ABSTRACT
Although highly infectious respiratory viral infections spread rapidly, humans have evolved a precise and complex immune mechanism to deal with respiratory viruses, with strong intrinsic, highly adaptive and specific humoral and cellular immunity. At the same time, vaccination against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is one of the most cost-effective and efficient means of preventing morbidity, severe illness, and death from Coronavirus disease 2019 (COVID-19). As the global epidemic of COVID-19 continues to evolve and vaccines are being developed, it is important to conduct studies on immunization strategies to optimize vaccination strategies when appropriate. This review was conducted to investigate the relationship between the immune response and the protective effect of different vaccination scenarios (including booster, sequential and hybrid immunity), and to provide a basis for the optimization of vaccination strategies and the development of new vaccines in the future.
Introduction
Since December 2019, COVID-19 has become a major public health event with the global transmission. According to public data from the World Health Organization (WHO) website, as of 3 May, 2023, there were 765,222,932 cumulative confirmed cases of COVID-19, 6,921,614 cumulative deaths, and 13,343,360,939 cumulative reported vaccination doses worldwide.Citation1 As the pandemic continues to spread and mutations continue to accumulate, the WHO has identified seven variants of concern (VOC), all of which are Omicron variants. The Omicron variant, with up to 40 mutations in the stinger protein alone, is highly transmissible and immune evasive and has rapidly replaced other strains as the dominant strain since 4 May, 2023.Citation2 The new Omicron subtypes are often the result of amino acid mutations in their ancestral Omicron subtypes that further evade the immune response.Citation3
Vaccines are essential for preventing and controlling infectious diseases, particularly viral infections. When it comes to SARS-CoV-2, the spike protein on its surface is the primary target for neutralizing antibodies. Many COVID-19 vaccines have been developed to specifically target this spike protein, as it plays a crucial role in the virus’s entry into host cells. () The immune response elicited by vaccines is not solely reliant on neutralizing antibodies. T cells, another component of the immune system, play a crucial role in recognizing and eliminating infected cells. T cells are less affected by changes in the spike protein and can provide protection against severe disease, even if the virus has evolved to partially evade neutral of existing vaccines against different variants. The COVID-19 vaccine is mainly divided into whole virus vaccines and virus component vaccines. Whole virus vaccines include attenuated and inactivated vaccines. Viral component vaccines include protein subunit vaccines, virus-like particle vaccines, DNA vaccines, RNA vaccines, non-replicating virus vector vaccines, replicating virus vector vaccines, etc. ().
Figure 1. The whole process of SARS-CoV-2 life cycle. First step: after the S protein ACE2 of SARS-CoV-2 binding to the host cell’s receptor, the S protein ACE2 is cleaved by the protease TMPRSS2, which induces a change in the conformation of the S protein; after the virus is endocytized by the cell, the S protein ACE2 is further cleaved by the host protease, to be exposed the envelope fusion region of S2, thereby mediating fusion of viral outer envelope with host cell membrane. Second step: the protein precursor encoded by the gene ORF1ab of the virus undergoes a series of cleavage and modification to produce non-structural proteins (NSPs), which can rapidly inhibit the translation of host mRNA, and at the same time start to replicate and translate the virus’s own RNA. Third step: SARS-CoV-2 induces the formation of double membrane vesicles (DMVs) in the intracellular membrane system to provides a safe environment for viral RNA replication and to simultaneously translate viral structural proteins. Fourth step: the structural proteins and genomic RNA of the virus are assembled into a complete virus particle in ERGIC; and virus particles are secreted out of the cell by vesicles.
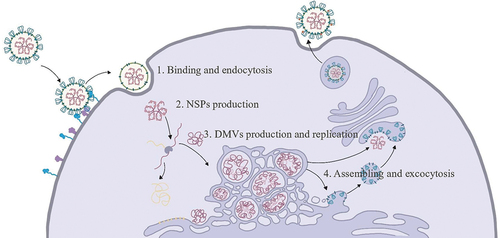
Table 1. Vaccines for SARS-COV-2.
Neutralizing antibodies recognize and stop viruses in body fluids from invading cells, while killer T cells are responsible for attacking and clearing infected cells when a few have escaped the antibody-invading cells. Antibody levels in the serum will gradually decline over time, and T cells and memory B cells will play a long-term protective role in the future when antibody levels drop lower and viral invasion is encountered again.Citation4
Immunoglobulin G (IgG) is the most abundant antibody in the blood and is produced by B cells in response to an antigen. After vaccination, IgG antibodies are produced by plasma cells and can be detected in the blood. It has been shown that the half-life of IgG antibodies in people recovering from mild COVID-19 is approximately 73 days (52–120 days).Citation5 This phenomenon could potentially account for the continued occurrence of breakthrough infections following vaccination, considering the emergence of variants with enhanced immune evasion abilities that are engaged in a competitive relationship with the host’s immune system. Furthermore, a decline in human antibody levels over time, particularly in more vulnerable populations, increases the likelihood of falling below the protective threshold. However, it remains unclear why breakthrough infections among vaccinated individuals are generally less likely to progress to severe disease. One plausible explanation is the rapid activation of the body’s T cells and memory B cells. In light of this context, it becomes imperative to examine whether vaccination strategies can be effectively modified to align with the immune response conferred by the vaccine, ultimately achieving optimal prevention of Omicron infections. This paper will examine the relationship between the different immune responses and the protective effects of different vaccination strategies, namely booster, sequential, and hybrid immunity.
