ABSTRACT
LYB001 is an innovative recombinant SARS-CoV-2 vaccine that displays a repetitive array of the spike glycoprotein’s receptor-binding domain (RBD) on a virus-like particle (VLP) vector to boost the immune system, produced using Covalink plug-and-display protein binding technology. LYB001’s safety and immunogenicity were assessed in 119 participants receiving a booster with (1) 30 μg LYB001 (I-I-30 L) or CoronaVac (I-I-C), (2) 60 μg LYB001 (I-I-60 L) or CoronaVac in a ratio of 2:1 after two-dose primary series of inactivated COVID-19 vaccine, and (3) 30 μg LYB001 (I-I-I-30 L) after three-dose inactivated COVID-19 vaccine. A well-tolerated reactogenicity profile was observed for LYB001 as a heterologous booster, with adverse reactions being predominantly mild in severity and transient. LYB001 elicited a substantial increase in terms of the neutralizing antibody response against prototype SARS-CoV-2 28 days after booster, with GMT (95%CI) of 1237.8 (747.2, 2050.6), 554.3 (374.6, 820.2), 181.9 (107.6, 307.6), and 1200.2 (831.5, 1732.3) in the I-I-30 L, I-I-60 L, I-I-C, and I-I-I-30 L groups, respectively. LYB001 also elicited a cross-neutralizing antibody response against the BA.4/5 strain, dominant during the study period, with GMT of 201.1 (102.7, 393.7), 63.0 (35.1, 113.1), 29.2 (16.9, 50.3), and 115.3 (63.9, 208.1) in the I-I-30 L, I-I-60 L, I-I-C, and I-I-I-30 L groups, respectively, at 28 days after booster. Additionally, RBD-specific IFN-γ, IL-2, IL-4 secreting T cells dramatically increased at 14 days after a single LYB001 booster. Our data confirmed the favorable safety and immunogenicity profile of LYB001 and supported the continued clinical development of this promising candidate that utilizes the VLP platform to provide protection against COVID-19.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to evolve with the emergence of Omicron and its sublineages, outcompeting other variants of concern (VOCs), resulting in several variant-driven waves of breakthrough infections. Vaccination is the most cost-effective tool for tackling the coronavirus disease 2019 (COVID-19) pandemic. To date, most approved vaccines against COVID-19 target the prototype SARS-CoV-2 sequence, including those based on the mRNA, adenovirus vectors, protein/adjuvant subunits, and inactivated virus platforms. These vaccines have demonstrated reduced protective effectiveness against COVID-19 over time due to waning immunity and the emergence of immune-evading SARS-CoV-2 VOCs.Citation1–4 In the absence of Omicron-adapted vaccines, optimizing the delivery of first-generation vaccines using a heterologous booster strategy (mix and match) appears to induce a better immune response than a homologous booster strategy.Citation5–10 Two inactivated COVID-19 vaccines (ICVs), developed by Sinovac and Sinopharm in China, accounted for approximately 45% of the global delivered doses in 2021 and notably contributed to worldwide vaccine coverage.Citation11 These ICVs have also proven to be highly effective against severe COVID-19 disease outcomes.Citation12,Citation13 However, they exhibited poor or even absent neutralizing antibody (NAb) activity and effectiveness against infection with Omicron sublineages after the two-dose primary series, a primary booster, or even a secondary booster.Citation14–17
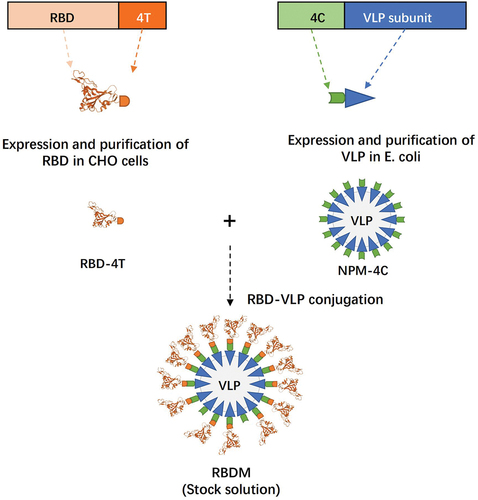
The receptor-binding domain (RBD) on the spike glycoprotein of SARS-CoV-2 is an immunodominant antigen that contains epitopes for most neutralizing antibodiesCitation18 and LYB001 is an innovative recombinant vaccine displaying repetitive RBDs on the surface of a virus-like particle (VLP) vector.Citation19 The array of RBD on the VLP was achieved using Covalink plug-and-display protein binding technology (isopeptide bond 4T/4C conjunction in ), similar to platforms described in other studies.Citation20 Because VLP and RBD can be expressed separately, the modular production of VLP in Escherichia coli and RBD in CHO cells is highly scalable. This platform also offers a shortened research and development cycle for a variant-adapted vaccine against rapidly evolving pathogens. Thus, vaccine adaptation can be easily accomplished, offering a major advantage in tackling the major global health challenges associated with human infectious diseases. Additionally, the highly repetitive antigen array (mimicking an actual virus) and relatively large particle size can enhance B-cell receptor cross-linking and antigen-presenting cell uptake and presentation, leading to strong stimulation of immune cells in the draining lymph nodes and overcoming the insufficient immunogenicity that can occur with soluble or monomeric recombinant subunit vaccines. Furthermore, the optimal orientation of the neutralizing epitope displayed on the VLP surface can result in a higher proportion of neutralizing antibodies.Citation21
The majority of the Chinese population had completed two- or three-dose ICV vaccinations at the beginning of the study, which made it challenging to obtain dose-response data in a naïve population. Additionally, the cross-NAb response against emerging variants, generated by the primary series of ICV targeting the prototype, waned substantially 6 months after the primary vaccination. A booster vaccination strategy was rolled out to restore immunity against emerging variants. In the absence of Omicron-adapted vaccines, we first explored the effects of a heterologous LYB001 booster at different doses compared with a homologous ICV booster among participants who completed two-dose ICV as dose-finding studies. Subsequently, we evaluated the safety and immunogenicity profiles of a heterologous LYB001 booster at a specific dose among participants who completed the three-dose ICV regimen. Our objective was to investigate whether alterations in reactogenicity and peak immunity occurred in relation to different primary ICV vaccinations. Herein, we present the safety and immunogenicity results of LYB001 as a booster vaccine at interval of 6–12 months in two- or three-dose ICV recipients.
