ABSTRACT
The field of mRNA vaccines has witnessed rapid development in recent years, leading to significant changes in global scientific collaboration. In this study, a bibliometric and social network analysis was conducted to reveal the evolution of global scientific collaboration in mRNA vaccine research. Altogether 6974 articles published since 2010 were retrieved and categorized into Period 1 (2010–2019), Period 2 (2020–2021) and Period 3 (2022–2023). During Period 2 and 3, there was a significant rise in the proportion of publications involving domestic inter-institutional cooperation (42.0%, 54.0% and 59.1%, respectively in Period 1, 2, and 3), while a significant decrease in international cooperation (32.1%, 23.7% and 21.0%). More countries participated in international collaboration during Period 2 and 3, with the US, the UK and Germany remaining top three throughout all periods, while some other countries like Italy, Japan, and China experiencing significant shifts. Significant correlations between collaboration type and publication impact and between geographical distance and collaborative publication counts were detected. Furthermore, significant changes in research focuses and institutions that are major contributors in the mRNA vaccine development have been observed. In conclusion, the mRNA vaccine field has experienced rapid development over the past decade, with significant evolutions of global scientific collaboration detected in our study.
Background
Collaboration plays a vital role in scientific research as individual scientists often lack the necessary skills and resources to address complex research problems.Citation1 In recent decades, driven by several factors, there has been a substantial increase in collaboration among scientists, research groups, and institutions. Firstly, the escalating complexity and cost of scientific challenges necessitate collaboration to achieve meaningful progress. Secondly, advancements in travel and communication technologies have made cross-institutional and cross-national exchanges more accessible and affordable. Finally, there is a growing recognition that collaboration among individuals with diverse abilities, cultures, and experiences can enhance team creativity and individual research performance.Citation1–3
However, on January 30, 2020, the World Health Organization (WHO) declared the Coronavirus Disease 2019 (COVID-19) as a Public Health Emergency of International Concern (PHEIC), thereby potentially impacting global scientific research and international collaboration.Citation4–6 The pandemic could have had both positive and negative implications for international collaboration. On one hand, the pandemic could have led to a reduction in global scientific collaboration, probably due to three reasons.Citation7 Firstly, travel restrictions and lockdown measures implemented to contain the spread of the virus may have reduced opportunities for researchers to have face-to-face interactions and collaborations, leading to a significant increase in the cost of collaboration.Citation8 Secondly, the closure of laboratories and research facilities during the pandemic could have disrupted the progress of international collaborative projects.Citation9 Lastly, the shifting international relations brought about by the pandemic could have affected cooperation between countries. On the other hand, the pandemic may have had varying effects on international collaboration across different fields.Citation10 Specifically, the urgent global health needs resulting from the COVID-19 crisis redirected research resources and efforts toward pandemic-related studies,Citation11,Citation12 potentially leading to a significant increase in research related to COVID-19 while a decrease in research on other areas.Citation7,Citation13
The COVID-19 pandemic has highlighted the importance of global scientific collaboration in addressing global health challenges. In response to the outbreak, a substantial number of scientists have engaged in international collaborative research on COVID-19, particularly in countries most affected by the crisis or with relatively lower GDP.Citation12,Citation14 Studies have confirmed a sudden increase in international collaborative research related to COVID-19, evident from bibliometric indicators such as an increase in the number of countries mentioned in scientific publications and a significant expansion of countries involved in international collaboration networks.Citation12,Citation15 Vaccines, as one of the key measures to combat the COVID-19 pandemic, often rely on international collaboration in scientific research. Druedahl et al. discovered that approximately one-third of the candidate vaccines listed by the World Health Organization for controlling the COVID-19 outbreak were developed through collaboration.Citation4
Among the various types of vaccines, mRNA vaccines have demonstrated remarkable potential in preventing infectious diseases and treating tumors compared to traditional vaccines.Citation16–18 They have received significant attention and recognition globally due to their effect in combating the COVID-19 pandemic.Citation16,Citation18,Citation19 A co-citation analysis conducted by our group in 2022 demonstrated that the evolution of mRNA vaccines has gone through several distinct periods, each marked by significant advancements.Citation20 In the initial exploratory phase, predating the early 2010s, research primarily revolved around assessing mRNA’s capability to trigger protein expression and immune responses.Citation21,Citation22 Transitioning into the growth phase, spanning from the early 2010s to 2019, efforts concentrated on enhancing the stability and immunogenicity of mRNA vaccines and advancing their development for infectious diseases and cancer applications.Citation23–28 Despite extensive efforts, no approvals were obtained during this period. However, in the rapid maturation phase following the COVID-19 outbreak, two mRNA vaccines, BNT162b2 and mRNA-1273, received authorization for the first time.Citation16,Citation29,Citation30 In recent years, research on mRNA-based infectious disease vaccines has expanded, with studies focusing on optimizing mRNA modification and delivery, addressing real-world challenges with approved vaccines, exploring mRNA vaccines for additional infectious diseases, and developing self-amplifying or thermostable vaccine solutions.Citation20 Similarly, in the field of mRNA-based therapeutic cancer vaccines, ongoing research involves the identification of suitable neoantigens, improving mRNA delivery to antigen-presenting cells, and devising strategies to overcome the suppressive tumor microenvironment.Citation20
Although the World Health Organization declared on May 5, 2023, that the COVID-19 pandemic no longer constitutes a PHEIC, it remains crucial to examine the evolution in international scientific collaboration in mRNA vaccine research. Firstly, the COVID-19 pandemic has posed unprecedented challenges for scientists in conducting research, collaborating with colleagues, and accessing resources.Citation31 Analyzing the changes in collaboration before and after the crisis can provide valuable insights into the impact of these challenges and help identify strategies to overcome them. Secondly, the pandemic has fostered new collaborations among researchers from different countries.Citation15,Citation31 Analyzing the shifts in global collaboration can unveil knowledge gaps and opportunities for collaboration, facilitating the discovery of new targets and indications for mRNA vaccines. Thirdly, analyzing the changes in international collaboration can provide insights into the positioning of countries within the global collaboration network of mRNA vaccine research and help identify the leaders and followers in the field.Citation5,Citation32 This assists policymakers and investors in allocating resources more effectively, thereby enhancing the speed and efficiency of vaccine development and ultimately improving public health outcomes. Lastly, understanding the changes in collaboration in mRNA vaccine research during the global COVID-19 crisis allows for the identification of successful collaborators, which may enable us to uncover best practices during crises and potentially replicate them in other research fields, thereby accelerating the development of other vaccines.Citation32
