on varicella-related primary healthcare encounters is scarce and often lacks detailed information regarding cost components and/or the impact of complications, age and other factors on HCRU and costs. Further research is needed to describe the burden of varicella and associated costs in the primary care setting across differing health systems, populations, and geographies.
Despite the fact that varicella is usually considered a benign infection, it may lead to complications and hospitalizations both in children and adults. Hospitalization rates were similar across countries and regions and in general <1%. Hospitalization rates were highest in the youngest age group < 1 year, consistent with other SLRs: In Europe, hospitalization rates reported in the SLR by Riera-Montes et al. varied between 0.05% and 3.5% with the highest proportion of hospitalizations observed in children aged <5 years.Citation7 In South America, hospitalization rates of approximately 1% were reported for Colombia and Brazil (pre-vaccination).Citation9
Large variations in hospitalization costs per varicella episode were observed depending on country, type of complication and duration of hospitalization. Average LoS ranged between 2 and 48 days; LoS could be longer in individual patients, mostly driven by the severity of the complication. As a result, hospitalization costs correlated with LoS, manifesting a right skewed distribution. In general, hospitalizations accounted for 2% to 25% of total direct costs. Differences could be due to methodological differences, i.e., the granularity used in breaking down cost components or country-specific differences, i.e., healthcare expenditure, income levels, country-specific treatment guidelines, healthcare resource availabilities, and physician practices.Citation23 Pawaskar et al. reviewed the economic burden of varicella in Europe.Citation8 Average LoS was 4.85 days in high-income countries and 5.89 days in low- and middle-income countries and hospitalization costs accounted for 2% to 21% of total costs, including indirect costs.Citation8 In an SLR specific to Latin America and the Caribbean, average LoS ranged between 3 and 8.5 days.Citation9 Also, severe complications could potentially lead to long-term sequelae such as persisting motion deficits (hemiparesis, nerve palsy, convulsion), scarring, bone and joint defects after infectious complications.Citation58,Citation67 Only few studies reported on costs related to long-term disability and further studies are needed to evaluate the burden of varicella-related sequelae.Citation19,Citation25,Citation29,Citation45,Citation48
Indirect costs, mostly valued through workdays lost, accounted for approximately two thirds of total costs. A major contributor were workdays lost which for uncomplicated varicella varied between 0.3 and 2.5 days/case for children and between 2.5 and 5.0 days/episode in people >15 years. In case of complications, number of workdays lost were higher (up to 26.1 days/case). The proportion of children attending daycare centers, employment rates and average wages were shown to influence indirect costs and lead to differences between countries. In the SLR by Pawaskar, indirect costs contributed between 36% and 77% of total costs incurred by varicella, emphasizing the high burden of varicella due to indirect costs.Citation8 Further research is needed to assess quantify the extent of school and work absenteeism and productivity losses incurred due to varicella.
More precise estimates of the size of varicella-related HCRU and costs would be of interest to policymakers when considering the introduction of varicella vaccine in their universal mass vaccination (UMV) program. Several countries have already introduced UMV since the availability of varicella vaccines on the market, including the US, Canada, Germany, Finland, Italy, Spain, Greece and Luxembourg.Citation68 Other countries (e.g. UK, Switzerland, Netherlands) have been hesitant due to concerns regarding the size of the burden of disease and the economic benefit of preventing a disease perceived as mild.Citation15,Citation36,Citation69,Citation70 The strength of this study is to provide a comprehensive summary of the economic burden of varicella using current evidence from high-quality studies.
There are several limitations to this SLR. We have opted to include both observational and cost-effectiveness studies to retrieve epidemiological, HCRU and costs data. This approach may lead to bias since cost-effectiveness studies rely on a variety of sources including published literature. We have not systematically assessed the quality of these sources. Information about complications and complication rates is non-exhaustive and requires additional research with focus on complications. Reporting of outcomes (e.g., HCRU, costs, complications), and break-down into different age-groups is very heterogeneous: some publications engage into a granular reporting approach both in terms of age category and type of complications; others only include main categories of complications and stratify the population into children and adults.