Methods
Ethical approval
The study was registered with ClinicalTrials.gov (NCT05928455) and approved by the Institutional Review Boards of Chengdu Xinhua Hospital and Chongqing Red Cross Hospital. The trial was conducted in accordance with Good Clinical Practice guidelines and the principles stated in the Declaration of Helsinki. Written informed consent was obtained from each participant prior to any study-related procedures.
Study design and participants
This study was conducted at Chengdu Xinhua Hospital Affiliated to North Sichuan Medical College and Chongqing Red Cross Hospital (People’s Hospital of Jiangbei District). This study aimed to evaluate the safety and immunogenicity of a heterologous LYB001 booster at interval of 6–12 months following two or three doses of ICV in healthy participants aged 18–59 years. The study was performed in two parts: In part 1, a randomized, open-label, positive-controlled design was utilized to evaluate the safety and immunogenicity profile following different heterologous booster doses (30 and 60 μg) of LYB001 using a dose-escalation design. This was compared to a homologous booster dose of CoronaVac in adults aged 18–59 years who had completed a two-dose primary series of ICV 6–12 months earlier. Ninety participants (n = 45 per dose group) were planned to be enrolled in part 1, randomized 2:1 to receive LYB001 (30 and 60 μg) or CoronaVac. In part 2, a designated dose (30 μg) of LYB001 based on the preliminary results from part 1 was used as booster in 30 adults aged 18–59 years who had completed a three-dose primary series of ICV 6–12 months earlier. Participants with a known COVID-19 vaccination history other than ICV, history of SARS-CoV-2 infection, history of severe, uncontrolled chronic disease, or other conditions that, per the judgment of the investigator, might interfere with safety and immunogenicity assessment or pose possible risks to participants, were excluded from the study. For the full list of screening criteria, see the inclusion and exclusion criteria in the Supplementary Materials.
Randomization and masking
In part 1, participants were recruited using a dose-escalation study design. Participants who had completed two-dose primary series of ICV were randomly assigned in a ratio of 2:1 either to receive 30 μg LYB001 or a CoronaVac booster. After confirmation of an acceptable 7-day safety profile in this cohort, the study proceeded to the cohort of two-dose ICV recipients randomly assigned in a ratio of 2:1 either to receive 60 μg LYB001 or CoronaVac booster. Randomization of participants and vaccines was performed by an independent statistician using SAS statistical software version 9.4 or higher via the block randomization method, with a block of five and block size of nine. Randomization numbers were allocated to eligible participants in order of enrollment. Participants were randomly allocated to each group according to the randomization table. In part 2, randomization was not applicable because this was a single-arm study.
Blinding and masking were not applicable in this open-label study because the CoronaVac booster information of each participant had to be mandatorily recorded in the national vaccination system. However, all laboratory staff responsible for immunogenicity assessment and laboratory safety measures were blinded to the group allocation.
Procedures
The design of the investigational vaccine is shown in . Briefly, LYB001 is a recombinant vaccine made using a procedure that expresses the VLP vector (NPM-4C) in Escherichia coli and the RBD (RBD-4T from the spike glycoprotein of the SARS-CoV-2 prototype strain) in CHO cells. Purified stock solutions of RBD-4T and NPM-4C were mixed to enable conjugation via isopeptide binding to produce final VLP (RBDM). Following the final purification of VLP, it was adsorbed onto the aluminum hydroxide adjuvant. The LYB001 (0.5 mL, doses of 30 or 60 μg) and CoronaVac (0.5 mL) vaccines were administered through intramuscular injection.
Safety assessments
In this trial, participants were required to stay at the trial site for a 30 min safety observation period for the potential development of immediate adverse events (AEs) after the vaccine booster. During the observation period, participants were instructed to fill out the diary card and were given a thermometer and a measurement scale for recording the AEs experienced within 7 days after the booster, including solicited local/systemic and unsolicited AEs. Solicited local AEs included injection-site pain, induration, redness, swelling, rash, and pruritus, whereas solicited systemic AEs included fever, diarrhea, nausea, vomiting, headache, myalgia (non-injection site), cough, fatigue, and acute allergic reaction. On day 8 after the booster, participants returned to the trial site to submit diary cards that were reviewed by the investigator, and contact cards were dispensed to participants to record unsolicited AEs within 8–28 days after the booster. The intensity of AEs was graded using appropriate guidelines issued by the National Medical Products Administration (NMPA) of China, and causality was assessed by the investigators.
Immunogenicity assessments
Blood samples for humoral immunogenicity assessment were drawn from participants at baseline (day 0 before vaccination) and at days 14, 28, and 90 after the booster, and used to determine: (1) spike glycoprotein binding IgG levels, and (2) NAb titers against prototype SARS-CoV-2 and circulating VOCs. Blood samples for cellular immunity were drawn from the participants at baseline and 14 days after the booster. Spike glycoprotein-binding IgGs were measured using ELISA, and the values were reported as binding antibody units (BAUs), in accordance with the manufacturer’s recommendations and the World Health Organization (WHO) International Standard and International Reference Panel for anti-SARS-CoV-2 immunoglobulin. NAb titers against the prototype SARS-CoV-2 and dominant VOCs were determined using vesicular stomatitis virus (VSV)-based pseudovirus-neutralizing assays by Chongqing Medleader Bio-Pharm Co., Ltd. Seroconversion was defined as either a four-fold increase in post-boost antibody levels from a seropositive (≥ cutoff value) baseline, or a seropositive conversion from a seronegative (< cutoff value) baseline. The cellular immune response was detected using enzyme-linked immunospot (ELISpot) assay, and was presented as the counts of spot forming cells (SFCs) per 3 × 105 peripheral blood mononuclear cells (PBMCs) secreting interferon (IFN)-γ, interleukin (IL)-2, and IL-4 when stimulated by the RBD peptide pool ex vivo. Further details of the methodology used for the immunogenicity assays are provided in Supplementary Methods.
