ABSTRACT
Herpes zoster (HZ) brings a significant economic burden. The HZ vaccine was introduced in China for the first time in 2020, and there is a lack of up-to-date information on the hospitalization costs and characteristics prior to vaccination. This study aimed to describe the characteristics and economic burden of HZ inpatients in Hunan Province, China, and analyze the factors influencing the length of stay (LOS) and costs. This was a retrospective study and we extracted information from the Chinese National Health Statistics Network Reporting System on HZ inpatients in Hunan Province, China from 2017 to 2019. Spatial join tools and Global or Local Moran’s Index were used for the geographic analysis of hospitalized HZ incidence. Multivariate linear regression models were used to analyze the factors influencing LOS and costs. There were 44,311 HZ inpatients included in this study, incurring a total of $31,857,734 medical costs. These patients had a median LOS of 8 days and a median expenditure of $573.47. Older age, more comorbidities, and the presence of complications with nervous system involved were all significantly associated with longer LOS and higher costs. HZ infection resulted in a large direct medical cost and heavy disease burden, especially in patients with advanced age or underlying medical conditions. The HZ vaccine has the potential to effectively reduce the disease burden and should be widely popularized especially among high-risk groups.
Introduction
Herpes zoster (HZ) is characterized with unilateral clusters of blisters based on erythema, which is secondary to reactivation of varicella-zoster virus (VZV) infection.Citation1,Citation2 Primary VZV infection might cause chickenpox, which is usually self-limited, leaving with VZV latent in the dorsal root ganglion cells. When triggered by immune dysfunction or reinfection, VZV might be reactivated and replicated, leading to a blister rash and pain along the nerve distribution. According to the survey, the percentage of serologically positive VZV in the population exceeds 60% in most regions, and even reaches more than 90% in many areas.Citation3 In other words, they may be potentially susceptible to HZ. About 20–30% of people develop HZ at least once in their lifetime. Furthermore, the incidence of HZ increases with age. The risk of developing HZ is higher for immunocompromised people, especially those over 50 years old.
Patients with HZ presented a high incidence in developing complications, especially in the immunocompromised people.Citation4 The risk of HZ complications also should not be ignored in immunocompetent people.Citation5 Postherpetic neuralgia is most commonly seen, demonstrating with paroxysmal or persistent referred pain distributed along with the innervation infected, which persists for variable periods of time or even for life. A study revealed that 5% to 30% of patients with HZ experience neuropathic pain for more than 3 months.Citation6 Long-term pain brings a significant notorious impact on the life quality of patients,Citation7 which might give rise to high risk of depression, anxiety, and even suicidal behavior.Citation8,Citation9 Besides, long term nerve injury and pain tolerance may cause changes in brain structure according to previous researches.Citation10 They may also have a high intendency in suffering from cardiovascular disease. HZ ophthalmicus is a relatively common complication, occurring in 10–20% of patients with HZ. It is brought on by reactivation of VZV affecting the ophthalmic branch of the trigeminal nerve. It usually manifests as painful blisters around the eye, and may present as keratitis, conjunctivitis, iritis, uveitis, acute retinal necrosis, which can impair vision or even cause blindness.Citation11,Citation12 Other complications consist of bacterial superinfections, peripheral nerve palsies, encephalitis or meningitis, and disseminated HZ, increasing the difficulty in comprehensive management.Citation1,Citation2,Citation13
Although most patients infected with HZ can be treated with traditional therapy with outpatient care,Citation2 it is a challenge to manage the complications and secondary infections for hospitalized HZ patients. Especially, persistent pain can be sometimes refractory and remain as an annoying issue for most patients after varied treatments including analgetic drugs, physiotherapy, neurectomy and etc. Sustaining pain might increase risk in developing severe depression,Citation14 which is more common in older populations.Citation15 In addition, disseminated VZV infection or complication with encephalitis or meningitis remarkably increased the risk in disability, or even death. In all, HZ can pose a high threat to both physical and mental health of the patients. However, the consequence has not been well acquainted by the public that HZ has always been overlooked and not well treated.Citation16 Delay in treatment might develop in poor prognosis and increase the socio-economic burden. HZ has caused significant economic burden, with Germany, France, and the UK paying 199.87 million, 205.7 million, and 271.21 million euros annually, respectively.Citation17 In the United States, HZ and its complications even account for more than $2.4 billion in annual medical costs.Citation18
In China, the current situation of HZ is not optimistic. Epidemiologic surveys show that the overall incidence of HZ in Chinese population is about 6.64/1000 person-years, and 11.2% of patients develop complications.Citation19 Considering the large population base and the uneven distribution of medical resources in China, HZ presents a widespread impact on the health of Chinese population and generates a large economic burden. With aging in China, the incidence of cancer and chronic diseases increases, the expansion of immunocompromised population is bound to increase the risk of developing HZ. However, there are few studies on the economic burden of HZ in China, and there are no latest data on separate inpatient admissions. As the proportion of the elderly population in China increases, hospitalization rates for patients with HZ are likely to rise. For most people, the cost of hospitalization is a great pressure. At this time, medical insurance becomes an extremely important safeguard to ensure the smooth progress of treatment and reduce the financial burden on patients. It is necessary to understand the LOS and costs of hospitalized patients with HZ in China, so that we can take timely targeted measures, such as vaccine,Citation20 to alleviate the pressure in the future.