Booster immunity
It is a natural part of the immune response that the immune protection induced by either type of vaccine will fade over time after vaccination. Booster immunity is a vaccine dose that is administered after completion of the vaccination and supplemented according to the waning of antibodies to maintain the body’s immunity to SARS-CoV-2. A study tracking the prevalence of Omicron in the UK in November-December 2021 found that Omicron positivity rates were significantly lower in children who had received two doses of vaccine compared to unvaccinated children, and Omicron positivity rates were significantly lower in adults after the 3rd dose of vaccine. As a population-wide epidemiological study, this study confirms the public health significance of vaccination boosters for the prevention of Omicron.Citation6 The UK Department of Health HAS reported real-world vaccine efficacy (VE) of the vaccine three months after the 3rd dose of vaccine rollout, with no significant decrease in protection over the three months the study was conducted in the Delta and Omicron epidemics. Protection against hospitalization and death was 97–99% for the 3rd dose, suggesting the importance of a 3rd dose of highly protective vaccine to prevent infection and serious illness in high-risk countries.Citation7 A study reported an increase in immunogenicity after the 3rd dose of BNT162b2, they found a 1.7-fold increase in receptor-binding domain (RBD) specific IgG, an increase in affinity from 61.1% to 96.3% and a 6.1-fold increase in neutralizing titers after the 3rd dose compared to the 2nd dose. The 3rd dose of vaccination increased protection by 85.6% relative to the two doses of vaccine.Citation8 In addition, the Food and Drug Administration (FDA) reported a neutralizing effect of serum on Omicron after booster vaccination, and found that serum neutralized Omicron at a titer of only 22 after two BNT162b2 vaccinations and recovered to 70 after three vaccinations.Citation9 These studies suggest a high protective effect on Omicron after booster vaccination.
Previously, a study examined whether T-cell responses induced by different vaccine platforms (mRNA-1273, BNT162b2, Ad26.COV2.S, NVX-CoV2373) cross-recognized the SARS-CoV-2 mutant strain. It was found that T-cell recognition of the mutant strain was preserved, but that neutralizing antibodies significantly decreased recognition and response to the mutant strain. In subjects, approximately 6 months post-vaccination, an average of 90% of true CD4+ and 87% of CD8+ memory T cell responses to the mutant strain were preserved, with 84% of CD4+ and 85% of CD8+ responses to Omicron being preserved and T cell immunity being slightly affected by the mutant strain.Citation10 While vaccine-induced neutralizing antibody levels decreased over time, the frequency of SARS-CoV-2 memory B and T cells was stable, which could explain its longer-lasting effectiveness in preventing severe diseases. The above studies provide an important basis for understanding the vaccine-induced immune response.Citation11 Another study investigated the role of Spike-specific memory T cell responses induced by BNT162b2 inoculation against Omicron mutant strains. The study analyzed cells from health care workers following two or three doses of BNT162b2 inoculation or two doses of inoculation after infection. The study found that Spike-specific memory T cells induced by BNT162b2 inoculation had a significant functional response to Omicron and a response comparable to wild-type viral Spike, including IFN-γ, IL-2 and TNF secretion. This study demonstrates that BNT162b2-induced memory T cell pairs are persistently multifunctional and have a significant immune response to Omicron mutants.Citation12 A study found that the immunogenicity results of phase 2 clinical trial of the 4th dose of mRNA vaccination showed that the T cell response was also enhanced after the 4th dose, with a 7.32 and 6.22 fold increase in ELISpot SFU/million cells in the BNT162b2 and mRNA-1273 groups, respectively.Citation13 mRNA vaccination was also found to significantly enhance T-cell responses compared to natural infection, but additional boosters on top of this did not enhance T-cell responses any further. The application of mRNA homologous or heterologous vaccination significantly enhanced the Omicron Spike-specific T-cell response, and multifunctionality and specificity of T-cell interferon secretion were all significantly better than the T-cell response induced by natural infection. If a 3rd dose of vaccine is administered on top of this, antibody titers increase significantly, while T-cell responses remain stable.Citation14 This suggests that the immune response does not improve indefinitely and immune responses concentrated in one subunit and shifts to other subunits are suppressed after repeated doses of a particular vaccine. In a review, Duane Wesemann reported on the process of differentiation and maturation of human B cells and production of high-affinity antibodies after vaccination and concluded that antibody titers over time after vaccination. It is suggested that although antibody titers decrease over time after vaccination, B cells