Outcomes
The primary objective of this study was to assess the safety and immunogenicity of LYB001 following a heterologous booster in adults aged 18–59 years who had previously completed two- or three-dose primary courses of ICV vaccination. The primary immunogenicity outcomes were the geometric mean titer (GMT), geometric mean fold rise (GMFR), and seroconversion rate (SCR) of spike glycoprotein-binding IgGs, NAb titers against the prototype SARS-CoV-2, and circulating VOCs at baseline and 14 and 28 days after the booster. The primary safety outcomes were immediate AEs within 30 min after the booster, solicited local/systemic AEs within 7 days, and unsolicited AEs within 28 days after the booster.
The secondary objective was to assess the immune response durability, which included the GMT and SCR of spike glycoprotein-binding IgGs, NAb titers against prototype SARS-CoV-2, and circulating VOCs measured 90 days after the booster. Secondary safety outcomes were severe adverse events (SAEs), adverse events of special interest (AESIs) within 90 days after the booster, and safety laboratory measures 3 days after the booster. The exploratory outcome was to assess the cellular immune response following a heterologous booster dose of LYB001, and the corresponding exploratory outcome was the RBD-specific IFN-γ, IL-2, and IL-4 secreting T cell response as measured by ELISpot assay at baseline and 14 days after the booster.
Statistical analysis
The sample size of this trial was not based on formal statistical hypotheses. Safety analyses were evaluated using the safety set (SS), which included all participants who received the booster dose. Immunogenicity analysis was performed in the per-protocol set for immunogenicity (I-PPS) following an intention-to-treat principle, including participants who had completed the booster immunization with immunogenicity results at day 0 before vaccination and at least one available post-boost immunogenicity result with no major protocol deviations. The counts and percentages of participants who experienced AEs were presented in the safety analyses, including solicited local/systemic AEs, unsolicited AEs, AEs graded as grade 3 or worse, AEs leading to participant withdrawal, SAEs, and AESIs. The NAb GMTs against the prototype SARS-CoV-2 and circulating VOCs at different time points after the booster were calculated with Clopper-Pearson 95% confidence intervals (CIs), and the t-test was used to compare log-transformed antibody titers between groups. Additionally, GMFRs and SCRs at different time points after the booster, relative to baseline, were calculated along with their Clopper-Pearson 95% CIs. The cellular immune responses (cytokine-secreting T cells by ELISpot assay) and their changes from baseline were statistically analyzed for each group at 14 days after the booster, and the differences were statistically tested using the Wilcoxon rank-sum test. The χ2 test or Fisher’s exact test was used to analyze other categorical data. Given the exploratory nature of this study, no adjustments were made for multiple comparisons or multiplicity. Statistical analysis was performed using GraphPad Prism 9.0 (GraphPad Software Inc., California, USA).
Results
Participants
Between May 14 and June 30, 2022, 215 individuals were screened, and 120 eligible participants aged 18–59 years who had competed a two- or three-dose ICV 6–12 months earlier were enrolled in this study (). One participant withdrew before the booster vaccination due to prior receipt of a two-dose recombinant protein subunit vaccine other than the ICV against COVID-19 (ZF2001). A total of 119 participants aged 18–59 years received the booster vaccination and were included in the analysis set. The mean (SD) participants’ age was 29.4 (8.2), 29.6 (7.8), 30.1 (9.1), and 30.3 (9.4) years, with a consistent sex distribution in the groups of participants receiving a booster with 30 μg LYB001 (I-I-30 L), 60 μg LYB001 (I-I-60 L), or CoronaVac (I-I-C) after two-dose primary series of ICV, or 30 μg LYB001 (I-I-I-30 L) after three-dose primary series of ICV, respectively. The mean (SD) prime-boost intervals were 290.2 (37.0), 267.8 (31.9), 276.9 (25.0), and 216.4 (37.3) days in the I-I-30 L, I-I-60 L, I-I-C, and I-I-I-30 L groups, respectively. In each group, there was no medication (or vaccination) or medical (or allergy) history, which, in the opinion of the investigator, might compromise the participants’ well-being or confound protocol-specified assessments. The NAb titers against the prototype SARS-CoV-2 and Omicron BA.4/5 variants were low or absent at baseline; these were comparable across the I-I-30 L, I-I-60 L, and I-I-C groups and were lower than that of the I-I-I-30 L group ().
Figure 2. Study profile.
Table 1. Baseline characteristics of the participants.
Safety
Overall adverse events/reactions
LYB001 as a heterologous booster after a two- or three-dose ICV was safe and well tolerated, with adverse reactions (vaccination-related AEs) being predominantly mild in severity (only two participants reported grade 2 adverse reactions) (). The majority of these adverse reactions were those commonly anticipated for intramuscularly administered vaccines and spontaneously resolved/recovered within a median duration of 2 days after symptom onset. The overall incidence rates of adverse reactions were 76.7% (23/30), 66.7% (20/30), 31.0% (9/29), and 63.3% (19/30) in the I-I-30 L, I-I-60 L, I-I-C, and I-I-I-30 L groups, respectively, which were predominantly contributed by solicited local adverse reactions.
