ABSTRACT
This study aimed to conduct a bibliometric analysis in the field of bladder cancer (BC) immunotherapy, and explore the research trends, hotspots and frontiers from 2000 to 2022. VOSviewer software was used to analyze the collaborative relationships between authors, institutions, countries/regions, and journals through citation, co-authorship, and co-citation analysis, to identify research hotspots and frontiers in this field. Researchers based in the United States of America have published a total of 627 papers with 27,308 citations. Indeed, the USA ranked first among the top 10 most active countries and showed the most extensive collaboration with other countries. The University of Texas MD Anderson CANC CTR has published 58 articles, making it the top most institution in terms of published articles and active collaborative research. Kamat AM and Lamm DL were the most active and co-cited authors with 28 papers and 980 co-citations, respectively. Chang Yuan and Xu le were the most active collaborative authors with a total link strength of 195. The J UROLOGY was the most active and frequently co-cited journal, with 100 papers and 6,668 co-citations. Studies of BC immunotherapy can be broadly classified into three categories: “basic research”, “clinical trial”, and “prognosis”. Our findings provide an overview of the research priorities and future directions of BC immunotherapy. Tumor microenvironment and immune checkpoint inhibitors (ICIs) of BC, as well as the combination of ICIs with other drugs, may become the main direction of future research.
Introduction
Bladder cancer (BC) is the fourth most common and eighth most lethal malignancy among men in the United States of America (USA), with an estimated 81,180 new cases and 17,100 deaths reported in 2022.Citation1 In patients with muscle-invasive bladder cancer (MIBC), despite the survival advantage conferred by neoadjuvant chemotherapy, metastasis continues to remains pose a significant therapeutic challenge, with a 5-year overall survival (OS) rate of only 4.8%.Citation2,Citation3
Cancer immunotherapy augments the body’s anti-tumor immune response and improves its ability to eliminate cancer cells. The introduction and mainstreaming of immunotherapy have transformed the cancer treatment landscape over the past decades. In particular, cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) targeted immunotherapeutic agents represent a breakthrough in tumor immunotherapy.Citation4 Non-targeted immunotherapeutic agents such as Bacille Calmette-Guerin (BCG), interferon, and interleukin (IL) are also widely used to treat BC.Citation5 For example, BCG has shown successful results in the treatment of NMIBC for more than 30 years. BC cells may play a role in the attachment and internalization of BCG. The subsequent presentation of BCG and cancer antigens to immune system cells may induce the release of cytokines and chemokines, contributing to its therapeutic effect.Citation6,Citation7 Therefore, immunotherapy is of great significance in BC and is expected to revolutionize the landscape of BC therapeutics.Citation8,Citation9
Bibliometrics entails a combination of mathematical and statistical methods for quantitative analysis of published literature in a specific field. This approach has been widely used for the analysis of research output, and to identify the hotspots and research trends. Bibliometrics helps to understand the knowledge base and research frontiers of a particular research field.Citation10,Citation11 Bibliometrics is widely used in the field of assessment research, to evaluate the research strength and influence of a research institution or researcher, and to identify hot topics and emerging research directions in a certain field. At the same time, bibliometrics also and plays an important role in the biomedical field.
The past decades have witnessed many scientific advances in BC immunotherapy research. However, no bibliometric analysis has been performed in this field. In this study, we performed a bibliometric analysis of studies related to BC immunotherapy published in the past 23 years. Our findings may provide insights into the current state of the field and identify new research directions.
Materials and methods
Data source and search strategy
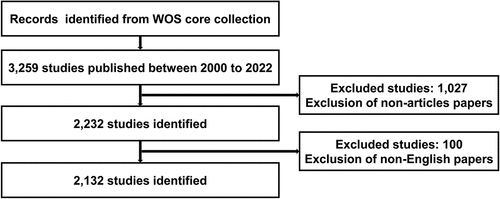
We searched for all publications related to BC immunotherapy in the Web of Science (WOS) core collection database from 1 January 2000 to 31 December 2022. The search strategies were as follows: TS = ((“bladder cancer” OR “bladder carcinoma”) AND (“immunotherapy” OR “immunotherapeutic”)). The types of documents were limited to articles, and the language of publication was limited to English. Detailed data retrieval and inclusion procedures are shown in .
Data collection and analysis
The file information was downloaded from the WOS core collection database. Full records and cited referenc es (titles, keywords, authors, journals, abstracts, references, and citations, etc.) were obtained. The documents were downloaded in TXT format. VOSviewer 1.6.18 software was used to analyze the collaborative relationships among authors, institutions, countries/regions, and journals through citation, co-authorship, and co-citation. The VOSviewer also produced a cluster map showing high-frequency co-cited references and keywords. These maps were presented through network, overlay, and density visualization. In network visualization, different colors indicate different clusters, the size of nodes is positively correlated with the frequency of total link strength or occurrence, and the straight lines between nodes represent the strength of the connection.
Microsoft Excel 2019 was used to analyze and plot the annual number of publications related to BC immunotherapy, as well as the top 10 most active authors, co-cited authors, institutions, and countries/regions, including the number of publications and citations. In addition, main journal-related data such as the number of published papers, countries, total citations, impact factor (IF), and journal citation reports were also analyzed and plotted. The top 10 co-cited articles related to BC immunotherapy, including authors, countries, year of publication, corresponding journals, and IF, are presented in Excel tables.