Hunan is a representative province in central China with dense population and upper middle economy but highly unbalanced regional development. In this study, we retrospectively investigated the annual hospitalization rate, the length of stay (LOS), economic burden, as well as the influencing factors, of hospitalized HZ patients in Hunan Province.
Methods
Data sources and study design
This was a retrospective study analyzing data extracted from the Chinese National Health Statistics Network Reporting System (CNHSNRS) in Hunan province.Citation21,Citation22 All data in CNHSNRS were proofread to exclude inaccurate or incomplete data following the criteria posted by the National Health Commission of China.Citation23 According to the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10), all inpatients from 372 hospitals across Hunan province with a primary diagnosis of HZ (ICD-10 codes: B02.0 to B02.9) between 1 January 2017 and 31 December 2019 were included.
We collected information on patient gender, age, type of complications, number of comorbidities, type of hospital (western medicine hospitals or Chinese medicine hospitals), hospital grade, overall hospital costs (including medication, examination, nursing, and hospital bed costs, etc), type of payer (insurance or out-of-pocket (OOP) payment) and LOS. The patients hospitalized at Chinese medicine hospitals were assumed to have received traditional Chinese medicine (TCM) therapy. Patients were separated into three age groups: less than 45 years, 45 to 64 years, and 65 years or older. In order to explore the impact of different complications on hospital cost and LOS, they were divided into five groups: Zoster encephalitis or meningitis (ICD-10 codes: B02.0, B02.1), Zoster with other nervous system involvement (B02.2), Zoster ocular disease (B02.3), Disseminated zoster or Zoster with other complications (B02.7, B02.8), and Zoster without complication (B02.9).
In China, according to the differences in hospital scale, equipment, and technical level, hospitals were classified into three levels (from low to high: primary, secondary, and tertiary). Because there were very few inpatients in primary hospitals, we classified hospital levels into tertiary and non-tertiary in this study. To facilitate cost comparison, we adjusted the hospital costs by Consumer Price Index (CPI) in Hunan in 2017 (the base year) in accordance with the National Bureau of Statistics of China and converted 1 Chinese yuan (CNY) to 0.148 US dollars (USD) based on the average exchange rate in 2017.
Statistical analysis
When it comes to the population level, the Sankey diagram is applied to depict the costs of patients classified by age groups, type of payer, and complication types, and the pyramid diagram is used to describe the cost by age, gender, and payer. The characteristics of patients stratified by the type of hospitalized hospital were tabulated in detail. The continuous variables with normal distribution and categorical variables were compared by t-test and Chi-square test, respectively.
When it comes to the individual level, LOS and costs with skewed distributions (examined by Kolmogorov-Smirnov test) were presented by medians and quartiles. The Kruskal-Wallis test or Mann-Whitney U test was employed to analyze the differences between groups. To investigate the risk factors associated with LOS and hospital costs of HZ and its complications, multivariate linear regression models were conducted with LOS and costs naturally log-transformed before analysis.
All statistical analyses were performed using R software (version 4.1.3). A P value of less than .05 was considered statistically significant.
Geographic analysis and mapping for hospitalized HZ incidence
First, we converted the available home address of hospitalized HZ patients (N = 40912) into longitude and latitude by Baidu Web service API of “Geocoder” with Python version 3.8.3.Citation21 Then, spatial join analysis was conducted to analyze the number of hospitalized patients in each county or district. The annual hospitalized HZ incidence was calculated as dividing the number of patients by the approximate total population in corresponding county or district which was obtained according to census results in 2020 (https://www.citypopulation.de/en/china/hunan/admin/).