Table 2. Overall adverse events or reactions after booster administration.
Solicited local/systemic reactions and unsolicited reactions
Solicited local adverse reactions were reported by 70.0% (21/30), 56.7% (17/30), 17.2% (5/29), and 60% (18/30) of the participants and the most common (reported in ≥ 10% of the population in at least one group) solicited local adverse reaction was injection-site pain (predominantly mild in severity), which was reported by 70.0% (21/30), 56.7% (17/30), 17.2% (5/29), and 60% (18/30) of the participants in the I-I-30 L, I-I-60 L, I-I-C, and I-I-I-30 L groups, respectively. The solicited systemic adverse reactions were reported by 20.0% (5/30), 16.7% (5/30), 3.4% (1/29), and 6.7% (2/30) of the participants and the most common solicited systemic adverse reaction was fatigue, which was reported by 16.7% (5/30), 10.0% (3/30), 3.4% (1/29), and 6.7% (2/30) of the participants in the I-I-30 L, I-I-60 L, I-I-C, and I-I-I-30 L groups, respectively. The unsolicited adverse reactions within 28 days after the booster were reported by 7 (23.3%), 5 (16.7%), 4 (13.8%), and 8 (26.7%) participants in the I-I-30 L, I-I-60 L, I-I-C, and I-I-I-30 L groups, respectively, which were comparable across groups.
Safety laboratory measures
Regarding the safety laboratory measures, the changes at day 3 after the booster from baseline did not indicate a particular trend concerning hematology, blood chemistry, urinalysis, and coagulation function parameters, with only a few significantly abnormal safety laboratory parameters reported (). All abnormal values spontaneously returned to normal at a subsequent visit without any clinical consequences.
SAEs, AESIs, and other significant AEs
No SAEs, AESIs, deaths, or AEs that led to withdrawal were reported within 90 days of booster administration. Only one participant in the 60 μg LYB001 booster group experienced a grade 3 or worse AE (preferred terms: pyrexia); however, this was judged as unrelated to the investigational vaccine.
Neutralization antibodies
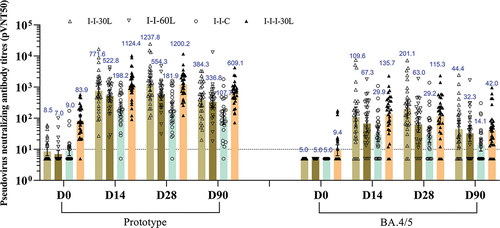
As shown in and Table S1, the heterologous LYB001 booster elicited a potent NAb response against the prototype SARS-CoV-2 and cross-NAb response against the Omicron BA.4/5 strain, which was dominant during the study period, in participants previously immunized with two- or three-dose ICV. The NAb response was low or undetectable at baseline, increased considerably at day 14 or 28 after the booster, and moderately declined by day 90 after the booster. In every time points after the booster vaccination, the NAb responses against the prototype SARS-COV-2 were higher in the heterologous LYB001 booster groups (I-I-30 L, I-I-60 L, I-I-I-30 L) compared to the homologous CoronaVac booster group (I-I-C). The peak pseudovirus NAb GMTs (95% CI) against the prototype SARS-COV-2 were 1237.8 (747.2, 2050.6), 554.3 (374.6, 820.2), 181.9 (107.6, 307.6), and 1200.2 (831.5, 1732.3) at 28 days after the booster in the I-I-30 L, I-I-60 L, I-I-C, and I-I-I-30 L groups, respectively. The peak pseudovirus NAb GMTs (95% CI) against the Omicron BA.4/5 were 109.6 (52.3, 229.6), 67.3 (36.5, 124.0), 29.9 (17.4, 51.4), and 135.7 (75.6, 243.5) at 14 days; 201.1 (102.7, 393.7), 63.0 (35.1, 113.1), 29.2 (16.9, 50.3), and 115.3 (63.9, 208.1) at 28 days after the booster in the I-I-30 L, I-I-60 L, I-I-C, and I-I-I-30 L groups, respectively. Given the same two-dose primary course of ICV vaccination, the 30 μg LYB001 booster elicited the highest NAb response against prototype and BA.4/5, followed by the 60 μg LYB001, and then the CoronaVac, from a comparable (against prototype) or identical (against BA.4/5) baseline immunity. At 28 days after the booster, the pseudovirus NAb titers of the I-I-30 L group were 6.8 times (1237.8 vs 181.9) higher than that of the I-I-C group against prototype, and 6.9 times (201.1 vs 29.2) higher than that of the I-I-C group against BA.4/5. When evaluating the impact of different primary dose regimens (two-dose ICV vs three-dose ICV), the NAb responses of the I-I-30 L group were comparable or even higher than that of the I-I-I-30 L groups: (1237.8 vs 1200.2 against prototype, and 201.1 vs 115.3 against BA.4/5 at 28 days after booster), despite the higher baseline NAb titers in the I-I-I-30 L group. This indicated that a single 30 μg LYB001 booster could substantially restore the NAb response within a prime-boost interval of 6–12 months, irrespective of undetectable or partially detectable baseline antibody levels. Despite the decline in the NAb response at 90 days after the booster, the SCRs of NAb titers in the I-I-30 L group still remained 100% against prototype, and 80.0% against BA.4/5.
Figure 3. VSV-based neutralizing antibody titers against prototype SARS-CoV-2 and Omicron BA.4/5 strain.