Results
Summary of the extracted literature
Analysis of publication outputs
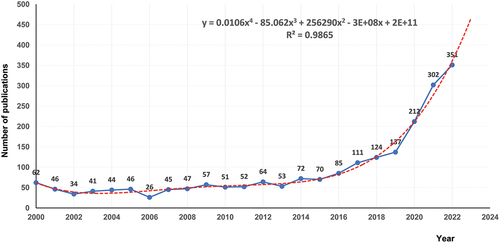
A total of 2,132 publications related to BC immunotherapy between 2000 and 2022 were retrieved from the WOS core collection database. As shown in , the lowest number of published papers in any year was 26 in 2006, and the highest was 351 in 2022, with an annual average of 93. A statistically significant relationship between year and number of publications was observed by fitting a mathematical function to the curve of annual number of publications (R2 = 0.9865). According to the fitting curve, 470 papers related to BC immunotherapy are expected to be published globally in 2023. There was no obvious trend of change in the number of published articles from 2000 to 2015. However, the number of articles published showed an increasing trend since 2015. Therefore, this topic has received great attention in recent years with widening research prospects.
Analysis of countries/regions
These publications were contributed by 70 countries/regions. The top 10 most active countries/regions involved in research related to BC immunotherapy are listed in . The USA is the most active country in this field, with 627 publications and 27,308 citations, far more than any other country. The People’s Republic of China (China) ranked second with 509 publications. Japan, Germany, and Italy ranked third, fourth, and fifth, respectively. This suggests a close relation between the number of publications and the level of economic development.
Table 1. Top 10 most active countries/regions of BC immunotherapy.
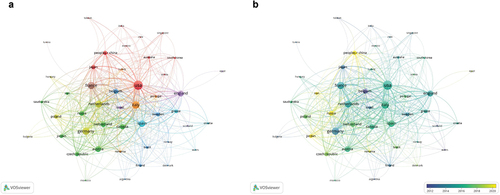
A total of 46 countries/regions with more than five publications each were selected for the co-authorship analysis. In the network visualization map (), the USA was the most productive contributor and the most extensive collaborator. In the field of BC immunotherapy, the USA has linkages with 43 countries, with a total link strength of 617. The USA has the closest cooperation with Italy, with a link strength of 65, followed by Germany and China.
Figure 3. Bibliometric analysis of the co-authorship of countries/regions in the field of BC immunotherapy. (a) Network visualization map of countries/regions collaborations in the field of BC immunotherapy. (b) Overlay visualization map of countries/regions collaborations in the field of BC immunotherapy.
Notably, a collaborative article titled “MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer” was published in the journal Nature in 2014 by researchers based in England, the USA, France, and Spain. This article has been cited 1,816 times, making it the most consistently cited and popular article. The study was a phaseIclinical trial of anti-PDL1 antibody MPDL3280A in the treatment of metastatic urothelial BC, which inhibits the interaction of PD-L1 with PD-1 (PDCD1) and B7.1 (CD80).Citation12
Austria ranked eighth in terms of total link strength, with only 41 publications. In contrast, China ranked second in terms of the number of publications (509), but in terms of the total link strength, it ranked 13th in the world. This likely reflects the fact that Austria produced many high-quality articles in close collaboration with other countries, while many articles in China were independently authored by Chinese scholars, and the degree of collaboration with scholars from other countries was not as good as that of some countries. China has a large population base and a large number of researchers in this field. Some related problems in this field can be solved through their own team. Moreover, researchers from some institutions may have relatively limited contact with foreign scholars due to various reasons, mainly focusing on their own group’s scientific research or collaborating with other domestic institutions. For example, researchers from Austria, Netherlands, Italy, and other countries have put forward some constructive suggestions on the optimal dose and duration of intravesical BCG vaccine for NMIBC through clinical trials.Citation13 These findings demonstrate that cooperation between countries is a powerful catalyst for knowledge renewal and innovation.
As can be observed in the overlay visualization map (), 12 countries/regions, including Germany, Japan, and Belgium, initiated research on BC immunotherapy before 2014. They were among the first countries to conduct research in this field. Since 2019, only a few more countries have joined the research in this field, including China, Russia, Romania, Jordan, and Iran. The recent years showed a declining trend of cooperation between countries/regions.
Analysis of institutions
There were 2,860 institutions involved in these publications. As shown in , the institution with the greatest output was the University of Texas MD Anderson Cancer Center, which published 58 publications. The MEM Sloan Kettering Cancer Center, second with 52 publications, was the most cited institution, with 3,393 citations. Of the top 10 institutions, the USA and China account for five each.
Table 2. Top 10 most active institutions related to BC immunotherapy.
A total of 106 institutions with more than 10 publications were selected for co-authorship analysis. In the network visualization map (), the University of Texas MD Anderson Cancer Center was the most collaborative institution, linking 43 institutions with a total link strength of 100. This was followed by MEM Sloan Kettering Cancer Center with a total link strength of 97. These two institutions have their research teams and are close partners. One of their studies demonstrated the tolerability and promising antitumor activity of pembrolizumab monotherapy in patients with BCG-nonresponsive NMIBC.Citation14 By calculating the thickness of the connection line between the two institutions in , we found that the strongest link strength was between Shanghai Jiao Tong University and Fudan University. One of the studies, demonstrated the immunosuppressive and tumor-promoting effects of dendritic cell-specific C-type lectin (+) tumor-associated macrophages, indicating their potential use as prognostic indicators and therapeutic targets in MIBC.Citation15
Figure 4. Bibliometric analysis of the co-authorship of institutions in the field of BC immunotherapy. (a) Network visualization map of collaborations among institutions in the field of BC immunotherapy. (b) Overlay visualization map of collaborations among institutions in the field of BC immunotherapy.
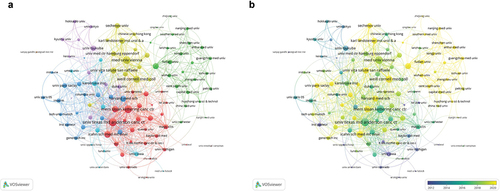
The distribution of institutions in different periods is presented in . It was observed that the University of Iowa, Institut Pasteur, and the University of Amsterdam were the major institutions conducting research in the field of BC immunotherapy before 2012. After 2020, many other institutions were involved in research in this field, including Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Fudan University, and the University of Washington.