Social interactions may also vary between countries, leading to different contact matrices and a shift in the peak varicella incidence across age groups. Some complications are rare and mentioned only in few publications. Therefore, no firm conclusion can be drawn with regards to rare complications, HCRU and costs by complication and age. In addition, varicella is a non-notifiable disease in most countries and primary care data are scarce.Citation20 Therefore, estimated varicella incidence relies mainly on seroprevalence studies. This could lead to uncertainty in true varicella incidence and primary healthcare use. Very limited information is available regarding long-term sequelae due to varicella. While rare, persistent deficits have a major impact on families and patients and lead to the need for long-term HCRU, which should be considered when assessing the burden of varicella. Finally, different methods of cost conversion exist, and the choice of method may have an impact on cost results. In this study, costs were first converted to $US using purchase power parity, which will directly reflect the basket of goods and services that could be obtained the same year, in the US.
Conclusion
Varicella and its complications can lead to substantial HCRU and costs in both outpatient and inpatients settings. Further research is needed to characterize complications rates in a population-wide setting, with special attention to HCRU, duration of hospitalization and long-term sequelae to allow for economic burden estimation.
Abbreviations
| CDC: | = | centers for disease control and prevention |
| CHEERS: | = | Consolidated Health Economic Evaluation Reporting Standards |
| ENT: | = | ear/nose/throat |
| ER: | = | emergency room |
| GP: | = | general practitioner |
| HCRU: | = | healthcare resource use |
| HZ: | = | herpes zoster |
| ISPOR: | = | Professional Society for Health Economics and Outcomes Research |
| LoS: | = | length of stay |
| LRTI: | = | lower respiratory tract infection |
| MMAT: | = | mixed methods appraisal tool |
| PRISMA: | = | Preferred Reporting Items for Systematic Reviews and Meta Analyses |
| UMV: | = | universal mass vaccination |
| VZV: | = | varicella zoster virus |
Author contributions
DA, IW, MG, NH, and NJ were involved in the conception and/or the design of the study. DA, IW, MG, NH, and NJ participated in the collection/generation of the study data. All authors had full access to the data and gave approval before submission. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The work described was carried out in accordance with the recommendations of the International Committee of Medical Journal Editors for conduct, reporting, editing, and publication of scholarly work in medical journals.
Data sharing statement
GSK makes available the anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicalstudydatarequest.com. To request access to patient-level data and documents for this study, please submit an enquiry via www.clinicalstudydatarequest.com. Information on GSK’s data sharing commitments and requesting access to anonymized individual participant data and associated documents can be found at www.clinicalstudydatarequest.com. A list of included studies in this systematic literature review is provided in supplementary information.
Supplemental Material
Download PDF (639.9 KB)Acknowledgments
The authors would like to thank the Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. The authors also thank Katrin Spiegel for providing medical writing support.
Disclosure statement
HS, IW, and NJ are employees of GSK. IW and HS hold shares in GSK. DA and NH received a grant and funding from GSK for performing project-related tasks. MG is an independent consultant with GSK affiliated to Hari Group limited (HGL). MG received funding from GSK, for the conduct of the study. Authors declare no other financial and non-financial relationships and activities.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2266225.
Additional information
Funding
References
- World Health Organization (WHO). Varicella: vaccine preventable diseases surveillance standards. Published 2018. https://www.who.int/publications/m/item/vaccine-preventable-diseases-surveillance-standards-varicella.
- Lee T, Suh J, Choi JK, Lee J, Park SH. Estimating the basic reproductive number of varicella in South Korea incorporating social contact patterns and seroprevalence. Hum Vaccines Immunother. 2021;17(8):2488–13. doi:10.1080/21645515.2021.1898917.
- Nardone A, de Ory F, Carton M, Cohen D, van Damme P, Davidkin I, Rota MC, de Melker H, Mossong J, Slacikova M, et al. The comparative sero-epidemiology of varicella zoster virus in 11 countries in the European region. Vaccine. 2007;25(45):7866–72. doi:10.1016/J.VACCINE.2007.07.036.
- Bollaerts K, Riera-Montes M, Heininger U, Hens N, Souverain A, Verstraeten T, Hartwig S. A systematic review of varicella seroprevalence in European countries before universal childhood immunization: deriving incidence from seroprevalence data. Epidemiol Infect. 2017;145(13):2666–77. doi:10.1017/S0950268817001546.
- Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, Grose C, Hambleton S, Kennedy PG, Oxman MN, Seward JF. Varicella zoster virus infection. Nat Rev Dis Prim. 2015;1(1):1–41. doi:10.1038/nrdp.2015.16.
- Banz K, Iseli A, Aebi C, Brunner M, Schmutz AM, Heininger U. Economic evaluation of varicella vaccination in Swiss children and adolescents. Hum Vaccin. 2009;5(12):847–57. doi:10.4161/hv.9898.