Spike protein-binding antibodies
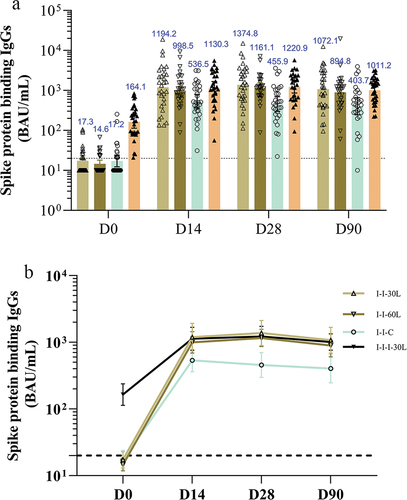
The spike glycoprotein-binding IgGs exhibited a similar trend to those seen for NAb responses, but with a slower waning 90 days after the booster (). Specifically, the peak spike protein-binding IgGs (95% CI) were 1374.8 (892.2, 2118.3), 1161.1 (863.6, 1561.2), 455.9 (297.5, 698.7), and 1220.9 (862.5, 1728.2) at 28 days; and slowly declined to 1072.1 (686.5, 1674.3), 894.8 (606.0, 1321.3), 403.7 (245.6, 663.7), and 1011.2 (762.6, 1341.0) at 90 days after the booster in the I-I-30 L, I-I-60 L, I-I-C, and I-I-I-30 L groups, respectively. The spike protein-binding IgG titers were comparable across the LYB001 booster groups (I-I-30 L, I-I-60 L, and I-I-I-30 L) regardless of different LYB001 dosages and primary vaccination schedules, all of which surpassed the concentrations observed in the I-I-C group.
Figure 4. The spike protein binding IgGs at baseline and 14, 28, and 90 days after booster administration.
Cellular immunity
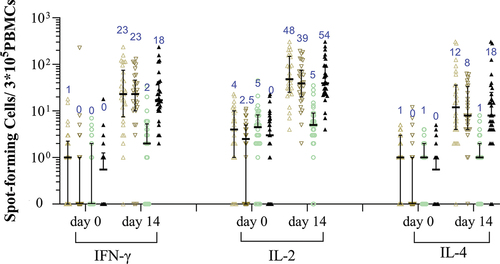
T cell responses detected in the LYB001 groups were compared with those in the CoronaVac control group. The majority of participants exhibited low preexisting responses ( and Table S2). Notably, the LYB001 booster induced significantly higher cytokine responses to the SARS-CoV-2 RBD peptide pool both in the 30 and 60 μg LYB001 groups as compared to the CoronaVac group, irrespective of administered dosages and primary vaccination regimens. SFCs per 3 × 105 PBMCs as indicated by median (Q1, Q3) were 23.0 (8.0, 68.0), 23.0 (10.0, 42.0), and 18.0 (5.0, 46.0) for IFN-γ; 48.0 (26.0, 145.0), 39.0 (21.0, 70.0), and 54.0 (30.0, 99.0) for IL-2; 12.0 (4.0, 36.0), 8.0 (4.0, 33.0), and 18.0 (7.0, 43.0) for IL-4 at 14 days after the booster in the I-I-30 L, I-I-60 L, and I-I-I-30 L groups, respectively. In contrast, the SFCs per 3 × 105 PBMCs were 2.0 (0.0, 4.0), 5.0 (3.0, 8.0) and 1.0 (0.0, 1.0) for IL-4, IL-2 and IL-4, respectively, at 14 days after the booster in the I-I-C group. While the cellular response measured in the I-I-C group was almost absent, the 30 and 60 μg LYB001 booster induced both Th1 (IFN-γ) and Th2 (IL-2 and IL-4) type cellular response, exhibiting over 10-fold increases versus the respective median SFCs at baseline.
Figure 5. RBD-specific IFN-γ, IL-2, or IL-4 secreting T-cells measured by ELISpot assay.
Discussion
In this study, we investigated the safety and immunogenicity of LYB001 as a heterologous booster following two or three doses of ICV in participants aged 18–59 years with a prime-boost interval of approximately 6–12 months. To the best of our knowledge, this is the first clinical trial conducted in China to report the preliminary safety, reactogenicity, and immunogenicity of a VLP-based vaccine against COVID-19. Overall, despite the higher incidence of reactogenicity events compared with the I-I-C group, AEs were mostly mild in severity and transient after a heterologous booster with LYB001 across the I-I-30 L, I-I-60 L, and I-I-I-30 L groups. The reported solicited adverse reactions represented reactogenicity events anticipated for intramuscularly administered vaccines, with local AEs including injection-site pain, swelling, redness, and pruritus, and systemic AEs including fatigue. In addition to mild reactogenicity, most AE symptoms resolve spontaneously, mostly within 48 h of onset. Those requiring treatment were managed using simple measures and widely available medications. No vaccination-related SAEs, AESIs, or AEs leading to participant withdrawal were reported within 90 days of booster administration. Although some participants reported abnormal safety laboratory measures with clinical significance 3 days after the booster, these abnormal values spontaneously returned to normal at the subsequent visit without any clinical consequences. In addition, these differences in laboratory results from baseline (calculated as values on day 3 after booster minus baseline values) for each participant in all groups did not show a particular trend. Furthermore, we found that the higher incidence rate of solicited local/systemic reaction for the heterologous LYB001 booster (in the I-I-30 L, I-I-60 L, and I-I-I-30 L groups) compared to the CoronaVac booster (in the I-I-C group) was predominantly due to injection-site pain. Injection-site pain accounted for 70.0% (n = 21), 56.7% (n = 17), 60.0 (n = 18), and 17.2% (n = 5) of the participants in the I-I-30 L, I-I-60 L, I-I-I-30 L, and I-I-C groups, respectively. The possible explanations for the increased incidence of injection-site pain are as follows: (1) the aluminum adjuvant content in LYB001 was higher than that of the inactivated vaccine (higher aluminum adjuvant was previously reported to correlate with a higher risk of pain),Citation22 or (2) LYB001, with the display of repetitive RBD on the VLP vector, has a larger particle size, which may result in a longer local recruitment time of relevant molecules of the innate immune system and activation of antigen-presenting cells.Citation21 Similarly, mRNA vaccines elicit transient increases in C-reactive protein (CRP) levels, an indicator of vaccine adjuvant activity.Citation23 Our results were consistent with a self-assembling, two-component nanoparticle vaccine (approved in South Korea) displaying the RBD of the SARS-CoV-2 spike glycoprotein in a highly immunogenic array. This vaccine demonstrated injection-site pain in 88.1% and 92.3% of participants receiving 10 μg GBP 510 and 25 μg GBP 510 in AS03 adjuvant, respectively.Citation24 In a phase III trial of another coronavirus-like particle (CoVLP) vaccine, injection site pain was reported in 85.0% of participants after the first dose of CoVLP with AS01 adjuvant versus 29.4% of participants in the placebo group.Citation25