Analysis of authors and co-cited authors
These 2,132 articles were authored by a total of 11,559 authors. The information of authors and co-cited authors was analyzed, and a density visualization map of the co-cited authors was drawn by VOSviewer software (). The number of co-citation authors in the figure showed a positive correlation with the density. In addition, the top 10 most active authors and the top 10 co-cited authors are listed in . Kamat AM is the most prolific author with 28 articles. Shariat SF has published six publications and is the most cited author with 3,324 citations. Among the top 10 co-cited authors, Lamm DL ranks first with 980 co-citations, much higher than other co-cited authors, followed by Powles T (552 co-citations), and Sylvester RJ (535 co-citations). These authors have made outstanding contributions to the field of BC immunotherapy.
Figure 5. Bibliometric analysis of the co-cited authors and co-authorship of authors in the field of BC immunotherapy. (a) Density visualization map of co-cited authors in the field of BC immunotherapy. (b) Network visualization map of authors collaboration in the field of BC immunotherapy. (c) Overlay visualization map of authors collaboration in the field of BC immunotherapy.
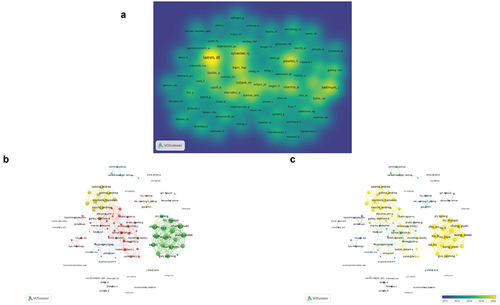
Table 3. Top 10 most active authors and co-cited authors related to BC immunotherapy.
A total of 105 authors who had published more than seven times were selected for network visualization analysis (). Chang Yuan and Xu le, who ranked first with a combined link strength of 195, belong to a research group. Their team also included Liu li, Wang Zewei, and Zhu Yu, and showed a close cooperative relationship. A study by their group showed that TIGIT(+) CD8(+) T-cell abundance can be used as an independent predictor of clinical outcome and a predictive biomarker of poor response to MIBC adjuvant chemotherapy.Citation16
It was observed that six authors Zu Xiongbing, Fradet Yves, Yao Xudong, Allory Yves, Chang In ho, and Bunimovich-Mendrazitsky S focused on individual studies and did not form a team. However, they have made some achievements in the field of BC immunotherapy through their own efforts. For example, a retrospective study by Zu et al.Citation17 compared the efficacy of neoadjuvant immunotherapy (tislelizumab), neoadjuvant chemotherapy (gemcitabine and cisplatin), and neoadjuvant combination therapy (tislelizumab+GC) and found that neoadjuvant combination therapy achieved the highest complete response rate and pathological downstaging rate compared to neoadjuvant immunotherapy or chemotherapy. Chang et al.Citation18 designed novel nanoparticles composed of liposome-encapsulated BCG cell wall skeleton (BCG-CWS). Compared with nonenveloped BCG-CWS, encapsulated BCG-CWS nanoparticles were more found to be effective in delivering BCG-CWS to the bladder and inhibiting tumor growth.
The overlay visualization map () demonstrates the increasing interest of several research teams and individuals in the field of BC immunotherapy in recent years. For example, Chang Yuan and Necchi A’s teams are in yellow in the figure, indicating their extensive contribution to this field in recent years.
Analysis of journals and co-cited journals
A total of 553 journals have published articles related to the field of BC immunotherapy; of these, 92 journals have published more than five papers each. lists the top 10 most prolific journals related to BC immunotherapy. The J UROLOGY has published 100 papers with 8,028 citations, far more than any other academic journal. We found that the EUR UROL from the Netherlands had the highest IF in 2023. The second was FRONT IMMUN from Switzerland. The IFs of the above three journals are 6.6, 23.4, and 7.3, respectively, and all three belong to the top quartile (Q1). Six out of the top 10 prolific journals were from the USA. This indicated that the USA had a high position in the field of BC immunotherapy, providing a reliable platform for the publication of related research papers.
Table 4. Top 10 most active journals related to BC immunotherapy.
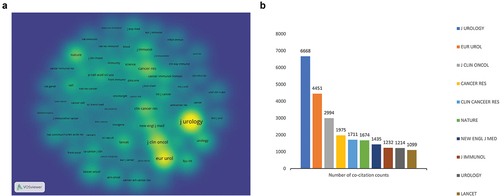
The density visualization map of co-cited journals was drawn by VOSviewer (). To date, 11 co-cited journals have been cited more than 1,000 times. The top 10 co-cited journals are plotted in . We noted that the J UROLOGY was the most co-cited journal with a total of 6,668 citations, far more than other co-cited journals. This was followed by EUR UROL (4,451 co-citations) and the J CLIN ONCOL (2,994 co-citations) in 2022. To our surprise, the J UROLOGY ranked first both in terms of being the most prolific and most co-cited journal, indicating its high reputation in the field of BC immunotherapy. Moreover, the three journals were cited in both prolific and co-cited journals, including the J UROLOGY, EUR UROL, and UROLOGY. This result indicated a close relationship between journals and co-cited journals, and that prolific journals were more likely to be co-cited. These data will help scientists select journals when submitting manuscripts related to BC immunotherapy in the future.