continue to differentiate after the second dose of vaccine and mature in antibody affinity using somatic hypermutation (SHM). This antibody affinity maturation process is actually the most important way in which humans mutate against viruses.Citation15 Nussenzweig’s group reported that three doses of vaccination based on the original wild-type design (BNT162b2 and mRNA-1273) remained effective against Omicron, in large part due to the role of memory B cells. The significant increase in the frequency of RBD-specific memory B-cell distribution after the 3rd dose of vaccine resulted from the continued expansion of RBD-specific memory B-cells induced after the 2nd dose of vaccine and the induction of new memory B-cell clones after the 3rd dose of vaccine. More importantly, the 3rd dose of vaccination resulted in a higher broad spectrum and neutralizing activity of the antibodies secreted by B cells; in particular, the newly induced B cell clones after the 3rd dose of vaccination had significantly higher neutralizing activity of the antibodies secreted by these B cells. Thus, the 3rd dose of vaccine resulted in a significant increase in B-cell repertoire (BCR) diversity, allowing for the rapid production of neutralizing antibodies to a broad spectrum of highly neutralize mutants. Their study explained why 3rd dose of BNT162b2 and mRNA-1273 provides better protection against Omicron infection.Citation16 Another study evaluating the response of memory B cells after three doses of vaccine was reported by John Wherry’s group, which found that after two doses of mRNA-1273 or BNT162b2 vaccine, antibody decay stabilized after 6–9 months, but antibody affinity maturation gradually increased. Spike and RBD-specific memory B cells remained stable over time, and 40–50% of RBD-specific memory B cells could bind the four mutant strains Alpha, Beta, Delta and Omicron. Spike and RBD-specific memory B cells remained stable over time, with 40–50% of RBD-specific memory B cells able to bind the four mutant strains Alpha, Beta, Delta and Omicron. After the 3rd dose of mRNA-1273 or BNT162b2 vaccine, Omicron RBD-specific memory B cells were activated in subjects, and this activation was closely associated with a significant increase in the neutralization titer to Omicron. A very important finding was that antibody titers before 3rd dose were negatively correlated with the fold change in antibody boosters, suggesting that high levels of circulating antibodies may limit the additional protection afforded by short-interval boosters. This suggests that antibody titers should be tested before booster vaccination to determine whether a booster is required. The above studies provide data on the quantity and quality of antibodies and B cells over time through three or more antigen exposures, and these data are important for the development of immunization strategies.Citation17 Longer vaccination intervals allow the human immune system to compensate for vaccine deficiencies: allowing more somatic hypermutation of B cells in secondary lymphoid organs.Citation18 Another study showed that longer vaccination intervals significantly increased antibody titers at the expense of T-cell response, suggesting that vaccination intervals should be somewhat flexible.Citation19
There is another key factor to pay extra attention to when studying the relationship between the immune response induced by a vaccine and its effectiveness: the length of incubation of the virus. By incubation period, we usually refer to the time between exposure to the pathogen and the onset of symptoms. The incubation period varies greatly from disease to disease and the activation of memory B cells is required for 3–5 days. An important meta-analysis showed that the incubation periods of the different strains of COVID-19 virus were: 6.65 days for the original strain; 5.00 days for Alpha; 4.50 days for Beta; 4.41 days for Delta; and 3.42 days for Omicron.Citation20 So on the one hand there is a gradual decline in antibody levels after vaccination, and on the other hand, when Omicron is encountered, the body’s immune memory cannot be fully activated before the virus replicates in large numbers, and this is when breakthrough infection occurs. However, compared to unvaccinated patients, vaccinated patients are much better off in terms of duration and severity of illness and are much less likely to become seriously ill. This is where the role of T cells and memory B cells comes into play. A current hypothesis is that an important mechanism for the prevention of severe illness and death by the COVID-19 vaccine is the induction of a long-lasting T-cell response, not just neutralizing antibodies. This may explain the current effectiveness of the three-dose inactivated vaccine in preventing severe illness and death in some real-world studies (e.g. the BA.2 outbreak in Hong Kong, China), despite its low protective power against infection. However, on the other hand, the priority of a vaccine should be to prevent infection, rather than being limited to preventing severe illness and death. Timely adjustment of booster strategies and the development of new vaccines are therefore essential for outbreak control.