The results of our study indicated that one heterologous booster dose of LYB001 could profoundly restore the NAb response, irrespective of the baseline antibody levels. LYB001 elicited a comparable NAb response (1237.8 vs. 1200.2) against the prototype 28 days after the booster in the I-I-30 L group versus the I-I-I-30 L group, despite a significantly different baseline (8.5 vs. 83.9). The heterologous LYB001 booster also induced superior humoral immune responses compared to the homologous booster with CoronaVac. When compared with a recombinant fusion protein vaccine V-01 (IFN-PADRE-RBD-Fc dimer, approved in China), the peak GMR (heterologous investigational vaccine booster versus homologous ICV booster) for VSV-based pseudovirus NAb GMT was 6.8 (1237.8 vs. 181.9) against prototype in the I-I-30 L group, higher than 3.3 GMR (893 vs. 268) after the 10 μg V-01 booster. The peak GMR (heterologous booster versus homologous ICV booster) was 6.9 (201.1 vs. 29.2) for the VSV-based pseudovirus NAb GMT against Omicron BA.4/5, higher than 3.8 GMR (211 vs. 56) against BA.1 after the V-01 booster.Citation26 Additionally, for a recombinant protein vaccine ZF2001 (approved in China, Colombia, Indonesia, Uzbekistan), the GMR (25 μg ZF2001 booster versus homologous ICV booster) was 2.4 (537 vs. 225) for the VSV-based pseudovirus NAb GMTs against prototype SARS-CoV-2, 1.7 (108 vs. 63) times higher than the ICV booster for the pseudovirus NAb GMTs against Omicron BA.1.Citation27 Taking into consideration a similar NAb detecting technique (VSV-based pseudovirus NAb assay), trial population (participants aged 18–59 years who completed a two-dose primary series of ICV), and comparison based on the active comparator of ICV, the 30 μg LYB001 booster can elicit a favorable NAb response against the circulating variant during the study period.
Although LYB001 was designed using the RBD from the prototype SARS-CoV-2, it demonstrated satisfactory immunogenicity against the prototype SARS-CoV-2 and robust cross-neutralizing activity against the Omicron BA.4/5 - SARS-CoV-2 variants that showed extensive immune escape. The conserved neutralizing epitopes between Omicron BA.4/5 and prototype SARS-CoV-2 may contribute to cross-neutralization. Immunogenicity could also be augmented by an innovative platform using RBD-VLP protein binding technology, which enhanced B cell activation, APC uptake and presentation, and efficient draining to lymph nodes. The optimal orientation for maximizing neutralizing epitope display, leading to a higher proportion of functional antibodies, was also reflected by a higher fold increase in the ratio of NAb titer versus spike glycoprotein-binding antibody concentration, comparing the LYB001 booster with the CoronaVac booster. Thus, this innovative design enables LYB001 to elicit robust NAb responses against the prototype SARS-CoV-2 and cross-neutralizing activity against circulating Omicron BA.4/5. Although a correlate of protection has not been established for predicting the individual-level risk of SARS-CoV-2 infection, a spike glycoprotein-binding IgG concentration of 1148 BAU/mL (also reported as BAUs in accordance with the WHO standard) may provide 75% protection against symptomatic infection with BA.5.Citation28 This indicates promising efficacy against BA.5 after LYB001 booster.
T-cell responses are also important for controlling disease development in patients with COVID-19. Targeted T-cell epitopes are broadly conserved between the prototype SARS-CoV-2 variant and Omicron.Citation29,Citation30 Generally, the cellular immune response is absent or at least weak after booster administration in healthy adults receiving two-dose ICV.Citation31,Citation32 The results from this trial indicated that LYB001 booster induced robust cellular responses to the SARS-CoV-2 RBD-specific peptide pool in the I-I-30 L, I-I-60 L, and I-I-I-30 L groups, as compared to the weak cellular responses in the I-I-C group. The RBD-specific IFN-γ secreting T-cells measured by ELISpot assay were dramatically increased (>10 times versus baseline) after a single LYB001 booster, comparable to one shot of adenovirus type-5-vectored COVID-19 vaccine (a median of approximately 10 IFN-γ secreting SFCs per 1 × 105 PBMCs) which generally elicited robust cellular immune response.Citation33 Some participants in this study appeared to have preexisting cellular responses to the RBD-specific peptide pool used for PBMC restimulation. Such cross-reactive T-cell memory is possibly due to previous doses of ICV vaccination and/or previous exposure to common human coronaviruses.Citation34,Citation35
This study has some limitations. The safety and immunogenicity findings were based on a small sample size (approximately 30 participants/group); therefore, the results should be interpreted with caution. This is an exploratory study without multiplicity adjustment to control type I error; therefore, the statistical power may be diminished. Additionally, this is an open-label study (participants receiving the CoronaVac booster must be registered with the vaccination system of China, and the fourth dose of the CoronaVac booster, which was used as an active comparator in part 1, was not approved when the study began), so the evidence provided in this trial is weaker than that in a blinded randomized control trial (RCT). To attain a more comprehensive and precise validation of the outcomes, minimize sample bias, and enhance statistical robustness, blinded RCTs of LYB001 booster with larger sample size and age stratification (including older participants aged ≥60 years) have now been conducted. Additionally, we only detected NAb titers of emerging Omicron sublineages during our study period in China, such as BA.5. Other emerging VOCs, such as XBB and its sublineages, will be included in future studies.