Analysis of the basic content of extracted literature
Analysis of co-cited references
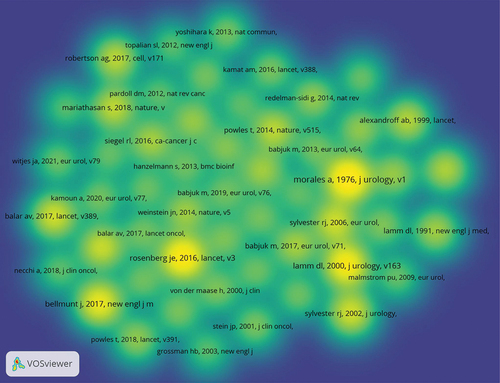
The density visualization map of the 53 most co-cited references was drawn by VOSviewer software (). We also summarized the top 10 co-cited references, including article title, corresponding author, country, year, co-citation frequency, journal, and IF (2023) (). In 1976, Morales et al.Citation19 published a paper in the J UROLOGY entitled “Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors,” which has been co-cited 275 times. This was followed by articles by Rosenberg et al.Citation20 and Lamm et al.Citation21 with 247 and 225 co-cited references, respectively. It was observed that 6 of the top 10 co-cited references were from the USA, two were from Belgium, and two were from Canada. In terms of journals, three were from the J UROLOGY, two from LANCET, and one each from the NEW ENGL J MED, LANCET ONCOL, EUR UROL, CELL, and NATURE. Sylvester RJ authored two papers among the top 10 articles.
Table 5. Top 10 co-cited references related to BC immunotherapy.
These 10 articles have had a significant impact on the field of BC immunotherapy. For example, in the BCG study, Morales et al.Citation19 found favorable changes in recurrence patterns after BCG treatment in nine patients with relapsed superficial BC. This was a milestone study that demonstrated the potential use of BCG for the treatment of BC, laying a foundation for further research. Lamm et al.Citation22 showed that maintenance BCG immunotherapy for BC was beneficial compared with standard induction therapy in patients with carcinoma in situ and in patients with stage Ta and T1. In the study by Sylvester et al.Citation23 intravesical BCG injection was found to significantly reduce the risk of progression after transurethral resection in patients with superficial BC receiving BCG maintenance therapy. Sharma et al.Citation24 reported a phase 2 trial of nivolumab in patients with metastatic UC who had received platinum therapy. The results showed that nivolumab was associated with an acceptable safety profile in previously treated patients with metastatic UC.
Analysis of keywords
The co-occurrence keywords network visualization map was composed of 146 high-frequency keywords, and the extraction frequency was more than 25 (). The keywords were divided into three categories: cluster 1: “basic research” (red), including expression, proliferation, induction, and growth; cluster 2: “clinical trials” (green), including safety, survival, PD-L1, phase-III trial, and pembrolizumab; cluster 3: “prognosis” (blue), including progression, risk, recurrence, complications, and BCG. Among the several high-frequency keywords, the co-occurrence frequency was highest for five important keywords: BCG, expression, progression, recurrence, and survival. These keywords indicate the key research directions in the field of BC immunotherapy.
Figure 8. Bibliometric analysis of the co-occurrence of all keywords in the field of BC immunotherapy. (a) Network visualization map of high frequency keywords in the field of BC immunotherapy. (b) Overlay visualization map of high frequency keywords in the field of BC immunotherapy.
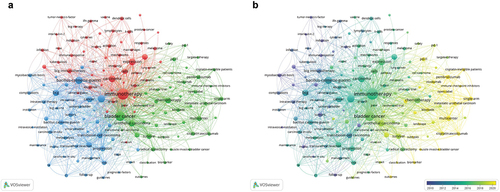
According to the overlay visual analysis of the keywords, the research direction of BC immunotherapy researchers may have shifted from basic research and prognosis to clinical research on new drugs (). It was observed that keywords such as pembrolizumab, atezolizumab, ICIs, tumor microenvironment (TME), landscape, and outcomes have become the research hotspots of BC immunotherapy in recent years.
Research hotspots and frontiers
Based on the above results, we listed some research hotspots and frontiers in the field of BC immunotherapy. According to the different stages of BC, immunotherapy drugs can be divided into three categories: “neoadjuvant,” “adjuvant,” and “metastatic.”
Neoadjuvant immunotherapy for BC
The successful outcomes of blockade of immune checkpoints targeting CTLA-4, PD-1, and PDL1 have made it an important direction of research on neoadjuvant immunotherapy for BC.Citation4 Carthon et al.Citation25 conducted the first pre-operative clinical trial of ipilimumab in 12 patients with localized urothelial BC and demonstrated a tolerable safety profile for anti-CTLA-4 therapy in a preoperative setting. In the ABACUS trial, two cycles of atezolizumab before radical cystectomy, 27 of 88 patients (31%) with muscle-invasive UC in an evaluable cohort receiving atezolizumab had a pathologic complete remission (pCR), and the pCR rate correlated with PD-L1 expression.Citation26 In a subsequent study of the ABACUS trial in 2022, the two-year DFS and OS were 68% (95% CI 58–76) and 77% (95% CI 68–85), respectively. This study found that patients with BC who received immunotherapy prior to cystectomy have good long-term outcomes.Citation27 In 2018, the PURE-01 study indicated that pembrolizumab may be a valuable neoadjuvant therapy for MIBC patients with PD-L1 positivity or high tumor mutation burden.Citation28 Two years later, the first survival outcomes from the PURE-01 study showed that postoperative event-free survival was better in patients who received pembrolizumab before radical cystectomy compared to those who received neoadjuvant chemotherapy before radical cystectomy, regardless of the biomarker status.Citation22 Dijk et al.Citation29 showed that a combination of CTLA-4 (ipilimumab) and PD-1 (nivolumab) blockade may provide an effective preoperative treatment strategy for locally advanced UC. Gao et al.Citation30 reported preliminary safety, efficacy, and biomarker data for neoadjuvant therapy in combination with anti-PD-L1 (durvalumab) plus anti-CTLA-4 (tremelimumab). This provided a further standard of care for patients with localized UC, especially high-risk patients who are not eligible for cisplatin therapy.