Sequential immunity
Homogeneity and heterogeneity of COVID-19 vaccines refer to the similarity or difference in the vaccine components, such as the antigenic proteins or viral vectors used to stimulate the immune system. Homologous vaccines are those that use the same vaccine components for both the primary and booster doses, while heterologous vaccines are those that use different vaccine components for the primary and booster doses, i.e. sequential immunization. The WHO Interim recommendations for heterologous COVID-19 vaccine schedules: interim guidance, published on 16 December 2021, recommends that Sequential immunity studies be conducted using COVID-19 vaccines that have been authorized for emergency use and that the COVID-19 vaccine sequential vaccination should be attempted and studied based on various factors such as country epidemiology, epidemic variant, and vaccine safety.Citation21
Iwasaki’s group reported data from two doses of CoronaVac followed by booster vaccination with BNT162b2, applying the gold standard Plague Reduction Neutralization Test (PRNT) to assess serum neutralization titers following vaccination. It was found that serum neutralizing titers PRNT50 after two doses of CoronaVac were less than Plague Reduction Neutralization Test (PRNT),Citation12 but 28 days after booster vaccination with BNT162b2, serum neutralizing titers PRNT50 rose 10.1-fold against the original wild type and 6.3-fold against Delta in vaccinees, comparable to two doses of BNT162b2. In contrast, for the Omicron mutant strain, there was no neutralizing activity after two doses of CoronaVac, and enhanced inoculation with BNT162b2 increased the neutralizing titer PRNT50 by 7.3-fold.Citation22 The presence of a pool of SARS-CoV-2-specific B cells in BNT162b2 groups is prone to respond to restimulation, which asserts the long-term effectiveness of the BNT162b2 vaccine in contrasting the severe form of the pathology and prevent COVID-19-associated hospitalization. Another group also evaluated sera from subjects who received two doses of inactivated BBIBP-CorV and CoronoVac vaccines followed by mRNA vaccines. This level is close to or at the same level as three doses of inactivated virus vaccine. This level approached or reached the total RBD antibody level of three doses of mRNA vaccine, or mRNA vaccination after infection. The study also found that the inactivated + mRNA mix significantly increased the levels of multifunctional T cells compared to single-dose mRNA vaccination.Citation23 The above study suggests that two doses of inactivated and mRNA vaccine as a booster is a proven protocol for boosting antibody titers with good immune efficacy.Citation24 China National Pharmaceutical Group Corp reported preclinical data on a new Omicron Spike full-length mRNA vaccine. Two mRNA vaccines, Delta-specific ZSVG-02 and Omicron BA.1-specific ZSVG-02-O were designed and given to mice already vaccinated with the inactivated virus vaccine BBIBP-CorV. ZSVG-02-O induced high titers of broad-spectrum neutralizing antibodies in mice, with 3, 10 and 30 ug inducing high titers of neutralizing antibodies against Omicron BA.1, BA.2 and BA.4/5, with 10 and 30 ug inducing titers of neutralizing antibodies against BA.5 at 1105 and 2188 respectively. 1105 and 2188, respectively, and two doses of 30 ug ZSVG-02-O induced a highly effective and broad-spectrum multifunctional T-cell response. This vaccination strategy has implications for the response to Omicron in populations receiving a full course of inactivated virus vaccine.Citation25
Chen Wei’s team reported the safety and immunogenicity results of a phase 1/2 clinical trial of a nebulized oral version of the CanSino Ad5 adenovirus vector vaccine, administered as a third booster dose on top of two doses of the inactivated virus vaccine CoronaVac. The study was divided into three groups, a low-dose oral vaccination group, a high-dose oral vaccination group and a comparison group of homologous intramuscular vaccination with CoronaVac. The incidence of adverse reactions was found to be 19% in the low-dose group, 24% in the high-dose group and 39% in the CoronaVac group (p < .0001). The study applied neutralizing antibody titers to assess the immunogenicity and found that 14 days after vaccination, the NAb GMT against wild-type SARS-CoV-2 was 744.4 in the low-dose group, 714.1 in the high-dose group and only 78.5 in the CoronaVac group. Oral Ad5 vaccine was significantly higher than CoronaVac (p < .0001). Efficient Spike-specific T-cell responses were induced in both the high and low-dose CoronaVac groups, significantly higher than in the CoronaVac group (p < .0001).Citation26 Another team reported data from a phase 4 clinical trial of a mix of Ad5 adenovirus vector vaccine (Convidecia) and inactivated virus vaccine (CoronaVac). 198 people received a second dose of Convidecia 3–6 months after a single dose, and 102 people received a second dose of Convidecia or a mix of CoronaVac as a second dose booster vaccination. Another group of 101 received one dose of CoronaVac, 50 received a second dose of CoronaVac, and 51 received a mixed dose of Convidecia as a second dose. The study assessed the effect of vaccination with a wild-type live virus neutralization test. Although there were more adverse events in the Convidecia group, they were all mild to moderate reactions. It was found that of all combinations, the best induction of neutralizing antibodies was achieved by two doses of CoronaVac followed by vaccination with Convidecia for heterologous boosting. In addition, Convidecia followed by one dose of CoronaVac induced the most significant T-cell response. In conclusion, the primary vaccination with inactivated virus vaccine and the booster vaccination with the Ad5 adenovirus vector was the most effective and had a good safety profile. This means that a booster vaccination with an existing Ad5 linear virus vector vaccine or a recombinant protein subunit vaccine on top of an inactivated virus vaccine can be expected to have good results.Citation27