Conclusions
Taken together, the LYB001 as a heterologous booster, after inactivated COVID-19 vaccine, elicited a strong humoral immune response, demonstrated cross-neutralizing activity against dominantly circulating BA.4/5 variants, and induced robust RBD-specific cytokine secreting T-cell responses, without compromising vaccine’s safety. The reactogenicity events were predominantly mild (grade 1) in severity and transient and were anticipated for intramuscularly administered vaccines. Thus, our study provides valuable evidence that the LYB001 vaccine, developed using RBD-VLP protein-binding technology, is a promising candidate for preventing COVID-19.
Author contribution
All authors have read and approved this version of manuscript. Yan Liu, Xiaolan Yong, Jun Liu, Ying Zeng, Xuelian Cui and Yilin Wang conceived and designed the clinical study. Xiaolan Yong, Jun Liu and Jing Nie performed the clinical trial. Tao Wang performed the immunological (humoral and cellular) assays. Yiyong Chen, Wei Kang and Yan Liu drafted and revised the manuscript. Zhonghua Yang reviewed data analysis and interpretated the data. All authors agreed to be accountable for all aspects of the work.
Supplemental Material
Download PDF (236.7 KB)Acknowledgments
The authors thank all the participants who volunteered to participate in this first-in-human study of LYB001.
Disclosure statement
Ying Zeng, Wei Kang, and Zhonghua Yang are employees of the Yantai Patronus Biotech Co., Ltd. The other authors declare no conflicts of interest.
Data availability statement
The data used in this study are available from the corresponding author upon reasonable request.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2267869.
Additional information
Funding
References
- Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Haas EJ, Milo R, Alroy-Preis S, Ash N, Huppert A. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. doi:10.1056/NEJMoa2114228.
- Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O’Connell A-M, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–12. doi:10.1056/NEJMoa2119451.
- Abu-Raddad LJ, Chemaitelly H, Bertollini R, National Study Group for C-V. Waning mRNA-1273 vaccine effectiveness against SARS-CoV-2 infection in Qatar. N Engl J Med. 2022;386:1091–3. doi:10.1056/NEJMc2119432.
- Zeng G, Wu Q, Pan H, Li M, Yang J, Wang L, Wu Z, Jiang D, Deng X, Chu K, et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis. 2022;22:483–95. doi:10.1016/S1473-3099(21)00681-2.
- Li J, Hou L, Guo X, Jin P, Wu S, Zhu J, Pan H, Wang X, Song Z, Wan J, et al. Heterologous AD5-nCOV plus CoronaVac versus homologous CoronaVac vaccination: a randomized phase 4 trial. Nat Med. 2022;28:401–9. doi:10.1038/s41591-021-01677-z.
- Munro APS, Janani L, Cornelius V, Aley PK, Babbage G, Baxter D, Bula M, Cathie K, Chatterjee K, Dodd K, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–76. doi:10.1016/S0140-6736(21)02717-3.
- Callaway E. COVID vaccine boosters: the most important questions. Nature. 2021;596:178–80. doi:10.1038/d41586-021-02158-6.
- Costa Clemens SA, Weckx L, Clemens R, Almeida Mendes AV, Ramos Souza A, Silveira MBV, da Guarda SNF, de Nobrega MM, de Moraes Pinto MI, Gonzalez IGS, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399:521–9. doi:10.1016/S0140-6736(22)00094-0.
- Pérez-Then E, Lucas C, Monteiro VS, Miric M, Brache V, Cochon L, Vogels CBF, Malik AA, De la Cruz E, Jorge A, et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med. 2022;28:481–5. doi:10.1038/s41591-022-01705-6.
- Cheng SMS, Mok CKP, Leung YWY, Ng SS, Chan KCK, Ko FW, Chen C, Yiu K, Lam BHS, Lau EHY, et al. Neutralizing antibodies against the SARS-CoV-2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat Med. 2022;28:486–9. doi:10.1038/s41591-022-01704-7.
- Dolgin E. Omicron thwarts some of the world’s most-used COVID vaccines. Nature. 2022;601:311. doi:10.1038/d41586-022-00079-6.
- McMenamin ME, Nealon J, Lin Y, Wong JY, Cheung JK, Lau EHY, Wu P, Leung GM, Cowling BJ. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis. 2022;22:1435–43. doi:10.1016/s1473-3099(22)00345-0.
- Jara A, Undurraga EA, Zubizarreta JR, González C, Pizarro A, Acevedo J, Leo K, Paredes F, Bralic T, Vergara V, et al. Effectiveness of homologous and heterologous booster doses for an inactivated SARS-CoV-2 vaccine: a large-scale prospective cohort study. Lancet Glob Health. 2022;10:e798–e806. doi:10.1016/s2214-109x(22)00112-7.
- Yu X, Wei D, Xu W, Li Y, Li X, Zhang X, Qu J, Yang Z, Chen E. Reduced sensitivity of SARS-CoV-2 Omicron variant to antibody neutralization elicited by booster vaccination. Cell Discov. 2022;8:4. doi:10.1038/s41421-022-00375-5.
- Melo-González F, Méndez C, Peñaloza HF, Schultz BM, Piña-Iturbe A, Ríos M, Moreno-Tapia D, Pereira-Sanchez P, Leighton D, Orellana C, Covarrubias C. Humoral and cellular response induced by a second booster of an inactivated SARS-CoV-2 vaccine in adults. medRxiv 2022:2022.08.22.22279080. doi:10.1101/2022.08.22.22279080.