Research on scientific collaboration often relies on analyzing co-authorship data.Citation33,Citation34 In this context, a publication is considered coauthored when it involves multiple authors, and it is regarded as internationally coauthored if the authors are affiliated with institutions from different countries.Citation35 Using such data as a measure of collaboration has some limitations. One limitation is that collaborative research does not always result in coauthored publications.Citation34 Another limitation is the limited coverage of literature databases, where articles published in books and domestic journals are often less likely to be included compared to those published in international journals, which particularly affects the fields of humanities and social sciences.Citation36,Citation37 However, the use of coauthored data offers unique and systematic insights into the structure of scientific collaboration compared to other methodologies.Citation34 One advantage is the ease of analyzing large datasets and the robustness of results. Additionally, the coauthored indicator encompasses both formal and informal types of collaboration, especially for the field of medical science. Various types of publications, such as articles, reviews, letters, notes, and editorial material, are commonly utilized, with articles being the most frequently employed and considered ideal for analyzing international research collaboration.Citation5,Citation38
Bibliometric indicators and social network analysis have been widely utilized in previous studies to quantitatively assess scientific research collaboration.Citation34,Citation39,Citation40 These methodologies provide valuable insights into the collaborative dynamics within the scientific community. Commonly used bibliometric indicators include the number, proportion and global share of collaborative publications, as well as the number of collaboration partners.Citation5,Citation40 These indicators offer a quantitative assessment of the scale and extent of collaboration in research endeavors. Social network indicators are also frequently used to analyze collaboration patterns. Measures such as network density, the number of nodes, and the number of links provide an overview of the general collaboration status within a particular field. Additionally, metrics like degree centrality, closeness centrality, betweenness centrality and clustering coefficient (CC) are always employed to evaluate the importance of individual nodes within the collaboration network.Citation32,Citation41
This study aims to trace the recent evolution of global scientific collaboration in mRNA vaccine research. At first, the mRNA vaccines field faced significant challenges due to the poor stability of mRNA, which impeded its progress.Citation21 Despite an early study dating back to 1990 have demonstrated the potential of injecting mRNA to produce proteins in vivo, progress in this field was sluggish in its early stages.Citation22 Following the discovery in 2008 that the incorporation of pseudouridine into mRNA could enhance its biological stability, coupled with the development of lipid nanoparticle technology in early 2010s, the mRNA vaccine research began to accelerate.Citation22 Therefore, this study utilizes scientific papers published since 2010 as its dataset, which is then divided into three sub-datasets (Period 1, Period 2 and Period 3). Period 1 covers the previously mentioned growth period from 2010 to 2019.Citation20 Period 2 covers the years 2020–2021, when the urgent demand generated by COVID-19 drove rapid maturation of mRNA vaccine technology, resulting in the successful development of the first two mRNA vaccines worldwide, namely BNT162b2 and mRNA-1273.Citation16,Citation18 Period 3 spans from 2022 to 2023. The reason for using the end of 2021 as a cutoff point is twofold. Firstly, with the introduction of BNT162b2 and mRNA-1273 to the market, there is bound to be a shift in the focus of mRNA vaccine research after 2021. Secondly, the emergence of the Omicron variant in late 2021 significantly altered the global pandemic landscape.Citation42,Citation43 Both of these factors have the potential to bring about significant changes in collaborative patterns.
By employing bibliometric and social network analyses, this research will delve into several key aspects. Firstly, the study will examine changes in the types of scientific collaboration and the size of teams in the field of mRNA vaccines and explore their correlation with the academic impact of publications, which will shed light on the collaborative dynamics that have shaped the field. Secondly, this study will assess transformations in the international scientific collaboration networks and identify shifts in the importance of top countries within these networks, which will provide insights into the global landscape of mRNA vaccine research collaboration and highlight countries at the forefront of scientific advancements. Thirdly, the study will investigate the correlation between geographical distance and the extent of collaborations, which will uncover potential barriers and opportunities in cross-border research partnerships. Fourthly, the evolution of research focuses in the mRNA field over time and the difference in research focuses among different types of collaborations will be visually presented. Finally, shifts in the institutions that are major contributors in the mRNA vaccine development will be analyzed descriptively. By conducting these analyses, this study will contribute valuable insights to the field, highlighting key trends and factors that have shaped scientific collaboration in this critical domain.
Methods
Literature search
On April 12, 2023, literature search was conducted in the Web of Science (WOS) Core Collection to identify publications related to mRNA vaccines. The search strategy involved two steps. In the first step, publications specifically related to the topic of “mRNA vaccine” were included. This included publications that had the terms “RNA OR mRNA” and “vaccine*” in the titles, abstracts, or keywords. In the second step, publications mentioning the product names of mRNA vaccines were retrieved as a supplement. The names of mRNA vaccines were obtained from the Cortellis database, which provided a comprehensive list of vaccine names. Some examples of the vaccine names included “mRNA 1273*,” “mRNA-1273*,” “Moderna COVID-19 Vaccin*,” M-1273, M1273, Elasomeran, “COVID-19 Vaccine Moderna,” Spikevax, TAK-919, TAK919, “TAK 919,” BNT162*, “BNT 162*,” “BNT-162*,” “Pfizer COVID-19 Vaccin*,” “Pfizer- BioNTech COVID-19 Vaccin*,” “BioNTech COVID-19 Vaccin*,” “ARCT 154,” zorecimeran, CV-07050101, CVnCoV, Tozinameran, Comirnaty, “mRNA-1647,” Pidacmeran, Abdavomeran, Ganulameran, “mRNA-1345,” “mRNA-1647,” “ARCoV,” “DS-5670,” “ARCT-021,” “ARCT-165,” “EXG-5003,” “mRNA-1010,” “mRNA-1020,” “mRNA-1030,” “mRNA-1283*,” “mRNA-1893,” “PTX-COVID19-B,” “HDT-301,” “BNT-161*,” “SYS-6006,” “CV2CoV,” “CV-7202,” “CVSQIV,” “nadorameran,” “GSK-3903133A,” “GSK-4108771A,” “mRNA-1172,” “mRNA-1189,” “mRNA-1574,” “mRNA-1644,” “mRNA-1653,” “SW-0123,” “mRNA-1388,” “mRNA-1440,” “mRNA-1851,” “VLPCOV-01,” “BNT-111,” “BNT-112,” “BNT-113,” “BI-1361849,” “BNT-122,” “mRNA-4157,” “NCI-4650,” “BNT-114,” “mRNA-5671,” etc. By utilizing this comprehensive search strategy, a wide range of publications related to mRNA vaccines were identified for further analysis.