We applied Global Moran’s Index to examine whether potential spatial autocorrelation existed. While the spatial randomness hypothesis was rejected by GlobalMoran’s Index with p-value < .05, we further identified significant spatial clusters by calculating Local Moran’s Index. Last, we used multivariate poisson regression model to investigate the association of healthcare resources availability (including number of doctors, registered nurses, and hospital beds per 10,000 population), disposable income per capita, and population density with the incidence of hospitalized HZ between high-high and low-low spatial clusters. All geographic analyses were conducted based on ArcGIS 10.8.1.
Results
Spending at the population level
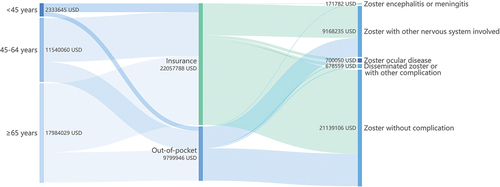
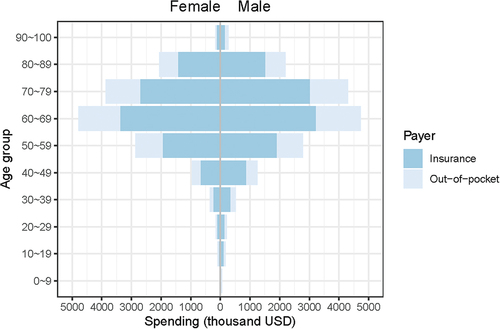
We included data of 44,311 hospitalized HZ patients from 2017 to 2019 for statistical analysis. Among 44,311 HZ inpatients 36,656 (82.7%) of them had ever been paid via insurance payment, while 7,655 (17.3%) of them were totally paid via OOP payment. The total hospitalization cost was $31,857,734, while the proportion paid by OOP was $9,799,946, accounting for 30.76% of the total costs. The composition of costs was summarized by age groups, payer, and complications as presented in . Most HZ patients presented no complication, while those with nervous system involved took up a large share of the hospitalization costs (). Besides, the population ranging from 60 to 69 years occupied the largest proportion of the hospitalization costs, which distributed similarly among different gender. (). The pyramid diagram shown for HZ inpatients residing on high-high or low-low spatial clusters revealed a similar distribution pattern as the overall sample (Figure S1–S2).
Geographic distribution of the hospitalized HZ incidence
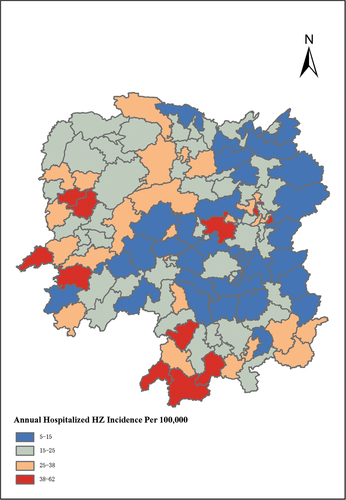
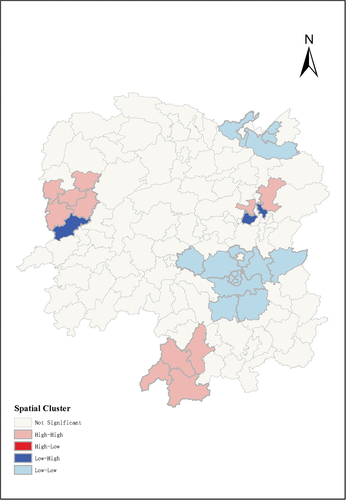
The annual incidence of hospitalized HZ in each county or district in Hunan province displayed spatial autocorrelation features as confirmed by Global Moran’s Index with statistically significance (Moran’s I = 0.306, Z-score = 4.796, p < .001) (). Spatial clusters analysis based on Local Moran’s Index showed that counties or districts with high incidence of hospitalized HZ were mainly located at west or southwest of Hunan, and the urban area of Changsha city. However, the incidence was relatively low in most contiguous area of Hengyang city and Yueyang city (). Results from multivariate poisson regression models indicated that higher number of hospital beds per 10,000 population and population density were significantly associated with a higher incidence of hospitalized HZ. However, increased number of doctors and registered nurses per 10,000 population and disposable income per capita had a negative impact on hospitalized HZ incidence. (Table S1)
Spending and LOS at the individual level
Among all patients included in the study (), there were 22,761 males (51.4%) and 21,550 females (48.6%). The mean age was 63.05 ± 15.27 years old. Only 4375 patients (9.9%) were under the age of 45, with 22,699 patients (51.2%) over the age of 65 and 17,237 patients (38.9%) between 45 to 64 years old. There were 12,372 (27.9%), 15595 (35.2%), and 16,344 (36.9%) patients recorded in 2017, 2018, and 2019, respectively. A total of 25,884 patients (58.4%) attended non-tertiary hospitals, while 18,427 patients (41.6%) chose tertiary hospitals. Among these patients 11,589 (26.2%) presented no comorbidities 16,669 (37.6%) had one or two comorbidities, and 16,053 (36.2%) had three or more comorbidities. In terms of complications, there were 962 (2.2%) with disseminated zoster or with other complications, 208 (0.5%) with meningitis or encephalitis, 8,841 (20%) with other nervous system involved, and 1320 (3%) with ocular disease. A total of 32,980 (74.4%) patients did not develop complications from HZ. Females, patients with younger age and less comorbidities, and hospitalized HZ with other nervous system involved or ocular diseases tended to choose Chinese medicine hospitals for treatment.