While the COVID-19 vaccine induces broad and efficient neutralizing antibodies and T-cell immunity, it has been less clear whether the vaccine induces a mucosal immune response in the respiratory tract, but this question is crucial and is directly related to the protective power of the vaccine against infection. A group found lower antibody titers to D614G, Delta, and Omicron in bronchoalveolar lavage fluid (BAL) from vaccinated patients, compared with higher BAL-neutralizing antibody titers from recovered patients. However, antibody responses in the peripheral blood of vaccinated patients were significant. In addition, there were few S-protein-specific T and B cells in the BAL of vaccinated patients compared to recovered patients. The study thus used a mouse model and found that the antibody response induced by vaccination alone was limited to the periphery and that it was difficult to induce an immune response in the respiratory mucosa; however, mRNA vaccination plus mucosal inoculation with adenoviral vector vaccine induced a broad spectrum and high titer of neutralizing antibodies against wild type and Omicron. This study first explains that one of the disadvantages of current vaccines for the prevention of infection is their inability to induce efficient mucosal immunity, and suggests a solution in the form of booster vaccines administered via the mucosal route.Citation28 Iwasaki’s group applied the Prime + Spike method, i.e. primary vaccination with an existing mRNA vaccine and booster with nasally inhaled Spike recombinant protein, and after booster vaccination, the method induced efficient virus-specific plasma IgG and IgA in mice, comparable to two doses of mRNA vaccination; this vaccination combination induced efficient nasal mucosal and lung IgA responses. The immune pathway also induced a virus-specific B-cell response and a mucosal memory T-cell response. In the attack virus model, the Prime + Spike method immunized mice survived 100% of the time and showed no weight loss. Viral titers in the lungs and nose were significantly lower than in the blank control and mRNA prime-immunized groups, and there was no pathological damage to the lungs. The method also provided good protection against the hamster attack model. Spike of SARS-CoV induced broad-spectrum peripheral blood and mucosal humoral and cellular immunity against SARS-CoV and SARS-CoV-2 if the mRNA vaccine was given to mice primed for immunization. This suggests that a single dose of mucosal vaccine can provide significant protection by applying existing vaccines and changing the route of immunization to the already widespread mRNA vaccination of the current population.Citation29
From the results of the available studies on Sequential immunity with COVID-19 vaccine, there is consensus that: (1) Sequential immunity with COVID-19 vaccine has been safe and effective in several countries, and no serious side effects have been observed; (2) Sequential immunity strategies have resulted in vaccine immunogenicity that meets or exceeds that of homologous vaccine boosters; (3) Sequential immunity is more effective than homologous booster immunization against some mutant strains; and (4) Given the safety of Sequential immunity and its effectiveness against some mutated strains, Sequential immunity is an exceptional tool to deal with severe epidemics, virus mutations and vaccine shortages, according to WHO guidelines.
Hybrid immunity
Hybrid immunity refers to the immune protection in individuals who have had one or more doses of a COVID-19 vaccine and experienced at least one SARS-CoV-2 infection before or after the initiation of vaccination. It appears to result in stronger protection than just infection or vaccination alone. Hybrid immunity is an exceptional tool to deal with severe epidemics, virus mutations and vaccine shortages, according to WHO guidelines.
A group reported on the neutralizing activity of inactivated booster sera against the four main Omicron subtypes BA.2, BA.2.12.1, BA.4 and BA.5 that are now prevalent throughout the world. The study applied a pseudovirus neutralization test to assess the effectiveness of the vaccine and found that the neutralizing activity of two doses of BBIBP-CorV against wild-type D614G was 89 NT50 and 274 NT50 for three doses. The neutralizing activity of ZF2001 booster after two doses of BBIBP-CorV was 255. 2 doses of BBIBP-CorV followed by Omicron BA.1 breakthrough infection resulted in a neutralizing activity of 1615 for D614G and 1712 for BA.2.2 breakthrough infection. The neutralizing activity of Omicron was 22 for BA.1, 35 for BA.2, 16 for BA.2.12.1 and 21 for BA.4/5 in three doses of BBIBP-CorV inoculated sera. 2 doses of BBIBP-CorV +1 dose of ZF2001 booster sera had a neutralizing activity of 36 for BA.1, 55 for BA.2 and 21 for BA.4/5. This suggests that the neutralizing activity against all subtypes of Omicron was low after the third dose of both vaccines. In contrast, the neutralizing activity was significantly higher in breakthrough infections. serum from those with BA.1 breakthrough infection after two doses of BBIBP-CorV had a neutralizing activity of 1360 against BA.1, 1059 against BA.2, 777 against BA.2.12.1 and 573 against BA.4/5. serum from those with BA.2 breakthrough infection after two doses of BBIBP-CorV had a neutralizing activity of 241 against BA.1. Thus, the highest titers of neutralizing antibodies and the broadest spectrum were produced after breakthrough BA.1 infection based on two doses of inactivated vaccine, which neutralized many of the currently prevalent Omicron subtypes, whereas the serum neutralizing activity after both vaccinations were lower. and activity after vaccination with both vaccines was lower.Citation30 In addition, it was found that serum activity and broad spectrum were significantly increased in breakthrough infections compared to vaccines without breakthrough infections (n = 47). In particular, breakthrough infections in three-dose vaccinees were associated with significant neutralizing activity against VOC mutants, and breakthrough infection with BA.1 triggered a strong immune recall response, rapidly promoting the expansion of memory B cells specific for conserved epitopes in various VOC mutants rather than inducing BA.1-specific B cells. Memory B cells are remarkably plastic and can be remodeled by different Spike(S antigen) exposures. This study confirms that memory B cells respond rapidly after breakthrough infection by amplifying conserved epitope-specific B cells, but not by increasing BA.1-specific responses.Citation31 A group reported on the B-cell response following Omicron infection in vaccine breakthrough-infected individuals, finding that early in the breakthrough infection, vaccinated individuals rapidly produced high levels of Spike antibodies and specific