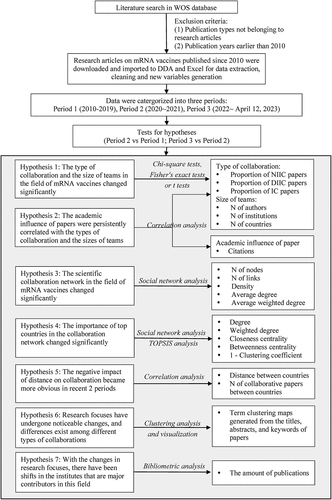
As displayed in the flow chart of this study in , considering scientific collaboration is the focus of this study, only research articles were included, while all other publication types such as reviews, letters, and editorial materials were excluded. As what has been illustrated above, articles published prior to 2010 were excluded.
Figure 1. The flow chart of this study.
Data collection and processing
As shown in , full bibliographic records of all articles were exported as plain text files, which were imported into Derwent Data Analyzer (DDA) to extract the following data for each article: title, abstract, keywords, publication year, countries, institutions, authors and citations.
Considering that the number of countries and institutions for each publication are key indicators for data analysis in this study, the names of countries and institutions were cleaned in DDA. The cleaning of countries was relatively easy, because there are fewer than 250 countries worldwide. However, the cleaning of institutions was difficult for the following reasons: first, there are numerous institutions conducting research on mRNA vaccines; second, different names may be used for the same institution; third, the hierarchy of institutions can be complex; fourth, the academic systems vary across different countries, making it difficult to identify the proper unit of analysis. Given these challenges, it is important to ensure transparency in data cleaning process, allowing readers to understand the potential for errors when counting the number of institutions for a publication.Citation44 In this study, we drew inspiration from the institution cleaning principles used in the CWTS Leiden Ranking, a widely recognized bibliometric ranking of major universities worldwide released by the Centre for Science and Technology Studies (CWTS) at Leiden University (for more information, refer to the following webpage: https://www.leidenranking.com/information/universities). It’s important to note that the CWTS Leiden Ranking only includes universities, whereas the institutions involved in our study cover all institutions publishing papers in our dataset, including universities, research institutes, and industrial companies, etc. In other words, we borrowed the institution cleaning principles instead of the institution list from the CWTS Leiden Ranking. Accordingly, the following measures were implemented for institution cleaning. First, we manually checked and standardized the names of institutions that appeared most frequently (approximately 80%); second, regarding institution hierarchy, particularly in cases of hospitals or research institutes affiliated with a university, if the university was clearly mentioned in the address, the publication was considered as an output of the university, otherwise not; third, for academic systems, we treated institutions of academic systems as they are perceived locally. For example, university systems in the US (e.g. the University of California) were treated as separated institutions.
The cleaned data were exported from DDA to Excel, and then a series of data processing procedures were conducted. First, considering the institutions and countries for a small proportion of publications were failed to be extracted in DDA, we collected the missing data from the official websites of journals and filled them in Excel. Second, several new variables were generated for each article, including the number of countries, the number of institutions, the number of authors, the publication period and the type of cooperation. As mentioned above, three publication periods were categorized, namely 2010 ~ 2019, 2020 ~ 2021, 2022 ~ 2023. Regarding the type of cooperation, publications authored by individuals from a single institution were classified as “no inter-institutional cooperation (NIIC),” while those authored by individuals from multiple institutions were classified as “inter-institutional cooperation (IIC).” The IIC category can be further divided into “domestic inter-institutional cooperation (DIIC)” and “international cooperation (IC).” The categories NIIC and DIIC are collectively referred to as “no international cooperation (NIC).”
Data analysis
The data analysis was conducted to test the following hypotheses.
Hypothesis 1:
The type of scientific collaboration and the size of teams in the field of mRNA vaccines changed significantly from period 1 to period 3.
For the type of collaboration, chi-square tests or Fisher’s exact tests were conducted to test the significance of changes in the proportion of publications in the categories of NIIC, DIIC and IC. For the size of teams, t tests were conducted to test the significance of changes in the number of authors, institutions and countries. Comparisons were made between period 2 and period 1, as well as between period 3 and period 2. The analyses were carried out using IBM SPSS Statistics 19, and a significance level of p < .05 (two-sided) was considered statistically significant. These analysis were conducted for the whole field and for each of the 25 countries with the most publications in the field of mRNA vaccines.
The proportion of NIIC publications was calculated by dividing the amount of publications authored by individuals from a single institution by the total number of publications.
The proportion of DIIC publications was calculated by dividing the number of publications authored by individuals from multiple institutions within the same country by the total number of publications.
The proportion of IC publications was calculated by dividing the number of publications authored by individuals from multiple countries by the total number of publications.
Hypothesis 2:
The academic influence of publications was persistently correlated with the types of collaboration and the sizes of teams from period 1 to period 3.
Spearman correlation tests were conducted to test the correlation between the citations received by publications and the type of collaboration and the size of teams. The analyses were performed using the entire database as well as separate databases for each time period. These tests aimed to answer the following questions: (1) whether IIC publications have a stronger academic influence compared to NIIC publications; (2) whether IC publications have a stronger academic influence compared to NIC publications; (3) whether publications published by larger teams (more authors, more institutions, or more countries) have a stronger academic influence compared to those published by smaller teams. These analysis were conducted using IBM SPSS Statistics 19 and a significance level of p < .05 from two-sided tests was considered statistically significant.
Hypothesis 3:
The inter-country scientific collaboration network changed significantly from period 1 to period 3.
To test this hypothesis, social network analysis was conducted to construct inter-country scientific collaboration networks for period 1, 2 and 3, respectively. The following indicators were used to quantify the networks: number of nodes, number of links, density, average degree, and average weighted degree, the meanings of which were explained below. The visualization of the networks were conducted using VOSviewer_1.6.10 and the calculation of quantitative indicators were conducted using Pajek.
Nodes. Nodes refers to countries in the networks.
Links: Links between two countries exist if there are scientific collaborations between them.
Density. Density measures the tightness of connections between nodes, which is calculated as the actual links among all nodes divided by the theoretical maximum links among all nodes.
Degree. The degree of a node refers to the number of nodes directly connected to it, which can reflect the diversity of collaboration partners.
Weighted degree. The weighted degree of a node refers to the total number of collaborations between that node and all other directly-connected nodes in the whole network, which can reflect the enthusiasm of collaboration.
Hypothesis 4:
The importance of top countries in the inter-country scientific collaboration network changed significantly from period 1 to period 3.
For each of these 25 countries with the most publications, five indicators measuring the importance of a node in a network were calculated using Pajek, including degree, weighted degree, closeness centrality, betweenness centrality and clustering coefficient. The meanings of these indicators were illustrated below. To test the significance of changes in the above-mentioned indicators, t tests comparing the average values in period 2 with those in period 1 and comparing the average values in period 3 with those in period 2 were conducted.
Degree. See above.
Weighted degree. See above.