Table 1. Characteristics of herpes zoster inpatients in Hunan from 2017 to 2019.
LOS of the hospitalized patients was at a median level of 8 days (IQR: 6–11), while the costs was at $573.47 (IQR: 378.33–871.70), as shown in . In addition, those patients with older age, more comorbidities, with nervous system involved, or visiting tertiary hospitals presented longer LOS and higher costs. However, zoster ocular diseases might take less hospitalization costs, with decreasing LOS compared to other HZ complications. Those patients hospitalized at Chinese medicine hospitals exhibited a relatively longer LOS and higher costs compared to those hospitalized at western medicine hospitals.
Table 2. Comparison of the LOS and hospital costs of HZ inpatients by different characteristics.
Factors for LOS and spending of hospitalized HZ
The multivariate linear regression models () that including potential influencing factors showed that older age, more comorbidities, visiting tertiary hospitals, or receiving TCM treatment were significantly associated with longer LOS and higher hospital costs. Compared with HZ without complications, zoster encephalitis or meningitis, and zoster ocular disease didn’t significantly affect LOS, but disseminated HZ or zoster with other complications (β = 0.10, 95%CI: 0.09, 0.11) and zoster with other nervous system involved (β = 0.07, 95%CI: 0.04, 0.11) significantly prolonged LOS.
Table 3. Multivariate linear models for risk factors associated with LOS and hospital costs (log transformed).
As for hospital costs of HZ, HZ patients with ocular disease had distinct lower hospitalization costs than those without complications (β=-0.21, 95%CI: −0.25, −0.18), whereas other HZ complications markedly increased hospitalization costs. There was no meaningful impact of gender on both LOS and hospitalization expenses.
Discussion
In this study, we analyzed the information of hospitalized patients with HZ in Hunan Province from 2017 to 2019, revealing the median LOS of hospitalized patients with HZ at 8 days, the median expenditure was $573.47, and a total medical expenditure of up to $31,857,734 in three years.
Our research indicates a consistent increase in the number of annual hospitalized HZ patients from 2017 to 2019. Previous studies have shown that the incidence of HZ also continues to increase before vaccination in many parts of the world. This phenomenon indicates the challenging situation of prevention and control, as well as the mounting medical burden.
According to our study, over half (51.2%) of the patients aged at 65 or older, while less than one-tenth (9.9%) were under the age of 45, revealing that aging significantly increases the risk in developing HZ. Identically, a multitude of prior studies substantiated aging as one of the primary risk factors for HZ, which might attribute to the fact that the elderly population possesses a diminished immune response in comparison to the youngsters. The body primarily relies on cell-mediated immune response against VZV. Compared with young people, VZV-specific T cells in the elderly showed reduced numbers, slower responses, and an increased proportion of dysfunctional T cells, which were prone to VZV reactivation.Citation24,Citation25 The increasing proportion of older individuals will continue to exert significant pressure in the foreseeable future.Citation26 Hence, prioritizing protective measures for older adults can lead to a significant reduction in the economic burden associated with HZ in the short term.
The number of comorbidities has a direct impact on both the LOS and costs. HZ patients who are hospitalized with three or more comorbidities experience significantly longer stays and higher costs, which is not surprising given that comorbidities often complicate the disease management. Alarmingly, 73.8% of patients in this study had at least one comorbidity, indicating a higher likelihood in developing HZ for those with underlying diseases. Several studies have found that chronic obstructive pulmonary disease, asthma, chronic kidney disease, inflammatory bowel disease, diabetes, polycystic ovary syndrome, hematologic tumors, HIV infection and autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis are associated with a higher risk of HZ. This may be due to impaired cell-mediated immunity caused by the underlying disease itself or receiving immunosuppressive therapy.Citation27,Citation28 In addition, HZ and the underlying disease will also affect each other, increasing the difficulty of treatment. Therefore, more comprehensive protection is very necessary for them.