- Riera-Montes M, Bollaerts K, Heininger U, Hens N, Gabutti G, Gil A, Nozad B, Mirinaviciute G, Flem E, Souverain A, et al. Estimation of the burden of varicella in Europe before the introduction of universal childhood immunization. BMC Infect Dis. 2017;17(1):1–16. doi:10.1186/s12879-017-2445-2.
- Pawaskar M, Méroc E, Samant S, Flem E, Bencina G, Riera-Montes M, Heininger U. Economic burden of varicella in Europe in the absence of universal varicella vaccination. BMC Public Health. 2021;21(1):1–11. Published online. doi:10.1186/s12889-021-12343-x.
- Arlant LHF, Garcia MCP, Avila Aguero ML, Cashat M, Parellada CI, Wolfson LJ. Burden of varicella in Latin America and the Caribbean: findings from a systematic literature review. BMC Public Health. 2019;19(1):1–18. doi:10.1186/s12889-019-6795-0.
- Damm O, Witte J, Wetzka S, Prosser C, Braun S, Welte R, Greiner W. Epidemiology and economic burden of measles, mumps, pertussis, and varicella in Germany: a systematic review. Int J Public Health. 2016;61(7):847–60. doi:10.1007/s00038-016-0842-8.
- Bozzola E, Bozzola M, Krzysztofiak A, Tozzi AE, Hachem M, El Villani A. Varicella skin complications in childhood: a case series and a systematic review of the literature. Int J Mol Sci. 2016;17(5):688. doi:10.3390/ijms17050688.
- PRISMA. PRISMA, transparent reporting of systematic reviews and meta-analyses. Published 2020. http://www.prisma-statement.org/.
- Hong Q, Fàbregues S, Bartlett G, Boardman F., Cargo M, Dagenais P, Gagnon MP, Griffiths F, Nicolau B, O’Cathain A, et al. The mixed methods appraisal tool (MMAT) version 2018 for information professionals and researchers. Educ Inf. 2018;34(4):285–91. doi:10.3233/EFI-180221.
- ISPOR. Consolidated health economic evaluation reporting standards (CHEERS) 2022 resources. Published 2022. https://www.ispor.org/heor-resources/good-practices/cheers.
- Bernal JL, Hobbelen P, Amirthalingam G. Burden of varicella complications in secondary care, England, 2004 to 2017. Eurosurveillance. 2019;24(42). doi:10.2807/1560-7917.ES.2019.24.42.1900233.
- Centers for Disease Control and Prevention (CDC). Chickenpox (varicella). Published 2021. https://www.cdc.gov/chickenpox/about/complications.html
- OECD. Health care prices and purchasing power parities. Published 2020. https://www.oecd.org/health/health-purchasing-power-parities.htm
- FRED EDSLF. Consumer price index for all urban consumers: medical care in U.S. Published 2020. https://fred.stlouisfed.org/series/CPIMEDSL
- Wutzler P, Neiss A, Banz K, Goertz A, Bisanz H. Can varicella be eliminated by vaccination? Potential clinical and economic effects of universal childhood varicella immunisation in Germany. Med Microbiol Immunol. 2002;191(2):89–96. doi:10.1007/s00430-002-0123-4.
- Wagenpfeil S, Neiss A, Banz K, Wutzler P. Empirical data on the varicella situation in Germany for vaccination decisions. Clin Microbiol Infect. 2004;10(5):425–30. doi:10.1111/j.1469-0691.2004.00853.x.
- Haugnes H, Flem E, Wisløff T. Healthcare costs associated with varicella and herpes zoster in Norway. Vaccine. 2019;37(29):3779–84. doi:10.1016/j.vaccine.2019.05.063.
- Giglio N, Monsanto H, Rampakakis E, Yang HK, Kuter BJ, Wolfson LJ. Economic burden of varicella in children 1–12 years of age in Argentina, 2009–2014. J Med Econ. 2018;21(4):416–24. doi:10.1080/13696998.2018.1431919.
- Wolfson LJ, Castillo ME, Giglio N, Meszner Z, Molnar Z, Vazquez M, Wysocki J, Altland A, Kuter BJ, Rickard J, et al. Varicella healthcare resource utilization in middle income countries: a pooled analysis of the multi-country MARVEL study in Latin America & Europe. Hum Vaccines Immunother. 2019;15(4):932–41. doi:10.1080/21645515.2018.1559687.