Closeness centrality. The closeness centrality of a node is calculated as the reciprocal value of the average distance between that node with all other nodes in the network. A higher closeness centrality indicates that the node is closer to other nodes in terms of network connectivity, suggesting its potential to act as a central node within the network.
Betweenness centrality. The betweenness centrality of a node measures the number of shortest paths between all pairs of nodes in the entire network that pass through that specific node, which is an indicator reflecting the potential of a node controlling the resource flows within the collaboration network. A higher betweenness centrality indicates a node’s greater importance in facilitating connections between other nodes.
1 - Clustering coefficient (1-CC). The clustering coefficient measures the degree to which the neighbors of a given node are connected to each other. It is calculated as the actual number of links among the neighbors divided by the maximum possible number of links. The larger the value, the less important the node is, which means that this node is not necessary for other nodes to be connected. For the convenience of explanation, one minus clustering coefficient (1-CC) was used in the TOPSIS analysis.
Based on the above-mentioned five indicators, TOPSIS analysis, with entropy weight method to determine the weight for each indicator, was conducted using SPSSPRO to obtain a comprehensive score for each country to measure the importance of them in the collaboration network. The TOPSIS analysis is a commonly used method for comprehensive evaluation, the basic idea of which is to identify the best and worst solutions from a finite set of options, and then, the distances between each evaluation object and the best and worst solutions can be calculated, respectively.Citation45–47 These distances are used to determine the proximity of each evaluation object to the best solution, serving as a basis for evaluating their ranking. The closer an evaluation object is to the best solution, the higher its ranking.Citation45–47 The entropy weight method is a commonly used approach for assigning weights to indicators. Its basic idea is that the greater the degree of dispersion of a particular indicator, the greater its impact (weight) on the overall evaluation result.Citation48 If a certain evaluation indicator has identical values across all evaluated objects, then that indicator lacks discriminative power and thus receiving a weight of 0. This method assigns weights based on the distribution of data, making it more objective compared to methods that involve subjective weight assignments.Citation48 After obtaining the TOPSIS rankings for the countries, we used Tableau for visualization. Furthermore, to further visualize the scientific collaborations among the top 25 countries, chord diagrams were drew for each of the three periods.
Hypothesis 5:
The negative impact of distance on collaboration tended to be more obvious in Period 2 and Period 3 as compared with Period 1.
The top 25 countries with the most publications were selected to test this hypothesis. The great circle distances, measured in kilometers (km), between the capital cities of countries were obtained from the Distance Calculator website (https://www.distancecalculator.net/). A scatter plot was created to illustrate the relationship between the distance between countries and the number of collaborative research papers. And then a correlation analysis between the distance and the number of collaborative publications was conducted using SPSS for Period 1, Period 2 and Period 3, respectively. Since the data were not normally distributed, Spearman correlation coefficients were calculated instead of Pearson correlation coefficients.
Hypothesis 6:
Research focuses have undergone noticeable changes, and differences exist among different types of collaborations.
Clustering analysis was conducted based on terms extracted from the titles, abstracts, and keywords of all papers published in each period. Based on the term clustering maps generated, the main research directions were summarized for each period. Further, on the term clustering maps in each period, overlay visualization maps by collaboration types were generated, so that the research directions for each collaboration type can be summarized. The VOSviewer version 1.6.10 was utilized for this analysis.
Hypothesis 7:
With the changes in research focuses, there have been shifts in the institutions that are major contributors in the mRNA vaccine development.
The amount of publications for institutions in the mRNA vaccine field was counted and ranked in descending order in each period. Institutions with a publication volume equal to or exceeding a predetermined threshold were considered major contributors in that period. Due to significant differences in publication volume across the three periods, different thresholds were set for each period, specifically 10 in Period 1, 20 in Period 2, and 40 in Period 3. Subsequently, major contributors from the three periods were de-duplicated, resulting in a consolidated list of major contributors, for whom the publication volumes for each of the three periods were visualized using the Tableau software. It is important to note that if an institution entered the major contributor list in only one or two periods, its publication volume for the other two or one periods was also displayed, regardless of whether it entered the major contributor list in those periods. Finally, the visualized results were interpreted to describe the shifts in institutions that are major contributor in mRNA vaccine development. This analysis is descriptive and does not involve statistical testing.
Results
Altogether 12,412 publications were obtained, among which 8105 were research articles and other 4307 were reviews, letters, meeting abstracts, editorial materials, etc. Among the 8105 research articles, 6947 (85.71%) published since 2010 were selected for analysis. The annual number of publications showed an increasing trend over time. From 2010 to 2019, there were less than 150 publications per year. However, in 2020, the number increased to 195, followed by a substantial increase to 1,640 in 2021, 3,631 in 2022, and 520 in 2023 by the literature retrieval time on April 12, 2023. Thus, the amount of publications in periods 2010–2019, 2020–2021 and 2022–2023 were 961, 1835 and 4151, respectively ().
Table 1. Change of indicators measuring the type of scientific collaboration and the size of teams in the field of mRNA vaccines.
Changes in the type of scientific collaboration and the size of teams
As shown in , the statistical analysis provides support for Hypothesis 1, indicating significant changes in the type of scientific collaboration and the size of teams in the field of mRNA vaccines in recent years, both for the overall field and for many top countries.
Table 2. Change of indicators measuring the type of scientific collaboration and the size of teams in the field of mRNA vaccines for top countries.
Comparing Period 2 to Period 1, significant changes in collaboration in the field of mRNA vaccines were observed. Regarding the type of scientific collaboration, there was a notable decrease in the proportion of NIIC publications and a significant increase in that of IIC publications. The increase in the proportion of IIC publications was primarily driven by a significant rise in the proportion of DIIC publications (increased from 42.0% to 54.0%, p < .001), rather than the proportion of IC publications (decreased from 32.1% to 23.7%, p < .001) (). When considering the size of teams, both the number of authors and the number of institutions showed significant increases. As to changes for individual countries, generally as with the whole field, the majority of top countries with the most publications in the field of mRNA vaccines exhibited significant changes in collaborative indicators in Period 2 compared to Period 1. Countries like Italy, Germany, and France exhibited such significant changes, as shown in . However, there were a few countries that demonstrated differences in certain indicators compared to the whole field. For example, Spain showed a significant increase in the proportion of NIIC publications, contrary to the trend observed in the entire field. It is important to note that due to the limited sample size, these changes may not hold significant meaning.