B-cell response. The antibodies had significant SHM but were specific for the original wild type used in the vaccine, suggesting that acute infection recalled the vaccination-induced memory B-cell response. Very interestingly, memory B cells primarily recognize the relatively conserved S2 subunit, but some time after breakthrough infection, the immunodominant antigen primarily recognized by the antibody shifts toward the RBD on S1, as evidenced by the tendency for RBD-specific neutralizing antibody clones isolated from Omicron breakthrough-infected individuals to become homogeneous. This finding is interesting in that it suggests that the immune memory established by vaccination tends to recognize the very conserved S2 subtype, but that there is a “clonal convergence” of antibodies to the most mutation-intensive RBD epitopes after breakthrough infection.Citation32 Other research group have reported that the magnitude and timing of recall immunity after breakthrough infection depends on the type of SARS-CoV-2 mutant strain. The study found different recall immunity in previously infected individuals vaccinated and in breakthrough infected individuals after vaccination, using a long-term observational cohort. While rapid and strong humoral and T-cell immunity was induced in previously infected individuals following vaccination, recall immunity in vaccine breakthrough infections was delayed and highly variable in magnitude, depending on the type of VOC mutant strain of the virus with which the individual was infected. In contrast, early recall response B-cell activation preceded the increase in neutralizing antibody titers in breakthrough-infected individuals. Omicron-binding memory B cells were efficiently reactivated by a 3rd dose of wild-type vaccine and correlated with the corresponding increase in neutralizing antibody titers. Although the delayed kinetics of immune recall provides a potential mechanism for the lack of complete prevention of viral infection by the vaccine in some patients, antibody recall occurs simultaneously with viral clearance, which may be the mechanism for the protective effect of the vaccine against severe diseases.Citation33 A group assessed the response of SARS-CoV-2 specific CD8+ T cells following antigenic stimulation based on single-cell sequencing in vaccinated, infected and breakthrough-infected individuals. It was found that the sequence of exposure determined the distribution of Spike-specific and non-Spike-specific responses, such that post-infection and post-vaccination resulted in the expansion and differentiation of Spike-specific T cells into CCR7-CD45RA+ effector cells. In contrast, individuals following breakthrough infection develop a strong non-Spike-specific response. Analysis of more than 4000 epitope-specific T cell antigen receptor TCR sequences in the study showed that all exposures triggered a broad spectrum of recognition of expanded TCRs. The findings of this study are important, namely that different sequences of infection and vaccination induce different specific CD8 T cell responses.Citation34
Veesler’s group reported on the immunological profile of breakthrough infections and assessed the serum-neutralizing activity of vaccinated and breakthrough-infected individuals. They also found that Omicron induced a very efficient and broad-spectrum neutralizing antibody response to SARS-CoV-2 VOC mutants including BA.5 and even SARS-CoV after breakthrough infection. The structure resolved by Cryo-em shows that the S2×324heavy chain binds to the open RBD K440-K444 region. Prophylactic administration of S2×324to hamsters prevented Omicron BA.2 and BA.5 infections. It was found that mucosal immunity was not induced locally in the respiratory tract by intramuscular vaccination, but that mucosal immunity was induced by vaccine breakthrough infection.Citation35 Humoral immunity is significantly enhanced by vaccination or hybrid immunity, but hybrid immunity (and natural infections) induces stronger mucosal immunity than vaccination alone, and mucosal immunity is an important shield against infection as the respiratory mucosa is the first point of entry for viruses into the body.Citation36 Individuals with hybrid immunity had the highest magnitude and durability of protection.
Conclusion
Vaccination providing long-lasting protection against SARS-CoV-2 is one of the most cost-effective and efficient options. Currently, humans have generally developed immunity to SARS-CoV-2, particularly antibody-mediated humoral immunity. This could lead to a situation similar to that of the 1918 H1N1 influenza, with each successive outbreak becoming weaker and weaker until it becomes a classic endemic. Due to viral evolution, it is important to evaluate the dynamic changes of humoral and cellular immune responses, protective effects and adverse reactions after different vaccination situations and to conduct studies on vaccination strategies in order to adapt and optimize vaccination strategies in a timely manner. Generally speaking, immune response, which dwindles in months, comes from B cells blocking SARS-CoV-2 from infecting cells via antibodies, and from T cells destroying infected cells and supporting other immune responses. This article provides a detailed framework for the above content, and these experiences will also provide important theoretical support for the development of universal vaccines by using new platforms and new generation vaccines with different technical routes. The development of pan-coronavirus vaccines targeting conserved antibody epitopes across coronaviruses and T-cell epitopes to prevent the emergence of new mutant strains and the development of transmucosal vaccines to induce respiratory mucosal immunity are the focus of the new COVID vaccine.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- World Health Organization. WHO Coronavirus(COVID 19) Dashboard. 2023 Oct 21. https://covid19.who.int/.
- Gangavarapu K, Latif AA, Mullen JL, Alkuzweny M, Hufbauer E, Tsueng G, Haag E, Zeller M, Aceves CM, Zaiets K, et al. Outbreak.Info genomic reports: scalable and dynamic surveillance of SARS-CoV-2 variants and mutations. 2023.
- Telenti A, Hodcroft EB, Robertson DL. The evolution and biology of SARS-CoV-2 variants. Cold Spring Harb Perspect Med. 2022;12(5):a041390. doi:10.1101/cshperspect.a041390.
- Jenni P, Shanron S, Patricia J, Judy O. Kuby immunology. 8thed. W. H. Freeman; 25 May 2018.
- Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Elliott J, Hofmann C, Hausner MA, Ferbas KG, Tobin NH, Aldrovandi GM, Yang OO. Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild COVID-19. N Engl J Med. 2020;383(11):1085–8. doi:10.1056/NEJMc2025179.
- Elliott P, Bodinier B, Eales O, Wang H. Rapid increase in Omicron infections in England during December 2021: REACT-1 study. Science. 2022 Mar 25;375(6587):1406–1411. doi:10.1126/science.abn8347.
- McMenamin ME, Nealon J, Lin Y, Wong JY, Cheung JK, Lau EHY, Wu P, Leung GM, Cowling BJ. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis. 2022;22(10):1435–43. doi:10.1016/S1473-3099(22)00345-0.
- Lustig Y, Gonen T, Meltzer L, Gilboa M, Indenbaum V, Cohen C, Amit S, Jaber H, Doolman R, Asraf K, et al. Superior immunogenicity and effectiveness of the third compared to the second BNT162b2 vaccine dose. Nat Immunol. 2022;23(6):940–6. doi:10.1038/s41590-022-01212-3.
- Lusvarghi S, Pollett SD, Neerukonda SN, Wang W, Wang R, Vassell R, Epsi NJ, Fries AC, Agan BK, Lindholm DA, et al. SARS-CoV-2 BA.1 variant is neutralized by vaccine booster–elicited serum but evades most convalescent serum and therapeutic antibodies. Sci Transl Med. 2022;14(645):eabn8543. doi:10.1126/scitranslmed.abn8543.
- Fleming-Dutra KE, Britton A, Shang N, Derado G, Link-Gelles R, Accorsi EK, Smith ZR, Miller J, Verani JR, Schrag SJ. Association of prior BNT162b2 COVID-19 vaccination with symptomatic SARS-CoV-2 infection in children and adolescents during Omicron predominance. JAMA. 2022;327(22):2210. doi:10.1001/jama.2022.7493.
- Humoral and cellular immune memory to four COVID-19 vaccines. 33.
- Jung MK, Jeong SD, Noh JY, Kim D-U, Jung S, Song JY, Jeong HW, Park S-H, Shin E-C. BNT162b2-induced memory T cells respond to the Omicron variant with preserved polyfunctionality. Nat microbiol. 2022;7(6):909–17. doi:10.1038/s41564-022-01123-x.
- Munro APS, Feng S, Janani L, Cornelius V, Aley PK, Babbage G, Baxter D, Bula M, Cathie K, Chatterjee K, et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): a multicentre, blinded, phase 2, randomised trial. Lancet Infect Dis. 2022;22(8):1131–41. doi:10.1016/S1473-3099(22)00271-7.
- Maringer Y, Nelde A, Schroeder SM, Schuhmacher J, Hörber S, Peter A, Karbach J, Jäger E, Walz JS. Durable spike-specific T-cell responses after different COVID-19 vaccination regimens are not further enhanced by booster vaccination. Sci Immunol. 2022;7(78):eadd3899. doi:10.1126/sciimmunol.add3899.
- Wesemann - 2022 - Omicron’s message on vaccines boosting begets bre.Pdf.
- Muecksch F, Wang Z, Cho A, Gaebler C, Ben Tanfous T, DaSilva J, Bednarski E, Ramos V, Zong S, Johnson B, et al. Increased memory B cell potency and breadth after a SARS-CoV-2 mRNA boost. Nature. 2022;607(7917):128–34. doi:10.1038/s41586-022-04778-y.
- Goel RR, Painter MM, Lundgreen KA, Apostolidis SA, Baxter AE, Giles JR, Mathew D, Pattekar A, Reynaldi A, Khoury DS, et al. Efficient recall of Omicron-reactive B cell memory after a third dose of SARS-CoV-2 mRNA vaccine. Cell. 2022;185(11):1875–87.e8. doi:10.1016/j.cell.2022.04.009.
- Zhao X, Zhang R, Qiao S, Wang X, Zhang W, Ruan W, Dai L, Han P, Gao GF. Omicron SARS-CoV-2 neutralization from inactivated and ZF2001 vaccines. N Engl J Med. 2022;387(3):277–80. doi:10.1056/NEJMc2206900.
- Shaw RH, Liu X, Stuart ASV, Greenland M, Aley PK, Andrews NJ, Cameron JC, Charlton S, Clutterbuck EA, Collins AM, et al. Effect of priming interval on reactogenicity, peak immunological response, and waning after homologous and heterologous COVID-19 vaccine schedules: exploratory analyses of Com-COV, a randomised control trial. Lancet Respir Med. 2022;10(11):1049–60. doi:10.1016/S2213-2600(22)00163-1.
- Wu Y, Kang L, Guo Z, Liu J, Liu M, Liang W. Incubation period of COVID-19 caused by unique SARS-CoV-2 strains: a systematic review and meta-analysis. JAMA Netw Open. 2022;5(8):e2228008. doi:10.1001/jamanetworkopen.2022.28008.
- Interim recommendations for heterologous COVID-19 vaccine schedules: interim guidance, 16 December 2021. 2021.