Adjuvant immunotherapy for BC
Adjuvant intravesical BCG therapy is a mature and successful adjuvant immunotherapy for superficial BC. BCG is the only Food and Drug Administration (FDA)-approved first-line treatment for patients with NMIBC.Citation31 The era of BC adjuvant immunotherapy was heralded by Morales et al.Citation19 who first reported the use of BCG in the treatment of BC in 1976. A meta-analysis by Han et al.Citation32 showed that adjuvant intravesical BCG with maintenance treatment is effective in preventing cancer recurrence in superficial BC. In addition, adjuvant intravesical BCG maintenance therapy showed good therapeutic effects in patients with papillary carcinomas. Buffen et al.Citation33 demonstrated that single nucleotide polymorphisms in the autophagy genes ATG2B (rs3759601) modulated the efficacy of in vivo BCG-induced trained immunity, and it is related to the progression and recurrence of BC after intravesical BCG infusion. These findings identify a critical role for autophagy in the nonspecific protective effect of BCG. Rentsch et al.Citation34 found that BCG Connaught was more immunogenic, hence more effective in preventing recurrence. Pérez-Jacoiste et al.Citation35 analyzed infectious complications of 282 BC patients after treatment with intravesical BCG. Disseminated infection (34.4%), urogenital infection (23.4%), and skeletal muscle infection (19.9%) were the most common manifestations in their cohort. Patients aged ≥65 years with disseminated infection and vascular involvement had higher attributable mortality.
Intravesical IFN alone has been used for the treatment of superficial BC. However, the combination of IFN and BCG therapy may have unexpected results. Lam et al.Citation36 found that intravesical BCG combined with IFN-alpha2B was an effective and tolerable alternative for patients with superficial BC. The benefits of this combination therapy may include a potential reduction in morbidity and improved clinical efficacy. Bunimovich-Mendrazitsky et al.Citation37 suggested that the non-responsive subpopulation may benefit from an intensive combination of BCG and IL-2 maintenance therapy. In the study by Bajorin et al.Citation38 for patients with muscle-invasive UC who underwent radical surgery, the median DFS in the adjuvant nivolumab group and placebo group was 20.8 months and 10.8 months, respectively, indicating better therapeutic effect of adjuvant nivolumab on muscle-invasive UC.
Metastatic immunotherapy for BC
Currently, five ICIs (atezolizumab, pembrolizumab, nivolumab, durvalumab, and avelumab) have been approved by the FDA for the treatment of patients with advanced UC who have progressed after platinum-based chemotherapy. Two ICIs (atezolizumab and pembrolizumab) were approved for first-line treatment in patients who were not eligible for cisplatin.Citation39 A single-arm phase 2 study by Rosenberg et al.Citation20 showed that atezolizumab has durable activity and is well tolerated in patients with locally advanced and metastatic UC. Increased PD-L1 expression on immune cells correlated with increased response. Balar et al.Citation40 reported a phase 2 trial of atezolizumab as first-line therapy in patients with advanced metastatic UC who were not eligible for cisplatin. The results showed encouraging sustained response rates, survival rates, and tolerability of atezolizumab in patients with untreated metastatic UC. In the IMvigor211 study, atezolizumab significantly improved OS in the entire study population and was better tolerated than chemotherapy.Citation41 Notably, atezolizumab did not significantly improve OS in people with high PD-L1 expression. The PD-L1 biomarker did not work as expected. Patients with high PD-L1 expression showed good results in both groups.Citation41
Bellmunt et al.Citation42 found that second-line pembrolizumab prolonged OS (approximately 3 months) and showed a lower incidence of treatment-related AEs than chemotherapy in patients with platinum-refractory advanced UC. In a phase 2 trial of pembrolizumab, 89 of 370 patients achieved a complete or partial response, and only one patient died from treatment-related AEs.Citation43
Recent advances in BC immunotherapy
Hu et al.Citation44 discovered a new cancer target, Siglec15, that is specifically overexpressed in the TME of various cancers. Anti-Siglec15 immunotherapy may be suitable for BC treatment as Siglec15 is associated with non-inflamed TME in BC. Burke et al.Citation45 showed that the combination of local Histone Deacetylase inhibition and systemic anti-PD-1 can induce a curable CD8 T cell immune response against primary lesions, and have long-lasting anti-tumor immunity against secondary and distal tumors. Goubet et al.Citation46 found that Escherichia coli -specific CXCL13 producing CD4+ T cells are associated with the clinical efficacy of neoadjuvant PD-1 blockade against BC. This immune response against Escherichia coli can also be used for future treatment strategies. Nikolos et al.Citation47 suggested that resistance to chemoimmunotherapy can be overcome by blocking the COX-2/prostaglandin E2 axis, thereby revitalizing the anti-tumor immune response. Zhu et al.Citation48 showed that porphyrin-based PLZ4-nanoparticles photodynamic therapy can synergize immunotherapy for locally advanced and metastatic BC. In mouse models of BC, Zhu et al.Citation49 found experiments that the combination of Pan-PI3K inhibition and ICI has significant antitumor effects irrespective of the activation of the PI3K pathway. Ji et al.Citation50 found that for NMIBC patients, rapamycin enhances BCG-specific gamma delta T cell immunity and boosts urinary cytokines during BCG treatment. Recent, Li et al.Citation51 and Cai et al.Citation52 successively reported that S100A5 and BCAT2 can shape a non-inflamed TME in BC by negatively regulating pro-inflammatory chemokines and the recruitment and cytotoxicity of CD8 T cells. S100A5 and BCAT2, as key molecules in TME, are emerging targets in combination with ICB, which can enhance the efficacy of ICB therapy in BC.
In addition, the hot keyword “landscape” attracted our attention, which mainly refers to immunological and metabolic landscapes related to cancer. Immunotherapy as a novel treatment method, can play an important role in the treatment of cancer. However, it is necessary to prevent AEs of immunotherapy. Many unknown aspects of immunotherapy are yet to be explored.