Comparing Period 3 to Period 2, the collaboration in the field of mRNA vaccines underwent further significant changes, following a similar trend observed from Period 1 to Period 2. As shown in , there was a significant increase in the proportion of IIC publications, which was also primarily driven by a significant increase in the proportion of DIIC publications (from 54.0% to 59.1%, p < .001), rather than that of IC publications (from 23.7% to 21.0%, p = .022). Regarding the size of teams, there was a further significant increase in the number of institutions, and a trend of an increase in the number of authors. As presented in , the changes for top countries in Period 3 were generally consistent with the overall field as well. The proportion of DIIC publications increased significantly for the US, Japan, Canada, the Netherlands, and Switzerland, and the proportion of IC publications decreased significantly for Japan, Canada, the Netherlands, and Turkey. In terms of team size, several countries, including Belgium and Sweden, witnessed significant increases in the number of authors, while the US saw a notable increase in the number of institutions.
Persistent correlation of the impact of publication with the types of collaboration and the sizes of teams
As shown in , the impact of publication is significantly correlated with the types of collaboration and with the sizes of teams. The analysis indicated that IIC publications, IC publications and those involving a greater number of authors, institutions or countries, received significantly more citations compared to NIIC publications, NIC publications and those involving fewer authors, institutions or countries. These correlations hold true for both the whole dataset including all publications since 2010, as well as for each of the three sub-datasets representing Period 1, Period 2, and Period 3.
Table 3. The spearman correlation coefficients between the citations received by publication and the type of collaboration and the size of teams.
Changes in the inter-country scientific collaboration network
During Period 1, scientific collaboration in the field of mRNA vaccines was limited to a select number of countries, forming a network with 69 nodes and 269 edges. The network density, representing the tightness of connections between nodes, was 0.113 (). On average, each country in the network had 7.80 partners, and the average collaboration frequency was 19.33. These statistics indicate that prior to the COVID-19 pandemic, there was relatively limited collaboration in the field of mRNA vaccine, with a restricted number of partners and low level of enthusiasm for collaboration among all countries involved.
Figure 2. The international collaboration network in the field of mRNA vaccine from 2010 to 2023.
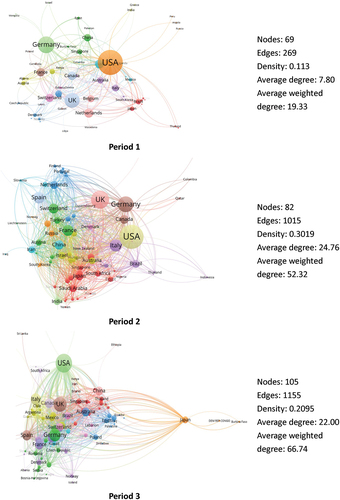
During Period 2, an increased number of countries participated in scientific collaboration in mRNA research, forming a network with 82 nodes and 1015 edges, resulting in a significantly higher network density of 0.3019 compared to Period 1 (). Both the average number of partners and the average collaboration frequency for countries increased significantly, from 7.80 to 24.76 and from 19.33 to 52.32, respectively. These findings suggest a substantial growth in collaboration within the mRNA vaccine field during Period 2. This increase can be attributed to the high demand for medical solutions driven by the COVID-19 pandemic and the continuous advancement of mRNA vaccine technology. More countries actively participated in international collaboration in this field, leading to an improved diversity of collaboration partners and an enhanced enthusiasm for collaborative efforts.
During Period 3, an even larger number of countries participated in scientific collaboration in mRNA research, forming a network with 105 nodes and 1155 edges (). The network density decreased from 0.3019 to 0.2095. The average number of partners per country remained relatively consistent compared to Period 2 (24.76 vs 22.00), but the average collaboration frequency per country further increased from 52.32 to 66.74. This indicates that there was no significant change in the diversity of collaboration partners over time, but there was an increase in the enthusiasm of collaboration. More countries actively engaged in international collaboration in the scientific research of mRNA vaccines. It is the participation of these countries that led to a slight sparsity in the collaboration network compared to Period 2.
Changes in the importance of top countries in the inter-country scientific collaboration network
Overall, the significant changes in the global collaboration network have also led to significant transformations in the importance of the top 25 countries within the network (). In Period 2, the following changes in social network indicators were observed for these countries: (1) both the number of collaboration partners (degree) and collaboration frequency (weighted degree) increased significantly, indicating a greater diversity of collaboration partners and a stronger willingness of collaboration; (2) as the network became denser, there were more direct connections between countries, resulting in shorter distances between them, which is reflected in the social network indicators as increased closeness and decreased betweenness; (3) due to the increased direct connections among the neighboring nodes of a certain node, the necessity of that certain node in connecting its neighbors decreased, which is reflected as a decrease in the 1-CC. In Period 3, the number of collaboration partners and collaboration frequency continued to increase for top countries, indicating a continued high level of collaboration enthusiasm. However, closeness, betweenness, and 1-CC have changed in the opposite direction compared to the previous period. This can be attributed to the significant influx of new countries into the network, without the formation of dense connections among them. As a result, the network has become sparser.
Table 4. Change in indicators measuring the importance of nodes in the collaboration networks in the field of mRNA vaccines for top countries.
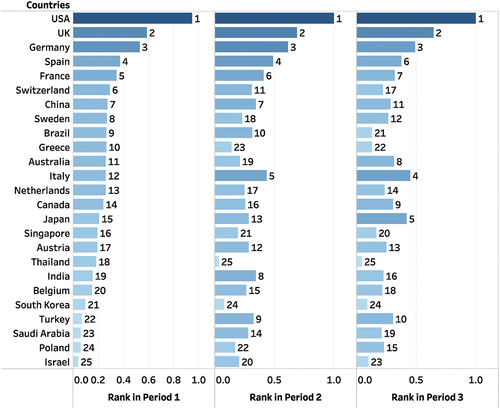
As to the TOPSIS analysis, the weights obtained for degree, weighted degree, closeness, betweenness, and 1- clustering coefficient according to the entropy weight method were as follows: 0.15556, 0.28843, 0.07477, 0.37941 and 0.10182, respectively in Period 1; 0.08645, 0.2737, 0.10119, 0.44343 and 0.09523, respectively in Period 2; and 0.09159, 0.28703, 0.10028, 0.41983, and 0.10127, respectively in Period 3. From these data, it can be observed that “betweenness” is the most discriminative indicator in all three periods, and therefore, it has received the highest weight. This means that the values of this indicator have the greatest impact on the overall score. According to the total scores and ranks shown in and , the US, the United Kingdom (the UK), and Germany consistently ranked among the top three countries in terms of their importance in the network. Italy improved its ranking from 12th in Period 1 to 5th in Period 2 and 4th in Period 3. Japan also made significant progress, moving from 15th initially to 5th in Period 3. However, China fell behind from 7th in Period 1 to 11th in Period 3. Additionally, a few Asian countries have also shown significant shifts in their positions. Turkey ascended from 22nd in Period 1 to 9th in Period 2. Saudi Arabia rose from 23rd in Period 1 to 14th in Period 2. India climbed from 19th in Period 1 to 8th in Period 2.