- Bilcke J, Christiaan M, Ogunjimi B, van Hoek AJ, Lejeune O, Callens M, Vancorenland S, Van Kerschaver E, Callaert K, Hens N, et al. Cost-utility of Vaccination against Chickenpox in Children and against Herpes Zoster in Elderly in Belgium. Brussels: Health Technology Assessment (HTA), Belgian Health Care Knowledge Centre (KCE); 2010. KCE Reports 151.
- Valentim J, Sartori AMC, de Soárez PC, Amaku M, Azevedo RS, Novaes HMD, de Soárez PC. Cost-effectiveness analysis of universal childhood vaccination against varicella in Brazil. Vaccine. 2008;26(49):6281–91. doi:10.1016/j.vaccine.2008.07.021.
- Brisson M, Edmunds WJ. The cost-effectiveness of varicella vaccination in Canada. Vaccine. 2002;20(7–8):1113–25. doi:10.1016/S0264-410X(01)00437-6.
- De Wals P, Blackburn M, Guay M, Bravo G, Blanchette D, Douville-Fradet M. Burden of chickenpox on families: a study in Quebec. Can J Infect Dis. 2001;12(1):27–32. doi:10.1155/2001/361070.
- Littlewood KJ, Ouwens MJNM, Sauboin C, Tehard B, Alain S, Denis F. Cost-effectiveness of routine varicella vaccination using the measles, mumps, rubella and varicella vaccine in France: an economic analysis based on a dynamic transmission model for varicella and herpes zoster. Clin Ther. 2015;37(4):830–41.e7. doi:10.1016/j.clinthera.2015.01.006.
- Banz K, Wagenpfeil S, Neiss A, Goertz A, Staginnus U, Vollmar J, Wutzler P. The cost-effectiveness of routine childhood varicella vaccination in Germany. Vaccine. 2003;21(11–12):1256–67. doi:10.1016/S0264-410X(02)00431-0.
- Banz K, Wagenpfeil S, Neiss A, Hammerschmidt T, Wutzler P. The burden of varicella in Germany: potential risks and economic impact. Eur J Heal Econ. 2004;5(1):46–53. doi:10.1007/s10198-003-0200-7.
- Meszner Z, Molnar Z, Rampakakis E, Yang HK, Kuter BJ, Wolfson LJ. Economic burden of varicella in children 1-12 years of age in Hungary, 2011-2015. BMC Infect Dis. 2017;17(1):1–11. doi:10.1186/s12879-017-2575-6.
- Thiry N, Beutels P, Tancredi F, Romanò L, Zanetti A, Bonanni P, Gabutti G, Damme PV. An economic evaluation of varicella vaccination in Italian adolescents. Vaccine. 2004;22(27–28):3546–62. doi:10.1016/j.vaccine.2004.03.043.
- Coudeville L, Brunot A, Giaquinto C, Lucioni C, Dervaux B. Varicella vaccination in Italy: an economic evaluation of different scenarios. Pharmacoeconomics. 2004;22(13):839–55. doi:10.2165/00019053-200422130-00003.
- Melegaro A, Marziano V, Del Fava E, Poletti P, Tirani M, Rizzo C, Merler S. The impact of demographic changes, exogenous boosting and new vaccination policies on varicella and herpes zoster in Italy: a modelling and cost-effectiveness study. BMC Med. 2018;16(1):1–13. doi:10.1186/s12916-018-1094-7.
- Wolleswinkel-van den Bosch JH, Speets AM, Rümke HC, Gumbs PD, Fortanier SC. The burden of varicella from a parent’s perspective and its societal impact in the Netherlands: an Internet survey. BMC Infect Dis. 2011;11(1):320. doi:10.1186/1471-2334-11-320.
- Boot HJ, de Melker HE, Stolk EA, de Wit GA, Kimman TG. Assessing the introduction of universal varicella vaccination in the Netherlands. Vaccine. 2006;24(37–39):6288–99. doi:10.1016/j.vaccine.2006.05.071.
- Wysocki J, Malecka I, Stryczynska-Kazubska J, Rampakakis E, Kuter B, Wolfson LJ. Varicella in Poland: economic burden in children 1-12 years of age in Poland, 2010-2015. BMC Public Health. 2018;18(1):1–0. doi:10.1186/s12889-018-5298-8.
- Blasco GP, Blasco Pérez-Aramendía J. A cost-benefit analysis of varicella vaccination in Aragón. Arch Argent Pediatr. 2017;115(5):432–8. doi:10.5546/aap.2017.432.