The complication is an integral part of the evaluation of HZ, which is also the main reason for the extremely high social and economic costs. This study further confirms the frequent occurrence of neurological complications, which accounts for 20% of the hospitalized patients and leads to a prolonged hospital stay and increased costs. This finding is in line with the previous concept that neurological complications, particularly postherpetic neuralgia, constitute a significant source of the substantial burden and bring about a grave impact on patients afflicted with HZ.Citation29,Citation30 Therefore, neural prosthetics could be of top priority when managing patients with HZ. Surprisingly, in our study, HZ ophthalmicus appeared to cause less hospital costs compared to HZ without complication. We believe that patients with HZ who have ocular involvement may pay more attention to the concern of vision loss and therefore receive treatment earlier to avoid the increase in treatment costs due to disease progression.
In Hunan Province, there is a geographic variation of the annual hospitalization rate of HZ. Previous research has found that lower socioeconomic status and income decline are associated with a higher incidence of HZ.Citation31,Citation32 Another study identified the population density as a determinant of VZV seroprevalence.Citation33 The higher density of doctors or nurses may help people gain better preventive or health knowledge, and seek for earlier medical visits, thus avoiding disease from progression to hospitalization. For the positive correlation between hospital beds supply and HZ hospitalization, it can be interpreted as the convenience to inpatient facilities preventing the treatment of HZ at the primary care level. It is noteworthy that Changsha, the provincial capital and economic center of Hunan Province, exhibits a high annual hospitalization rate which might attribute to its high population density and abundant hospital beds supply. Given the relationship between income and HZ, for patients with financial concerns, health insurance support is crucial. Hospitalization costs vary greatly by region, ranging from 774.66 to 31,026.22 euros in Europe and from 9041.36 to 23,219.82 euros in the United States.Citation34 The direct hospitalization cost in Latin America was approximately $763.19 USD.Citation30 In China, the average cost for hospitalized HZ patients ranged from 573.47 to 1179.6 USD.Citation19,Citation35 Because of the extensive coverage of medical insurance in China, our result showed that nearly 70% of the cost was paid for by medical insurance in general. As aging continues to deepen, healthcare insurance funds will face mounting pressure due to the higher risk in getting diseases, such as HZ.
Although early diagnosis and treatment can reduce the individual treatment cost, the overall cost remains a large burden owing to the growing number of patients. The HZ vaccine may be the key to solve this problem. Currently, there are two major HZ vaccines: the live attenuated Zostavax vaccine, which was approved by the FDA in 2006, and the recombinant Shingrix vaccine, which was approved by the FDA in 2017. Many studies have shown the promising efficacy of vaccines. Previous clinical trials have shown that the live attenuated herpes Zostavax vaccine can reduce the incidence of HZ by 51.3% and postherpetic neuralgia by 66.5% in people over the age of 60,Citation36 with good safety.Citation37 According to a survey by Ryan R Thompson et al., the incidence of HZ among people older than 60 years of age in the United States has decreased annually since the introduction of the live attenuated vaccine in 2007.Citation38 Moreover, effectiveness continues to increase with the development of new vaccines. Himal Lal et al. showed that the recombinant subunit vaccine had a high protective efficacy of 97.2% in people over 50 years of age and had the potential to greatly reduce the incidence of HZ.Citation39 The clinical trial of Celine Boutry et al. showed that the recombinant zoster vaccine had a protective efficacy of more than 84% for up to 7 years, demonstrating the ability of vaccines to provide long-term and stable protection.Citation40 At the same time, the severity of patients who unfortunately remained ill after vaccination decreased. Studies have found that getting vaccinated against HZ can even reduce the risk of stroke.Citation41 Unlike live attenuated vaccines, recombinant subunit vaccines also provide protection in immunocompromised persons with underlying diseases.Citation42 The FDA has approved Shingrix for immunocompromised persons older than 18 years of age.Citation43 More and more people have the opportunity to be protected by vaccines. However, many countries, including China, have not yet introduced HZ vaccine into their national immunization schedules. At the same time, people also face many concerns about paying for vaccination, which is difficult to effectively protect the relatively susceptible low-income population. One study showed that more than half of respondents over the age of 50 had no active intention to be vaccinated.Citation44 In 2020, Shingrix was listed in China at a price of 1600 RMB for people over 50 years old. On April 10, 2023, the first Chinese-developed HZ vaccine was marketed in China and met the immunization needs of people aged 40 to 50 years old. Although cheaper than the former, it is still too high for most people and became a huge obstacle to improving the vaccination rate of HZ. At this time, manufacturers’ reduction of vaccine prices, government funding support, and more active disease knowledge promotion may effectively improve the population coverage. The efforts of expanding vaccine coverage to all age groups and incorporating it into national immunization programs should be considered in the future to strengthen primary prevention.Citation45 Meanwhile, it is necessary to improve the primary health care (PHC) system, increase investment, and fully leverage the indispensable role of PHC in the prevention and control of diseases such as HZ, as well as in achieving universal health coverage, which can reduce the disease burden in a comprehensive manner.Citation46,Citation47 Our study measured the burden of disease from 2017 to 2019, when HZ vaccination was not implemented in China. The pre-vaccination baseline disease burden data was provided to support health economic evaluation of the HZ vaccine, enabling a cost-benefit analysis of vaccination promotion against the economic impact of the disease.