Figure 3. The comprehensive collaboration rankings of countries from 2010 to 2023.
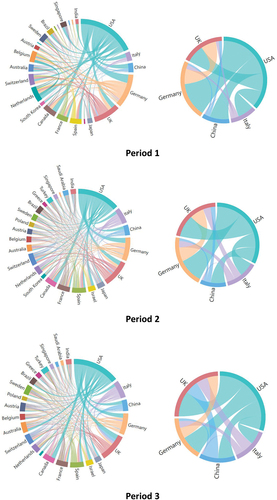
Seeing from the chord diagrams (), the US, Germany, and the UK consistently had the highest frequency of international collaborations, while Italy and China have undergone significant changes in their collaborations with other countries. For the US, the primary collaboration was with Germany followed by Canada and the UK in Period 1, while its collaboration with the UK was increased to surpass its collaboration with Germany in Period 3. For the UK, the primary collaboration was with the US followed by Germany in Period 1 and Period 2, while its collaboration with China and Italy was increased to exceed its collaboration with Germany in Period 3. For Germany, the primary collaboration partner remained the US followed by the UK throughout all three periods. Italy’s international collaboration with other countries was limited in Period 1, but was grew obviously in Period 2 and Period 3, primarily with the US, the UK, and Germany, as its publication output increased. China, ranking third in the amount of publications, exhibited relatively lower enthusiasm for international collaboration as compared to the US, the UK, Germany and Italy in Period 2 and Period 3. Its primary collaboration was with the US in all three periods, and its collaboration with the UK was increased to as frequent as with the US in Period 3. However, China’s collaboration with other countries remained minimal in all periods.
Figure 4. The chord diagrams showing the collaboration among top countries in the field of mRNA vaccine from 2010 to 2023.
Changes in the impact of distance on the number of collaborative publications between countries
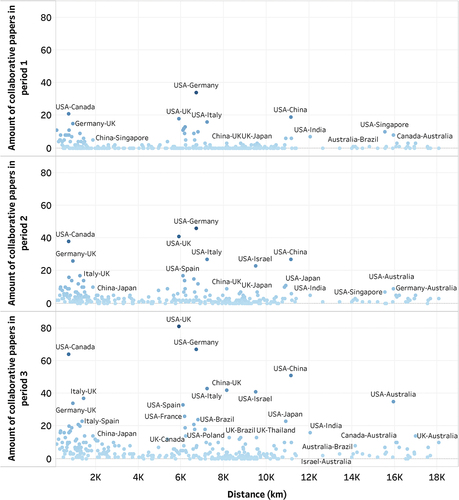
As shown in , there is an evident negative correlation between the distance between countries (X-axis) and the number of collaborative publications between countries (Y-axis), particularly in period 2 and period 3. The results of the Spearman correlation analysis further support this correlation, revealing a significant negative correlation coefficient of −0.121 in Period 1, −0.203 in Period 2 and −0.271 in Period 3. The increasing absolute value of the correlation coefficients indicated that longer distance between countries may probably affect the cooperation between the two countries, particularly following the onset of the COVID-19 pandemic.
Figure 5. Scatter plot demonstrating the relationship between the distance between countries and the amount of collaborative research papers.
Evolution of research directions in the mRNA field over time and the differences among different types of collaborations
As shown in , there are mainly three research directions in Period 1: (1) optimization of mRNA modification and delivery technologies; (2) primary research on mRNA vaccines against cancers; (3) primary research on mRNA vaccines against infectious diseases (e.g., HIV, HCV, and Zika virus). NIIC Papers tend to focus on the first direction, while IC papers tend to focus on the second direction. However, the differences among different types of collaborations were not that pronounced.
Figure 6. Visualization of mRNA vaccine research directions in scientific papers, categorized by time period and collaboration type.
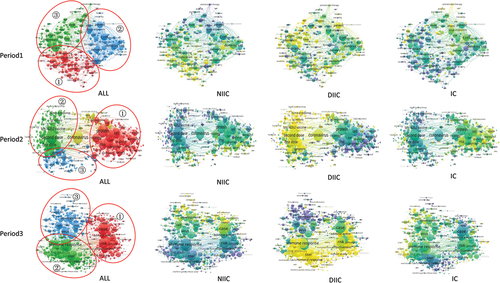
In Period 2, nearly all mRNA vaccine researches were centered on COVID-19, which can be categorized into three main directions: (1) design and development of mRNA vaccines targeting COVID-19; (2) analysis of immune responses following the first and second doses of mRNA vaccines; (3) investigation of adverse reactions related to mRNA COVID-19 vaccines. DIIC papers constitute the vast majority of publications during this period, focusing mainly on the 2nd and 3rd directions. However, IC papers during this period tended to focus on the first direction, which may be due to the urgent demand for vaccines during the COVID-19 pandemic.
In Period 3, as research transitioned from product development to assessing long-term vaccine efficacy, evaluating effectiveness against variants, and safety evaluation, more disease-oriented studies were conducted. Specifically, the three primary research directions in this period are as follows: (1) tracking the risk of adverse reactions to mRNA COVID-19 vaccines through cohort studies or case-control research; (2) testing the effect of mRNA vaccines to stimulate immune responses in long term or against new variants of SARS-COV-2; (3) development of mRNA cancer vaccines. During this period, a significant amount of DIIC research was conducted to address issues in the 1st and 2nd directions. NIIC Papers tend to focus on the 3rd direction, while IC papers tend to have involvement across all three directions.
Shifts in the institutions that are major contributors in the mRNA vaccine development
According to the predetermined thresholds, 29 institutions published as least 10 papers in Period 1, 28 institutions published at least 20 papers in Period 2 and 31 institutions published at least 40 papers in Period 3 were considered major contributors (). After de-duplicating, we compiled a list of 50 institutions. It’s worth noting that Pfizer did not appear in the aforementioned list, but we included it in the visualization in , due to its significant role as one of the primary developers of BNT162b2. As shown in , major contributors in the field of mRNA vaccines have undergone significant shifts.
Figure 7. Publication counts of major contributors in the mRNA vaccine field across three periods.
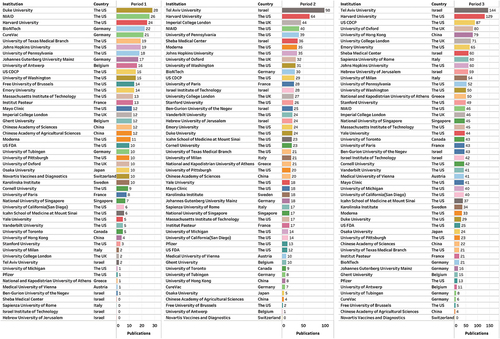
In Period 1, major contributors were primarily institutions from the US and Germany, including universities as well as companies like BioNTech, CureVac, and Moderna. This can be attributed to the fact that the field was in the stages of technical and methodological exploration, relying on universities and companies to achieve technological breakthroughs.