- Díez-Domingo J, Aristegui J, Calbo F, Gonzalez-Hachero J, Moraga F, Peña Guitian J, Ruiz Contreras J, Torrellas A. Epidemiology and economic impact of varicella in immunocompetent children in Spain. A nation-wide study. Vaccine. 2003;21(23):3236–9. doi:10.1016/S0264-410X(03)00264-0.
- Lenne X, Diez Domingo J, Gil A, Ridao M, Lluch JA, Dervaux B. Economic evaluation of varicella vaccination in Spain-results from a dynamic model. Vaccine. 2006;24(47–48):6980–9. doi:10.1016/j.vaccine.2006.04.051.
- Wolfson LJ, Daniels VJ, Pillsbury M, Kurugöl Z, Yardimci C, Kyle J, Dinleyici EC. Cost-effectiveness analysis of universal varicella vaccination in Turkey using a dynamic transmission model. PloS One. 2019;14(8):1–26. doi:10.1371/journal.pone.0220921.
- Turel O, Bakir M, Gonen I, Hatipoglu N, Aydogmus C, Hosaf E, Siraneci R. Children hospitalized for varicella: complications and cost burden. Value Heal Reg Issues. 2013;2(2):226–30. doi:10.1016/j.vhri.2013.05.003.
- Van Hoek AJ, Melegaro A, Gay N, Bilcke J, Edmunds WJ. The cost-effectiveness of varicella and combined varicella and herpes zoster vaccination programmes in the United Kingdom. Vaccine. 2012;30(6):1225–34. doi:10.1016/j.vaccine.2011.11.026.
- Walker JL, Andrews NJ, Mathur R, Smeeth L, Thomas SL. Trends in the burden of varicella in UK general practice. Epidemiol Infect. 2017;145(13):2678–82. doi:10.1017/S0950268817001649.
- Hsu HC, Lin RS, Tung TH, Chen THH. Cost-benefit analysis of routine childhood vaccination against chickenpox in Taiwan: decision from different perspectives. Vaccine. 2003;21(25–26):3982–7. doi:10.1016/S0264-410X(03)00270-6.
- Bilcke J, Ogunjimi B, Marais C, De Smet F, Callens M, Callaert K, Van Kerschaver E, Ramet J, Van Damme P, Beutels P, et al. The health and economic burden of chickenpox and herpes zoster in Belgium. Epidemiol Infect. 2012;140(11):2096–109. doi:10.1017/S0950268811002640.
- Esmaeeli S, Yaghoubi M, Nojomi M. Cost‑effectiveness of varicella vaccination program in Iran. Int J Prev Med. 2017;8(103):1–5. doi:10.4103/ijpvm.IJPVM_295_16.
- Zhou F, Ortega-Sanchez IR, Guris D, Shefer A, Lieu T, Seward JF. An economic analysis of the universal varicella vaccination program in the United States. J Infect Dis. 2008;197(Suppl 2):S156–S64. doi:10.1086/522135.
- Brisson M, Edmunds WJ, Law B, Gay NJ, Walld R, Brownell M, Roos LL, De Serres G. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect. 2001;127(2):305–14. doi:10.1017\S0950268801005921.
- Vazquez M, Perezbolde C, Monsanto H, Rampakakis E, Altland A, Wolfson LJ, Pastor V. The economic burden of varicella in Mexico. Value Heal. 2018;21:S152. doi:10.1016/j.jval.2018.04.1051.
- van Hoek AJ, Gay N, Melegaro A, Opstelten W, Edmunds WJ, van Hoek AJ. Estimating the cost-effectiveness of vaccination against herpes zoster in England and Wales. Vaccine. 2009;27(9):1454–67. doi:10.1016/j.vaccine.2008.12.024.
- Guillen JM, Gil-Prieto R, Alvaro A, Gil A. Burden of adult varicella hospitalizations in Spain (2001-2007). Hum Vaccin. 2010;6(8):659–63. doi:10.4161/hv.6.8.12014.
- Hobbelen PHF, Stowe J, Amirthalingam G, Miller L, van Hoek AJ. The burden of hospitalisation for varicella and herpes zoster in England from 2004 to 2013. J Infect. 2016;73(3):241–53. doi:10.1016/j.jinf.2016.05.008.
- Widgren K, Giesecke J, Lindquist L, Tegnell A. The burden of chickenpox disease in Sweden. BMC Infect Dis. 2016;16(1):1–8. doi:10.1186/s12879-016-1957-5.