There are some limitations to this study. First, this study indirectly reflects the situation in China through the situation in Hunan Province. However, due to differences in medical systems, medical resources, and race, the reference value for other countries and regions may be biased. It should be interpreted with caution when generalizing our results to other economically developed regions, which might suffer from reduced burden because of higher income and more healthcare workers. Nonetheless, our findings can still provide important reference for most regions of the world with uneven economic development, especially regions with inadequate primary care system. Second, the impact due to the different data quality and completeness in different hospitals is inevitable. The supervision undertaken by Hunan provincial Health Commission helps to guarantee the accuracy of data, while the omission of records may still exist, especially in relatively lower levels of hospitals, thus leading to an underestimation of the total economic burden incurred by HZ hospitalization.
Conclusion
In summary, neurological symptoms, complicated with underlying diseases, and aging significantly increase the economic or/and disease burden of HZ for hospitalization. HZ vaccination should be highly recommended and generalized to avoid the heavy disease burden.
Supplemental Material
Download PDF (110.9 KB)Supplemental Material
Download PDF (222.9 KB)Acknowledgments
The authors would like to thank the staffs from the Health Data Center of Hunan Health Commission for their assistance with access to the anonymous data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data will be made available on request. The corresponding author ([email protected]) will be responsible for reviewing and approving data applications.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2268990.
Additional information
Funding
References
- Le P, Rothberg M. Herpes zoster infection. BMJ. 2019;364:k5095. doi:10.1136/bmj.k5095.
- Schmader K. Herpes zoster. Ann Intern Med. 2018;169(12):ITC19–9. doi:10.7326/AITC201808070.
- Amjadi O, Rafiei A, Haghshenas M, Navaei RA, Valadan R, Hosseini-Khah Z, Omran AH, Arabi M, Shakib RJ, Mousavi T, et al. A systematic review and meta-analysis of seroprevalence of varicella zoster virus: a nationwide population-based study. J Clin Virol. 2017;87:49–59. doi:10.1016/j.jcv.2016.12.001.
- Bardach AE, Palermo C, Alconada T, Sandoval M, Balan DJ, Nieto Guevara J, Gómez J, Ciapponi A. Herpes zoster epidemiology in Latin America: a systematic review and meta-analysis. PloS One. 2021;16(8):e0255877. doi:10.1371/journal.pone.0255877.
- Kridin K, Cohen AD. The underestimated risk for complications in immunocompetent patients with herpes zoster: should we change our clinical practice? Br J Dermatol. 2021;184(6):994–5. doi:10.1111/bjd.19860.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833. doi:10.1136/bmjopen-2014-004833.
- Drolet M, Brisson M, Schmader KE, Levin MJ, Johnson R, Oxman MN, Patrick D, Blanchette C, Mansi JA. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. CMAJ. 2010;182(16):1731–6. doi:10.1503/cmaj.091711.
- Du J, Sun G, Ma H, Xiang P, Guo Y, Deng Y, Li S, Li X. Prevalence and risk factors of anxiety and depression in patients with postherpetic neuralgia: a retrospective study. Dermatology. 2021;237(6):891–5. doi:10.1159/000512190.
- Schlereth T, Heiland A, Breimhorst M, Fechir M, Kern U, Magerl W, Birklein F. Association between pain, central sensitization and anxiety in postherpetic neuralgia. Eur J Pain. 2015;19(2):193–201. doi:10.1002/ejp.537.
- Li H, Li X, Wang J, Gao F, Wiech K, Hu L, Kong Y. Pain-related reorganization in the primary somatosensory cortex of patients with postherpetic neuralgia. Hum Brain Mapp. 2022;43(17):5167–79. doi:10.1002/hbm.25992.
- Ting DSJ, Ghosh N, Ghosh S. Herpes zoster ophthalmicus. BMJ. 2019;364:k5234. doi:10.1136/bmj.k5234.