Strengths and limitations
To the best of our knowledge, this is the first systematic bibliometric analysis of BC immunotherapy. Our findings can help researchers to identify research collaborators, as well as provide some guidance for clinicians and researchers. However, some limitations of this study should be acknowledged. Firstly, the literature search was performed only in the WOS core collection database, which may have led to the omission of some pertinent literature. Secondly, only articles published in the English language were eligible for inclusion, which may have introduced an element of selection bias. Thirdly, articles published in 2023 were excluded due to insufficient data. But compared with the volume of literature from 2000 to 2022, new data may have little impact on the final results. Finally, some newly published high-quality papers may not receive as much attention and be cited less frequently than classic papers.
Conclusion
This study contributes to our understanding of the status of BC immunotherapy-related research during the period from 2000 to 2022. Bibliometric analysis revealed that the volume of published articles was relatively low and stable from 2000 to 2015. Since 2015, there has been a noticeable increasing trend in the number of published articles. Researchers based in the USA have made notable contributions to BC immunotherapy. J UROLOGY was the most active and the most frequently co-cited journal. Collaboration between authors, institutions, and countries/regions must continue to be strengthened. Countries should actively create opportunities for communication and cooperation and provide a good platform for researchers and institutions. TME and ICIs of BC seem to be the hotspots in recent years. Much research is currently underway on the combination of ICIs with other drugs, which may become the main research direction in the future.
Abbreviations
| BC | = | bladder cancer |
| USA | = | United States of America |
| NMIBC | = | non-muscle-invasive BC |
| MIBC | = | muscle-invasive BC |
| OS | = | overall survival |
| CTLA-4 | = | cytotoxic T lymphocyte-associated antigen 4 |
| PD-1 | = | programmed cell-death protein 1 |
| PD-L1 | = | programmed cell-death ligand 1 |
| FDA | = | Food and Drug Administration |
| ICIs | = | immune checkpoint inhibitors |
| UC | = | urothelial carcinoma |
| BCG | = | Bacille Calmette-Guerin |
| WOS | = | Web of Science |
| IF | = | impact factor |
| BCG-CWS | = | BCG cell well skeleton |
| TME | = | tumor microenvironment |
| RFS | = | recurrence-free survival |
| pCR | = | pathologic complete remission |
| AEs | = | adverse events |
Authors’ contributions
KW and YW conceived the manuscript; ZL and JH reviewed the information. ZL wrote the manuscript. ZL and YW prepared the figures. KW and YW critically reviewed the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022 Jan;72(1):7–12. doi:10.3322/caac.21708.
- Ritch CR, Velasquez MC, Kwon D, Becerra MF, Soodana-Prakash N, Atluri VS, Almengo K, Alameddine M, Kineish O, Kava BR, et al. Use and validation of the AUA/SUO risk grouping for nonmuscle invasive bladder cancer in a contemporary cohort. J Urol. 2020 Mar;203(3):505–11. doi:10.1097/JU.0000000000000593.
- Alfred Witjes J, Lebret T, Compérat EM, Cowan NC, De Santis M, Bruins HM, Hernández V, Espinós EL, Dunn J, Rouanne M, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017 Mar;71(3):462–75. doi:10.1016/j.eururo.2016.06.020.
- Zhang Z, Lu M, Qin Y, Gao W, Tao L, Su W, Zhong J. Neoantigen: a new breakthrough in tumor immunotherapy. Front Immunol. 2021 Apr 16;12:672356. doi:10.3389/fimmu.2021.672356.
- Vasekar M, Degraff D, Joshi M. Immunotherapy in bladder cancer. Curr Mol Pharmacol. 2016;9(3):242–51. doi:10.2174/1874467208666150716120945.
- Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer–a current perspective. Nat Rev Urol. 2014 Mar;11(3):153–62. doi:10.1038/nrurol.2014.15.
- Wołącewicz M, Hrynkiewicz R, Grywalska E, Suchojad T, Leksowski T, Roliński J, Niedźwiedzka-Rystwej P. Immunotherapy in bladder cancer: current methods and future perspectives. Cancers Basel. 2020 May 7;12(5):1181. doi:10.3390/cancers12051181.
- Wu Z, Liu J, Dai R, Wu S. Current status and future perspectives of immunotherapy in bladder cancer treatment. Sci China Life Sci. 2021 Apr;64(4):512–33. doi:10.1007/s11427-020-1768-y.
- Tulsi R, Haque MMU, Hanif FM, Devi A, Mubarak M, Hassan Luck N. Metastasis of duodenal adenocarcinoma to the urinary bladder presenting as hematuria. J Transl Int Med. 2021 Jan 29;9(2):143–5. doi:10.2478/jtim-2021-0010.
- Wallin JA. Bibliometric methods: pitfalls and possibilities. Basic Clin Pharmacol Toxicol. 2005 Nov;97(5):261–75. doi:10.1111/j.1742-7843.2005.pto_139.x.
- Guler AT, Waaijer CJ, Palmblad M. Scientific workflows for bibliometrics. Scientometrics. 2016;107(2):385–98. doi:10.1007/s11192-016-1885-6.
- Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng S-L, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014 Nov 27;515(7528):558–62. doi:10.1038/nature13904.
- Oddens J, Brausi M, Sylvester R, Bono A, van de Beek C, van Andel G, Gontero P, Hoeltl W, Turkeri L, Marreaud S, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. 2013 Mar;63(3):462–72. doi:10.1016/j.eururo.2012.10.039.
- Balar AV, Kamat AM, Kulkarni GS, Uchio EM, Boormans JL, Roumiguié M, Krieger LEM, Singer EA, Bajorin DF, Grivas P, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021 Jul;22(7):919–30. doi:10.1016/S1470-2045(21)00147-9.
- Hu B, Wang Z, Zeng H, Qi Y, Chen Y, Wang T, Wang J, Chang Y, Bai Q, Xia Y, et al. Blockade of DC-SIGN+ tumor-associated macrophages reactivates antitumor immunity and improves immunotherapy in muscle-invasive bladder cancer. Cancer Res. 2020 Apr 15;80(8):1707–19. doi:10.1158/0008-5472.CAN-19-2254.