- Zhou F, Harpaz R, Jumaan AO, Winston CA, Shefer A. Impact of varicella vaccination on health care utilization. J Am Med Assoc. 2005;294(7):797–802. doi:10.1001/jama.294.7.797.
- Tseng HF, Tan HF, Chang CK. Varicella Epidemiology and cost-effectiveness analysis of universal varicella vaccination program in Taiwan. Southeast Asian J Trop Med Public Heal. 2005;36(6):1450–8.
- Yawn BP, Yawn RA, Lydick E. Community impact of childhood varicella infections. J Pediatr. 1997;130(5):759–65. doi:10.1016/S0022-3476(97)80019-4.
- Losurdo G, Bertoluzzo L, Canale F, Timitilli A, Bondi E, Castagnola E. Varicella and its complications as cause of hospitalization varicella e sue complicanze come causa. Le Infez Med. 2005;4:229–34.
- Macias-Parra M, Rodriguez-Weber MA, Moreno-espinosa S, Ceron-Trujillo B, Ojeda-Diezbarroso K, DeAntonio R, Cortes-Alcala R, Martinez G, Carreño-Manjarrez R, Jiménez-Juárez RN Economic burden of varicella complications in two referral centers in Mexico. Hum Vaccines Immunother. 2018;14(12):2950–4. doi:10.1080/21645515.2018.1504541.
- Bozzola E, Guolo S, Macchiarulo G, Festa L, Spina G, Krzysztofiak A, Grandin A, Bozzola M, Raponi M, Villani A, et al. Hospitalization for acute cerebellitis in children affected by varicella: how much does it cost? Ital J Pediatr. 2020;46(1):1–4. doi:10.1186/s13052-020-00875-8.
- Azzari C, Massai C, Poggiolesi C, Indolfi G, Spagnolo G, De Luca M, Gervaso P, de Martino M, Resti M. Cost of varicella-related hospitalisations in an Italian paediatric hospital: comparison with possible vaccination expenses. Curr Med Res Opin. 2007;23(12):2945–54. doi:10.1185/030079907X242610.
- Büyükcam A, Çelik M, Cengiz M, Ceyhan M, Kara A. The chickenpox complications and financial burden in healthy children and with underlying a comorbidity during the pre vaccine and post vaccine era in a University hospital. Value Heal. 2016;19(7):A406–A7. doi:10.1016/j.jval.2016.09.349.
- Van Lier A, Van Erp J, Donker GA, Van der Maas NAT, Sturkenboom MCJM, De Melker HE. Low varicella-related consultation rate in the Netherlands in primary care data. Vaccine. 2014;32(28):3517–24. doi:10.1016/J.VACCINE.2014.04.034.
- Herrero-Arias R, Diaz E. A qualitative study on the experiences of southern European immigrant parents navigating the Norwegian healthcare system. Int J Equity Health. 2021;20(1):1–12. doi:10.1186/s12939-021-01384-8.
- Landsem MM, Magnussen J. The effect of copayments on the utilization of the GP service in Norway. Social Sci Med. 2018;205:99–106. doi:10.1016/J.SOCSCIMED.2018.03.034.
- Johansson N, Jakobsson N, Svensson M. Effects of primary care cost-sharing among young adults: varying impact across income groups and gender. Eur J Heal Econ. 2019;20(8):1271–80. doi:10.1007/s10198-019-01095-6.
- Ziebold C, von Kries R, Lang R, Weigl J, Schmitt H. Severe complications of varicella in previously healthy children in Germany: a 1-year survey. Pediatrics. 2001;108(e79):1–6. doi:10.1542/peds.108.5.e79.
- Trucchi C, Gabutti G, Rota MC, Bella A. Burden of varicella in Italy, 2001–2010: analysis of data from multiple sources and assessment of universal vaccination impact in three pilot regions. J Med Microbiol. 2015;64(11):1387–94. doi:10.1099/jmm.0.000061.
- Iseli A, Aebi C, Banz K, Brunner M, Schmutz AM, Heininger U. Prospective surveillance of varicella-zoster virus infections in an out-patient setting in Switzerland. Hum Vaccin. 2009;5(12):843–6. doi:10.4161/hv.9897.
- Harpaz R, Van Hoek AJ. Point–counterpoint: the Hope-Simpson hypothesis and its implications regarding an effect of routine varicella vaccination on herpes zoster incidence. JID. 2018;218(2):S57–S62. doi:10.1093/infdis/jiy418.