In Period 2, both Moderna and BioNTech continued to be major contributors with over 30 publications each, which could be due to the rapid development of their COVID-19 vaccine products. In contrast, CureVac lagged behind with only seven publications due to slower progress in product development. Pfizer rapidly entered this field through its collaboration with BioNTech on the development of BNT162b2, with its publication count increasing from 1 in Period 1 to 13 in Period 2. As mRNA vaccine products became available in the market, there was an increase in research regarding their real-world effectiveness and safety. Institutions like Tel Aviv University and Sheba Medical Center in Israel and the University of Milan in Italy emerged as new major contributors in this field.
In Period 3, as research transitioned from product development to assessing long-term vaccine efficacy, evaluating effectiveness against variants, and safety evaluation, the role of companies in this field somewhat diminished. Neither Moderna (33 papers), BioNTech (21 papers), nor CureVac (6 papers) made it to the list of major contributors. Instead, universities, research institutes, and CDCP dominated this list. For example, Tel Aviv University and Harvard University continued to hold the top two positions globally, and the US CDCP increased from the 12th position in Period 2 to the 3rd position in Period 3. Because research in this period focused more on disease-oriented studies, institutions from countries other than those with established technological advantages like the US and Germany also had the opportunity to join the research in this field, making the country distribution of major contributors more diversified.
Discussion
Our study has revealed a significant increase in the proportion of IIC publications in the field of mRNA vaccines since 2020. Alongside this trend, there has been an increase in the size of research teams, manifested by a significant increase in the number of authors and institutions involved. This trend is contrasted to that described in a previous study, which reported fewer nations and smaller teams in COVID-19 research compared to pre-COVID research.Citation12 This disparity may be explained by the necessity of conducting large-scale research to advance mRNA technology and the pressing clinical demands for mRNA vaccines brought about by the COVID-19 pandemic. As previously discussed in our earlier publication, the evolution of mRNA technology can be delineated into three distinct stages: the preliminary exploratory stage prior to early 2010, characterized by the exploration of mRNA’s potential to induce protein expression and immune responses; the growing up stage from the early 2010s to 2019, marked by a multitude of studies aiming to enhance mRNA modification and delivery; the rapid maturation stage subsequent to the COVID-19 outbreak, during when the first two mRNA vaccines, BNT162b2 and mRNA-1273, were rapidly developed and authorized for use in 2020.Citation20 It is explainable that a greater proportion of IIC publications involving more authors and institutions have been undertaken since 2020, for the reasons of technological development necessity and urgent clinical needs. For instance, the two publications disclosing the results of key Phase 3 clinical trials for BNT162b2 and mRNA-1273 involved as many as 29 and 37 authors, respectively.Citation16, Citation18
In contrast to the increasing proportion of IIC publications, it is noteworthy that the proportion of IC publications experienced a significant decline during Period 2, which can be observed both globally and among the top countries. This decline is likely attributed to the frequent implementation of international travel restrictions as a measure to curb the global spread of COVID-19.Citation49,Citation50 In Period 3, the proportion of IC publications continued to decrease, indicating a sustained and increasingly evident impact of the COVID-19 pandemic on international cooperation, even in the field of mRNA vaccines which is so crucial for curbing COVID-19. Consistent with our findings, a previous study indicated that the COVID-19 pandemic had a positive effect on national collaboration but a negative effect on international collaboration.Citation31 Although the World Health Organization declared in May 2023 that COVID-19 no longer constitutes a PHEIC and travel restrictions in various countries have been canceled, the current data on publications are insufficient to analyze the recovery of international collaboration following the easing of travel restrictions. However, this is an area of study that warrants further investigation in the coming years.
Aligning with findings from previous studies, our analysis revealed that NIC publications received significantly fewer citations compared to IC publications, which holds true across all three periods. Two studies published in 2019 demonstrated the positive and statistically significant effects of international collaboration on citation impacts.Citation51,Citation52 Considering the aforementioned decrease in the proportion of IC publications following the onset of the COVID-19 pandemic and the positive effect of international collaboration on publication impact, a significant issue that deserves discussion is whether the COVID-19 pandemic primarily hindered the technological advancement of mRNA vaccines by affecting international collaboration or, conversely, stimulated technological progress by prompting increased resource allocation to mRNA vaccine development. From a negative perspective, the pandemic may have impacted international collaboration in the mRNA vaccine field through the following three ways: (1) the travel restrictions and lockdown measures implemented may have reduced opportunities for researchers to have face-to-face interactions and collaborationsCitation8; (2) the closure of laboratories and research facilities during the pandemic could have disrupted the progress of international collaborative projectsCitation9; (3) the shifting international relations brought about by the pandemic could have affected cooperation between countries.Citation10 However, from a positive standpoint, during the pandemic, a significant amount of resources flowed into mRNA vaccine research, which included government resources, corporate investments, and contributions from the broader community. Additionally, the opening of certain expedited regulatory pathways, such as emergency use authorization, played a crucial role in promoting advancements in mRNA vaccine technology.Citation53 When we carefully consider the fundamental reasons for international collaboration, it’s essentially about overcoming individual resource and technological limitations through complementary efforts. We can reasonably infer that the substantial resource allocation during the pandemic may have compensated for the impact on international collaboration.Citation11 In other words, even if international collaboration was not as extensive during the pandemic as it is during normal times, some issues that typically require collaboration to resolve could still be addressed due to the substantial resource allocation during the pandemic. Looking at the development and emergency use authorization of BNT162b2 and mRNA-1273, which took only a few months, it can be concluded that the COVID-19 pandemic, on the whole, accelerated the advancement of mRNA vaccine technology.Citation16,Citation18,Citation54
From the perspective of inter-country collaboration networks, an increase in the number of nodes and an elevation in the density of network were observed in Period 2 as compared to Period 1. This can be attributed to a substantial increase in the total volume of collaborative publications in the field of mRNA vaccines, driven by the urgent medical demands brought about by the COVID-19 pandemic and the advancement of mRNA vaccine technology itself. It has been demonstrated that inter-country collaboration facilitated the rapid progress of technologies. For instance, the COVID-19 vaccine BNT162b2, developed through collaboration among countries including the US, Argentina, Brazil, Germany, South Africa and Turkey, was launched within a year after the initiation of the first study for COVID-19 indication.Citation16 In Period 3, the collaboration networks became relatively sparse compared to Period 2. This can be attributed to the increased participation of more countries in mRNA vaccine research as mRNA vaccines became widely used globally. Scientific research in Period 3 expanded beyond technological aspects and focused on solving real-world challenges, such as characterizing variant-adapted mRNA vaccines, addressing problems including decreased level of neutralizing antibodies over time, safety concerns, and vaccine shortages.Citation20,Citation55–57 In other words, the sparsity of the network in Period 3 is mainly due to an increase in the number of network nodes rather than a decrease in the cooperation enthusiasm of existing nodes, which is supported by the degree and weighted degree of top countries in .