- Ranzani OT, Hitchings MDT, de Melo RL, de França GVA, Fernandes CDFR, Lind ML, Torres MSS, Tsuha DH, David LCS, Said RFC, et al. Effectiveness of an inactivated Covid-19 vaccine with homologous and heterologous boosters against Omicron in Brazil. Nat Commun. 2022;13:5536. doi:10.1038/s41467-022-33169-0.
- Huang Z, Xu S, Liu J, Wu L, Qiu J, Wang N, Ren J, Li Z, Guo X, Tao F, et al. Effectiveness of inactivated and Ad5-nCov COVID-19 vaccines against SARS-CoV-2 Omicron BA. 2 variant infection, severe illness, and death. BMC Med. 2022;20:400. doi:10.1186/s12916-022-02606-8.
- Dai L, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21:73–82. doi:10.1038/s41577-020-00480-0.
- Li Y, Zhang Y, Zhou Y, Li Y, Xu J, Ai Y, Xu L, Xiao X, Zhang B, Jin J, et al. An RBD virus-like particle vaccine for SARS-CoV-2 induces cross-variant antibody responses in mice and macaques. Signal Transduct Target Ther. 2023;8:173. doi:10.1038/s41392-023-01425-4.
- Bruun TUJ, Andersson AC, Draper SJ, Howarth M. Engineering a rugged nanoscaffold to enhance plug-and-display vaccination. Acs Nano. 2018;12:8855–66. doi:10.1021/acsnano.8b02805.
- Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–96. doi:10.1038/nri2868.
- Lin YJ, Shih YJ, Chen CH, Fang CT. Aluminum salts as an adjuvant for pre-pandemic influenza vaccines: a meta-analysis. Sci Rep. 2018;8:11460. doi:10.1038/s41598-018-29858-w.
- Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, Baum A, Pascal K, Quandt J, Maurus D, et al. COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature. 2020;586:594–9. doi:10.1038/s41586-020-2814-7.
- Song JY, Choi WS, Heo JY, Lee JS, Jung DS, Kim SW, Park K-H, Eom JS, Jeong SJ, Lee J, et al. Safety and immunogenicity of a SARS-CoV-2 recombinant protein nanoparticle vaccine (GBP510) adjuvanted with AS03: a randomised, placebo-controlled, observer-blinded phase 1/2 trial. EClinicalMedicine. 2022;51:101569. doi:10.1016/j.eclinm.2022.101569.
- Ward BJ, Gobeil P, Seguin A, Atkins J, Boulay I, Charbonneau PY, Couture M, D’Aoust M-A, Dhaliwall J, Finkle C, et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat Med. 2021;27:1071–8. doi:10.1038/s41591-021-01370-1.
- Zhang Z, He Q, Zhao W, Li Y, Yang J, Hu Z, Chen X, Peng H, Fu Y-X, Chen L, et al. A heterologous V-01 or variant-matched bivalent V-01D-351 booster following primary series of inactivated vaccine enhances the neutralizing capacity against SARS-CoV-2 Delta and Omicron strains. J Clin Med. 2022;11:11. doi:10.3390/jcm11144164.
- Ai J, Wang X, He X, Zhao X, Zhang Y, Jiang Y, Li M, Cui Y, Chen Y, Qiao R, et al. Antibody evasion of SARS-CoV-2 Omicron BA.1, BA.1.1, BA.2, and BA.3 sub-lineages. Cell Host Microbe. 2022;30:1077–83 e4. doi:10.1016/j.chom.2022.05.001.
- Nilles EJ, Paulino CT, de St Aubin M, Duke W, Jarolim P, Sanchez IM, Murray KO, Lau CL, Gutiérrez EZ, Ramm RS, et al. Tracking immune correlates of protection for emerging SARS-CoV-2 variants. Lancet Infect Dis. 2023;23:153–4. doi:10.1016/s1473-3099(23)00001-4.
- Lang-Meli J, Luxenburger H, Wild K, Karl V, Oberhardt V, Salimi Alizei E, Graeser A, Reinscheid M, Roehlen N, Reeg DB, et al. SARS-CoV-2-specific T-cell epitope repertoire in convalescent and mRNA-vaccinated individuals. Nat Microbiol. 2022;7:675–9. doi:10.1038/s41564-022-01106-y.
- Keeton R, Tincho MB, Ngomti A, Baguma R, Benede N, Suzuki A, Khan K, Cele S, Bernstein M, Karim F, et al. Author correction: T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022;604:E25. doi:10.1038/s41586-022-04708-y.
- Assawakosri S, Kanokudom S, Suntronwong N, Auphimai C, Nilyanimit P, Vichaiwattana P, Thongmee T, Duangchinda T, Chantima W, Pakchotanon P, Srimuan D. Neutralizing activities against the Omicron variant after a heterologous booster in healthy adults receiving two doses of CoronaVac vaccination. J Infect Dis. 2022;226:1372–1381. doi:10.1101/2022.01.28.22269986.
- Filardi BA, Monteiro VS, Schwartzmann PV, Do Prado Martins V, Zucca LER, Baiocchi GC, Malik AA, Silva J, Hahn AM, Chen NF, et al. Age-dependent impairment in antibody responses elicited by a homologous CoronaVac booster dose. Sci Transl Med. 2023;15:eade6023. doi:10.1126/scitranslmed.ade6023.
- Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, Hou LH, Li J-X, Yang B-F, Wang L, Wang W-J, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–88. doi:10.1016/s0140-6736(20)31605-6.
- Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L, Jadi RS, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–501 e15. doi:10.1016/j.cell.2020.05.015.
- Sette A, Crotty S. Pre-existing immunity to SARS-CoV-2: the knowns and unknowns. Nat Rev Immunol. 2020;20:457–8. doi:10.1038/s41577-020-0389-z.