- Freund PR, Chen SH. Herpes zoster ophthalmicus. CMAJ. 2018;190(21):E656. doi:10.1503/cmaj.180063.
- Cohen JI. Clinical practice: herpes zoster. N Engl J Med. 2013;369(3):255–63. doi:10.1056/NEJMcp1302674.
- Chen MH, Wei HT, Su TP, Li CT, Lin WC, Chang WH, Chen T-J, Bai Y-M. Risk of depressive disorder among patients with herpes zoster: a nationwide population-based prospective study. Psychosom Med. 2014;76(4):285–91. doi:10.1097/PSY.0000000000000051.
- Pickering G, Leplege A. Herpes zoster pain, postherpetic neuralgia, and quality of life in the elderly. Pain Pract. 2011;11:397–402. doi:10.1111/j.1533-2500.2010.00432.x.
- Paek E, Johnson R. Public awareness and knowledge of herpes zoster: results of a global survey. Gerontology. 2010;56(1):20–31. doi:10.1159/000240046.
- Gater A, Uhart M, McCool R, Preaud E. The humanistic, economic and societal burden of herpes zoster in Europe: a critical review. BMC Public Health. 2015;15(1):193. doi:10.1186/s12889-015-1514-y.
- Harvey M, Prosser LA, Rose AM, Ortega-Sanchez IR, Harpaz R. Aggregate health and economic burden of herpes zoster in the United States: illustrative example of a pain condition. Pain. 2020;161(2):361–8. doi:10.1097/j.pain.0000000000001718.
- Sun X, Wei Z, Lin H, Jit M, Li Z, Fu C. Incidence and disease burden of herpes zoster in the population aged ≥50 years in China: data from an integrated health care network. J Infect. 2021;82(2):253–60. doi:10.1016/j.jinf.2020.12.013.
- Mick G. Vaccination: a new option to reduce the burden of herpes zoster. Expert Rev Vaccines. 2010;9:31–5. doi:10.1586/erv.10.32.
- Chen P, Huang J, Li S, Tang Y, Xiao Y, Zou B, Chen X, Li J, Zhao Z, Shen M, et al. Nitrogen dioxide and hospital length of stay and cost for systemic lupus erythematosus in Hunan, China. Sci Total Environ. 2023;856:159013. doi:10.1016/j.scitotenv.2022.159013.
- Deng X, Zou B, Li S, Wu J, Yao C, Shen M, Chen J, Li S. Disease specific air quality health index (AQHI) for spatiotemporal health risk assessment of multi-air pollutants. Environ Res. 2023;231:115943. doi:10.1016/j.envres.2023.115943.
- Yang J, Wan SQ, Huang L, Zhong WJ, Zhang BL, Song J, Ma Y-H, Hu M. Analysis of hospitalization costs and length of stay for oral cancer patients undergoing surgery: evidence from Hunan, China. Oral Oncol. 2021;119:105363. doi:10.1016/j.oraloncology.2021.105363.
- Weinberg A, Canniff J, Rouphael N, Mehta A, Mulligan M, Whitaker JA, Levin MJ. Varicella-zoster virus–specific cellular immune responses to the live attenuated zoster vaccine in young and older adults. J Immunol. 2017;199(2):604–12. doi:10.4049/jimmunol.1700290.
- Arvin A. Aging, immunity, and the varicella–zoster virus. N Engl J Med. 2005;352(22):2266–7. doi:10.1056/NEJMp058091.
- Varghese L, Standaert B, Olivieri A, Curran D. The temporal impact of aging on the burden of herpes zoster. BMC Geriatr. 2017;17(1):30. doi:10.1186/s12877-017-0420-9.
- Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc. 2017;92:1806–21. doi:10.1016/j.mayocp.2017.10.009.
- Qian J, Lassere MN, Heywood AE, Liu B. Use of disease-modifying antirheumatic drugs and the subsequent risk of herpes zoster in older adults. Rheumatology (Oxford). 2021;60(11):5042–51. doi:10.1093/rheumatology/keab538.
- Diez-Domingo J, Curran D, Cambronero MDR, Garcia-Martinez JA, Matthews S. Economic burden and impact on quality of life of herpes zoster in Spanish adults aged 50 years or older: a prospective cohort study. Adv Ther. 2021;38(6):3325–41. doi:10.1007/s12325-021-01717-7.
- Rampakakis E, Pollock C, Vujacich C, Toniolo Neto J, Ortiz Covarrubias A, Monsanto H, Johnson, KD. Economic burden of herpes zoster (“culebrilla”) in Latin America. Int J Infect Dis. 2017;58:22–6. doi:10.1016/j.ijid.2017.02.021.