- Pérez-Then E, Lucas C, Monteiro VS, Miric M, Brache V, Cochon L, Vogels CB, De la Cruz E, Jorge A, De los Santos M, et al. Immunogenicity of heterologous BNT162b2 booster in fully vaccinated individuals with CoronaVac against SARS-CoV-2 variants Delta and Omicron: the Dominican Republic Experience. 2021. http://medrxiv.org/lookup/doi/10.1101/2021.12.27.21268459.
- Muik A, Wallisch A-K, Sänger B, Swanson KA, Mühl J, Chen W, Cai H, Maurus D, Sarkar R, Türeci Ö, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine–elicited human sera. Sci. 2021;371(6534):1152–3. doi:10.1126/science.abg6105.
- Zuo F, Abolhassani H, Du L, Piralla A, Bertoglio F, de Campos-Mata L, Wan H, Schubert M, Cassaniti I, Wang Y, et al. Heterologous immunization with inactivated vaccine followed by mRNA booster elicits strong humoral and cellular immune responses against the SARS-CoV-2 Omicron variant. 2022. http://medrxiv.org/lookup/doi/10.1101/2022.01.04.22268755.
- Lu C, Zhang Y, Liu X, Hou F, Cai R, Yu Z, Liu F, Yang G, Ding J, Xu J, et al. Heterologous boost with mRNA vaccines against SARS-CoV-2 Delta/Omicron variants following an inactivated whole-virus vaccine. 30.
- Li J-X, Wu S-P, Guo X-L, Tang R, Huang B-Y, Chen X-Q, Chen Y, Hou L-H, Liu J-X, Zhong J, et al. Safety and immunogenicity of heterologous boost immunisation with an orally administered aerosolised Ad5-nCoV after two-dose priming with an inactivated SARS-CoV-2 vaccine in Chinese adults: a randomised, open-label, single-centre trial. Lancet Respir Med. 2022;10(8):739–48. doi:10.1016/S2213-2600(22)00087-X.
- Li J, Hou L, Guo X, Jin P, Wu S, Zhu J, Pan H, Wang X, Song Z, Wan J, et al. Heterologous AD5-nCOV plus CoronaVac versus homologous CoronaVac vaccination: a randomized phase 4 trial. Nat Med. 2022;28(2):401–9. doi:10.1038/s41591-021-01677-z.
- Tang J, Zeng C, Cox TM, Li C, Son YM, Cheon IS, Wu Y, Behl S, Taylor JJ, Chakaraborty R, et al. Respiratory mucosal immunity against SARS-CoV-2 after mRNA vaccination. Sci Immunol. 2022;7(76):eadd4853. doi:10.1126/sciimmunol.add4853.
- Mao T, Israelow B, Peña-Hernández MA, Suberi A, Zhou L, Luyten S, Reschke M, Dong H, Homer RJ, Saltzman WM, et al. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science. 2022;378(6622):eabo2523. doi:10.1126/science.abo2523.
- Yao L, Zhu K-L, Jiang X-L, Wang X-J, Zhan B-D, Gao H-X, Geng X-Y, Duan L-J, Dai E-H, Ma M-J. Omicron subvariants escape antibodies elicited by vaccination and BA.2.2 infection. Lancet Infect Dis. 2022;22(8):1116–17. doi:10.1016/S1473-3099(22)00410-8.
- Quandt J, Muik A, Salisch N, Lui BG, Lutz S, Krüger K, Wallisch A-K, Adams-Quack P, Bacher M, Finlayson A, et al. Omicron BA.1 breakthrough infection drives cross-variant neutralization and memory B cell formation against conserved epitopes. Sci Immunol. 2022;7(75):eabq2427. doi:10.1126/sciimmunol.abq2427.
- Kaku CI, Bergeron AJ, Ahlm C, Normark J, Sakharkar M, Forsell MNE, Walker LM. Recall of preexisting cross-reactive B cell memory after Omicron BA.1 breakthrough infection. Sci Immunol. 2022;7(73):eabq3511. doi:10.1126/sciimmunol.abq3511.
- Koutsakos M, Lee WS, Reynaldi A, Tan H-X, Gare G, Kinsella P, Liew KC, Taiaroa G, Williamson DA, Kent HE, et al. The magnitude and timing of recalled immunity after breakthrough infection is shaped by SARS-CoV-2 variants. Immunity. 2022;55(7):1316–26.e4. doi:10.1016/j.immuni.2022.05.018.
- Minervina AA, Pogorelyy MV, Kirk AM, Crawford JC, Allen EK, Chou C-H, Mettelman RC, Allison KJ, Lin C-Y, Brice DC, et al. SARS-CoV-2 antigen exposure history shapes phenotypes and specificity of memory CD8+ T cells. Nat Immunol. 2022;23(5):781–90. doi:10.1038/s41590-022-01184-4.
- Fraser R, Orta-Resendiz A, Mazein A, Dockrell DH. Upper respiratory tract mucosal immunity for SARS-CoV-2 vaccines. Trends Mol Med. 2023;29(4):255–67. doi:10.1016/j.molmed.2023.01.003.
- Mettelman RC, Allen EK, Thomas PG. Mucosal immune responses to infection and vaccination in the respiratory tract. Immunity. 2022;55(5):749–80. doi:10.1016/j.immuni.2022.04.013.