- Liu Z, Zhou Q, Wang Z, Zhang H, Zeng H, Huang Q, Chen Y, Jiang W, Lin Z, Qu Y, et al. Intratumoral TIGIT+ CD8+ T-cell infiltration determines poor prognosis and immune evasion in patients with muscle-invasive bladder cancer. J Immunother Cancer. 2020 Aug;8(2):e000978. doi:10.1136/jitc-2020-000978.
- Hu J, Chen J, Ou Z, Chen H, Liu Z, Chen M, Zhang R, Yu A, Cao R, Zhang E, et al. Neoadjuvant immunotherapy, chemotherapy, and combination therapy in muscle-invasive bladder cancer: a multi-center real-world retrospective study. Cell Rep Med. 2022 Nov 15;3(11):100785. doi:10.1016/j.xcrm.2022.100785.
- Whang YM, Yoon DH, Hwang GY, Yoon H, Park SI, Choi YW, Chang IH. Liposome-encapsulated Bacillus Calmette–Guérin cell wall skeleton enhances antitumor efficiency for bladder cancer in vitro and in vivo via induction of AMP-Activated Protein Kinase. Cancers Basel. 2020 Dec 8;12(12):3679. doi:10.3390/cancers12123679.
- Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976 Aug;116(2):180–3. doi:10.1016/S0022-5347(17)58737-6.
- Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O’Donnell PH, Balmanoukian A, Loriot Y, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016 May 7;387(10031):1909–20. doi:10.1016/S0140-6736(16)00561-4.
- Lamm DL, Blumenstein BA, Crissman JD, Montie JE, Gottesman JE, Lowe BA, Sarosdy MF, Bohl RD, Grossman HB, Beck TM, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest oncology group study. J Urol. 2000 Apr;163(4):1124–9. doi:10.1016/S0022-5347(05)67707-5.
- Bandini M, Gibb EA, Gallina A, Raggi D, Marandino L, Bianchi M, Ross JS, Colecchia M, Gandaglia G, Fossati N, et al. Does the administration of preoperative pembrolizumab lead to sustained remission post-cystectomy? First survival outcomes from the PURE-01 study☆. Ann Oncol. 2020 Dec;31(12):1755–63. doi:10.1016/j.annonc.2020.09.011.
- Sylvester RJ, van der Meijden AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002 Nov;168(5):1964–70. doi:10.1016/S0022-5347(05)64273-5.
- Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm M-O, Bracarda S, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017 Mar;18(3):312–22. doi:10.1016/S1470-2045(17)30065-7.
- Carthon BC, Wolchok JD, Yuan J, Kamat A, Ng Tang DS, Sun J, Ku G, Troncoso P, Logothetis CJ, Allison JP, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010 May 15;16(10):2861–71. doi:10.1158/1078-0432.CCR-10-0569.
- Powles T, Kockx M, Rodriguez-Vida A, Duran I, Crabb SJ, Van Der Heijden MS, Szabados B, Pous AF, Gravis G, Herranz UA, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med. 2019 Nov;25(11):1706–14. doi:10.1038/s41591-019-0628-7.
- Szabados B, Kockx M, Assaf ZJ, van Dam P-J, Rodriguez-Vida A, Duran I, Crabb SJ, Van Der Heijden MS, Pous AF, Gravis G, et al. Final results of neoadjuvant atezolizumab in cisplatin-ineligible patients with muscle-invasive urothelial cancer of the bladder. Eur Urol. 2022 Aug;82(2):212–22. doi:10.1016/j.eururo.2022.04.013.
- Necchi A, Anichini A, Raggi D, Briganti A, Massa S, Lucianò R, Colecchia M, Giannatempo P, Mortarini R, Bianchi M, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J Clin Oncol. 2018 Dec 1;36(34):3353–60. doi:10.1200/JCO.18.01148.
- van Dijk N, Gil-Jimenez A, Silina K, Hendricksen K, Smit LA, de Feijter JM, van Montfoort ML, van Rooijen C, Peters D, Broeks A, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med. 2020 Dec;26(12):1839–44. doi:10.1038/s41591-020-1085-z.
- Gao J, Navai N, Alhalabi O, Siefker-Radtke A, Campbell MT, Tidwell RS, Guo CC, Kamat AM, Matin SF, Araujo JC, et al. Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma. Nat Med. 2020 Dec;26(12):1845–51. doi:10.1038/s41591-020-1086-y.
- Sfakianos JP, Salome B, Daza J, Farkas A, Bhardwaj N, Horowitz A. Bacillus Calmette-Guerin (BCG): its fight against pathogens and cancer. Urol Oncol. 2021 Feb;39(2):121–9. doi:10.1016/j.urolonc.2020.09.031.
- Han RF, Pan JG. Can intravesical bacillus Calmette-Guerin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology. 2006 Jun;67(6):1216–23. doi:10.1016/j.urology.2005.12.014.
- Buffen K, Oosting M, Quintin J, Ng A, Kleinnijenhuis J, Kumar V, van de Vosse E, Wijmenga C, van Crevel R, Oosterwijk E, et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLoS Pathog. 2014 Oct 30;10(10):e1004485. doi:10.1371/journal.ppat.1004485.
- Rentsch CA, Birkhäuser FD, Biot C, Gsponer JR, Bisiaux A, Wetterauer C, Lagranderie M, Marchal G, Orgeur M, Bouchier C, et al. Bacillus Calmette-Guerin strain differences have an impact on clinical outcome in bladder cancer immunotherapy. Eur Urol. 2014 Oct;66(4):677–88. doi:10.1016/j.eururo.2014.02.061.