- Esteban-Vasallo MD, Dominguez-Berjon MF, Gil-Prieto R, Astray-Mochales J, Gil de Miguel A. Sociodemographic characteristics and chronic medical conditions as risk factors for herpes zoster: a population-based study from primary care in Madrid (Spain). Hum Vaccines Immunother. 2014;10(6):1650–60. doi:10.4161/hv.28620.
- Onizuka H, Fukuda H. Associations between income changes and the risk of herpes zoster: LIFE study. Social Sci Med. 2023;328:115981. doi:10.1016/j.socscimed.2023.115981.
- Sornchai S, Warachit W, Lolekha P, Kosuwan P, Sutra S, Tanthiphabha B, Sutra S, Chup-Upprakarn S, Bock HL, Kosuwan P. Effect of climatic factors and population density on varicella zoster virus epidemiology within a tropical country. Am J Trop Med Hyg. 2001;64(3):131–6. doi:10.4269/ajtmh.2001.64.131.
- Panatto D, Bragazzi NL, Rizzitelli E, Bonanni P, Boccalini S, Icardi G, Gasparini R, Amicizia D. Evaluation of the economic burden of Herpes Zoster (HZ) infection. Hum Vaccines Immunother. 2015;11(1):245–62. doi:10.4161/hv.36160.
- Yin D, Van Oorschot D, Jiang N, Marijam A, Saha D, Wu Z, Tang H, Diaz-Decaro J, Watson P, Xie X, et al. A systematic literature review to assess the burden of herpes zoster disease in China. Exp Rev Anti-Infect Ther. 2021;19(2):165–79. doi:10.1080/14787210.2020.1792290.
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–84. doi:10.1056/NEJMoa051016.
- Willis ED, Woodward M, Brown E, Popmihajlov Z, Saddier P, Annunziato PW, et al. Herpes zoster vaccine live: a 10 year review of post-marketing safety experience. Vaccine. 2017;35:7231–9. doi:10.1016/j.vaccine.2017.11.013.
- Thompson RR, Kong CL, Porco TC, Kim E, Ebert CD, Acharya NR. Herpes zoster and postherpetic neuralgia: changing incidence rates from 1994 to 2018 in the United States. Clini Infect Dis. 2021;73(9):e3210–e7. doi:10.1093/cid/ciaa1185.
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–96. doi:10.1056/NEJMoa1501184.
- Boutry C, Hastie A, Diez-Domingo J, Tinoco JC, Yu CJ, Andrews C, Beytout J, Caso C, Cheng H-S, Cheong HJ, et al. The adjuvanted recombinant zoster vaccine confers long-term protection against herpes zoster: interim results of an extension study of the pivotal phase 3 Clinical trials ZOE-50 and ZOE-70. Clin Infect Dis. 2022;74(8):1459–67. doi:10.1093/cid/ciab629.
- Yang Q, Chang A, Tong X, Merritt R. Herpes zoster vaccine live and risk of stroke among medicare beneficiaries: a population-based cohort study. Stroke. 2021;52(5):1712–21. doi:10.1161/STROKEAHA.120.032788.
- Bastidas A, de la Serna J, El Idrissi M, Oostvogels L, Quittet P, Lopez-Jimenez J, Vural F, Pohlreich D, Zuckerman T, Issa NC, et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized Clinical trial. JAMA. 2019;322(2):123–33. doi:10.1001/jama.2019.9053.
- Anderson TC, Masters NB, Guo A, Shepersky L, Leidner AJ, Lee GM, Kotton CN, Dooling KL. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the advisory committee on immunization practices — United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(3):80–4. doi:10.15585/mmwr.mm7103a2.
- Jiang B, Wang Q, Wang Z, Xu Y, Yang T, Yang W, Jia M, Feng L. Willingness to accept herpes zoster vaccines and the influencing factors in China. BMC Infect Dis. 2022;22(1):888. doi:10.1186/s12879-022-07840-2.
- Lahariya C, Bhardwaj P. Adult vaccination in India: status and the way forward. Hum Vaccin Immunother. 2020;16:1508–10. doi:10.1080/21645515.2019.1692564.
- Lahariya C. ‘Ayushman Bharat’ program and universal Health coverage in India. Indian Pediatr. 2018;55(6):495–506. doi:10.1007/s13312-018-1341-1.
- Lahariya C. Health & wellness centers to strengthen primary Health care in India: concept, progress and ways forward. Indian J Pediatr. 2020;87(11):916–29. doi:10.1007/s12098-020-03359-z.