- Pérez-Jacoiste Asín MA, Fernández-Ruiz M, López-Medrano F, Lumbreras C, Tejido Á, San Juan R, Arrebola-Pajares A, Lizasoain M, Prieto S, Aguado JM. Bacillus Calmette-Guerin (BCG) infection following intravesical BCG administration as adjunctive therapy for bladder cancer: incidence, risk factors, and outcome in a single-institution series and review of the literature. Med. 2014 Oct;93(17):236–54. doi:10.1097/MD.0000000000000119.
- Lam JS, Benson MC, O’Donnell MA, Sawczuk A, Gavazzi A, Wechsler MH, Sawczuk IS. Bacillus Calmette-Guérin plus interferon-α2B intravesical therapy maintains an extended treatment plan for superficial bladder cancer with minimal toxicity. Urol Oncol. 2003 Sep-Oct;21(5):354–60. doi:10.1016/S1078-1439(03)00012-7.
- Bunimovich-Mendrazitsky S, Halachmi S, Kronik N. Improving bacillus Calmette-Guerin (BCG) immunotherapy for bladder cancer by adding interleukin 2 (IL-2): a mathematical model. Math Med Biol. 2016 Jun;33(2):159–88. doi:10.1093/imammb/dqv007.
- Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, Bamias A, Lebret T, Shariat SF, Park SH, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021 Jun 3;384(22):2102–14. doi:10.1056/NEJMoa2034442.
- Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin. 2020 Sep;70(5):404–23. doi:10.3322/caac.21631.
- Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL, et al. IMvigor210 Study Group. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017 Jan 7;389(10064):67–76. doi:10.1016/S0140-6736(16)32455-2.
- Powles T, Durán I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, Oudard S, Retz MM, Castellano D, Bamias A, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018 Feb 24;391(10122):748–57. doi:10.1016/S0140-6736(17)33297-X.
- Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee J-L, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, et al. KEYNOTE-045 investigators. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017 Mar 16;376(11):1015–26. doi:10.1056/NEJMoa1613683.
- Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, Plimack ER, Hahn NM, de Wit R, Pang L, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017 Nov;18(11):1483–92. doi:10.1016/S1470-2045(17)30616-2.
- Hu J, Yu A, Othmane B, Qiu D, Li H, Li C, Liu P, Ren W, Chen M, Gong G, et al. Siglec15 shapes a non-inflamed tumor microenvironment and predicts the molecular subtype in bladder cancer. Theranostics. 2021 Jan 1;11(7):3089–108. doi:10.7150/thno.53649.
- Burke B, Eden C, Perez C, Belshoff A, Hart S, Plaza-Rojas L, Delos Reyes M, Prajapati K, Voelkel-Johnson C, Henry E, et al. Inhibition of histone deacetylase (HDAC) enhances checkpoint blockade efficacy by rendering bladder cancer cells visible for T cell-mediated destruction. Front Oncol. 2020 May 15;10:699. doi:10.3389/fonc.2020.00699.
- Goubet AG, Lordello L, Alves Costa Silva C, Peguillet I, Gazzano M, Mbogning-Fonkou MD, Thelemaque C, Lebacle C, Thibault C, Audenet F, et al. Escherichia coli –specific CXCL13-producing TFH are associated with clinical efficacy of neoadjuvant PD-1 blockade against muscle-invasive bladder cancer. Cancer Discov. 2022 Oct 5;12(10):2280–307. doi:10.1158/2159-8290.CD-22-0201.
- Nikolos F, Hayashi K, Hoi XP, Alonzo ME, Mo Q, Kasabyan A, Furuya H, Trepel J, Di Vizio D, Guarnerio J, et al. Cell death-induced immunogenicity enhances chemoimmunotherapeutic response by converting immune-excluded into T-cell inflamed bladder tumors. Nat Commun. 2022 Mar 28;13(1):1487. doi:10.1038/s41467-022-29026-9.
- Zhu Z, Ma AH, Zhang H, Lin T-Y, Xue X, Farrukh H, Zhu S, Shi W, Yuan R, Cao Z, et al. Phototherapy with cancer-specific nanoporphyrin potentiates immunotherapy in bladder cancer. Clin Cancer Res. 2022 Nov 1;28(21):4820–31. doi:10.1158/1078-0432.CCR-22-1362.
- Zhu S, Ma AH, Zhu Z, Adib E, Rao T, Li N, Ni K, Chittepu VCSR, Prabhala R, Garisto Risco J, et al. Synergistic antitumor activity of pan-PI3K inhibition and immune checkpoint blockade in bladder cancer. J Immunother Cancer. 2021 Nov;9(11):e002917. doi:10.1136/jitc-2021-002917.
- Ji N, Mukherjee N, Reyes RM, Gelfond J, Javors M, Meeks JJ, McConkey DJ, Shu Z-J, Ramamurthy C, Dennett R, et al. Rapamycin enhances BCG-specific γδ T cells during intravesical BCG therapy for non-muscle invasive bladder cancer: a randomized, double-blind study. J Immunother Cancer. 2021 Mar;9(3):e001941. doi:10.1136/jitc-2020-001941.
- Li H, Chen J, Li Z, Chen M, Ou Z, Mo M, Wang R, Tong S, Liu P, Cai Z, et al. S100A5 attenuates efficiency of anti-PD-L1/PD-1 immunotherapy by inhibiting CD8+ T cell-mediated anti-cancer immunity in bladder carcinoma. Adv Sci. 2023 Sep;10(25):e2300110. doi:10.1002/advs.202300110.
- Cai Z, Chen J, Yu Z, Li H, Liu Z, Deng D, Liu J, Chen C, Zhang C, Ou Z, et al. BCAT2 shapes a noninflamed tumor microenvironment and induces resistance to anti-PD-1/PD-L1 immunotherapy by negatively regulating proinflammatory chemokines and anticancer immunity. Adv Sci. 2023 Mar;10(8):e2207155. doi:10.1002/advs.202207155.