From the perspective of the importance of countries in the collaboration network, the US, the UK and Germany have consistently maintained their significance. The US has made significant contributions to the field of mRNA vaccines, with American scientists pioneering the synthesis of mRNA and the use of lipid nanoparticles for delivery as early as the 1980s22. In the 2000s, they developed nucleoside modification techniques to enhance mRNA stability and evade cellular immunity.Citation21 The lipid nanoparticle encapsulation techniques and nucleoside modification techniques developed by American scientists remain core technologies for mRNA vaccine development.Citation58 Additionally, Moderna, founded by American scientists in 2010, has become a leading company in the field of mRNA vaccines. Germany follows closely behind the US in terms of accumulation in the field of mRNA vaccines. CureVac and BioNTech, two leading companies in the mRNA vaccine field, were established by German scientists before 2010.Citation16,Citation59 The UK has less accumulation in mRNA vaccines compared to the US and Germany, but holds a leading position in other vaccine platforms, with the ChAdOx1 nCOV-19 adenoviral vector vaccine developed by the University of Oxford being widely used worldwide.Citation60,Citation61 During Period 1, scholars in the field of mRNA vaccines from the UK collaborated primarily with the US and Germany. In Period 2 and Period 3, in addition to the US and Germany, China has emerged as a major collaborator for the UK, and collaborations focused on testing the effectiveness and safety of vaccines such as BNT162b2, mRNA-1273, ChAdOx1, or COVID-19 inactivated vaccines in real-world settings.Citation62,Citation63
Unlike the three leading countries, Italy, Japan and China have experienced significant changes in their importance in the international collaboration network in the field of mRNA vaccines. In Period 1, Italy published only 35 papers, significantly fewer than Germany’s 126 papers. However, in Period 2, Italy’s publication volume had become almost comparable to that of Germany. This is mainly because that Italy swiftly introduced COVID-19 mRNA vaccines for widespread domestic vaccination to address the COVID-19 pandemic, which led to a significant increase in Italy’s international collaborations and research activities focusing on evaluating vaccine effectiveness and safety among populations.Citation64,Citation65 In Period 1, Italy collaborated with the network central nodes, the US, Germany, and the UK, for 16, 4, and 3 times, respectively, which increased to 27, 12, and 14 times in Period 2, respectively, and to 43, 21, and 37 times in Period 3, respectively. As the collaborations with central nodes increased, Italy’s betweenness centrality also rose (0.025, 0.035, and 0.061), making it increasingly important in the network. Japan had a relatively low number of publications in the first two periods, much fewer than Germany and the UK. However, in Period 3, Japan surpassed both Germany and the UK. This increase can also be attributed to the significant rise in research related to mRNA vaccines due to the widespread mRNA vaccine administration in the Japanese population. The research primarily focused on the effectiveness and safety of mRNA vaccines after vaccination. Notably, Japan’s importance in the collaborative network rose to the 5th position during Period 3. This was mainly due to its two articles in collaboration with DEM REP CONGO, Burkina Faso, El Salvador, Cambodia, and Uzbekistan,Citation66,Citation67 making Japan become the unique and crucial bridge connecting these countries with others, leading to a significant increase in its betweenness centrality during Period 3, second only to the United States (In the lowest figure in , El Salvador, Cambodia, and Uzbekistan are not shown as they are hidden behind DEM REP CONGO and Burkina Faso). China, as one of the countries ranking in top five in terms of the total number of publications, exhibited a different change from the other four top countries in Period 3. As shown in , the number of countries engaged in mRNA vaccine research increased from 82 in Period 2 to 105 in Period 3. Meanwhile, the number of collaboration partners for the US rose from 68 to 80, for Italy from 54 to 61, for Germany from 57 to 65, and for the UK from 63 to 71. However, the number of collaboration partners for China decreased from 50 to 44. Consequently, the importance of China in the international collaboration network has decreased, potentially due to two reasons. First, other kinds of COVID-19 vaccines, especially inactivated vaccines instead of mRNA vaccines, have been widely administered among Chinese population.Citation68,Citation69 Consequently, there is limited data in China supporting research on the effect and safety of mRNA vaccines. Second, due to strict mobility control measures, it becomes challenging for China to establish collaborations with countries that newly entered the field of mRNA vaccine.Citation70 Although collaboration with existing partners such as the US and the UK has not decreased, China’s importance may decrease due to the lack of connections with the newly added nodes in the network. This highlights the significance of actively establishing collaboration with other countries during non-pandemic periods, which can facilitate continued cooperation and help maintain a positive position in the industry during pandemics.
Several Asian countries, including Turkey, Saudi Arabia, and India, have had limited publication output in the field of mRNA vaccines. However, there have been noticeable shifts in their positions within the collaboration networks, particularly from Period 1 to Period 2. In Period 1, Turkey had minimal involvement in mRNA vaccine-related research, with only sporadic contributions in a few articles, resulting in a very low ranking within the collaboration network. However, in Period 2, multiple Turkish institutions became clinical trial sites for BioNTech and Pfizer’s mRNA vaccine and actively participated in publishing related research articles.Citation71 Consequently, the number of publications for Turkey increased from 3 in Period 1 to 17 in Period 2, with collaborations involving 6 articles with the US, 5 with Germany, and 5 with the UK, all of which are central players in the network. Additionally, articles disclosing the results of the key trial for BNT162b2 (NCT04368728) also featured contributions from Turkish institution Hacettepe University.Citation16,Citation72 Similar to Turkey, Saudi Arabia also conducted very little mRNA vaccine research in Period 1. However, in Period 2, unlike Turkey, which participated as a clinical trial location during the development of BNT162b2, Saudi Arabia rapidly introduced BNT162b2 for population-wide vaccination after it became available on the market. This provided Saudi research institutions with the opportunity to collaborate internationally with countries such as the US, Germany and Italy to assess the vaccine’s effectiveness and adverse reactions in the population. As a result, its ranking in the importance increased from 23rd in the Period 1 to 14th in the Period 2.Citation64,Citation73 India’s importance in the collaboration network increased from 19th in Period 1 to 8th in Period 2. This significant rise was primarily due to India’s first report and wide-spreading of the Delta variant, which garnered international attention due to its immune evasion properties. Therefore, many countries collaborated with India to conduct research on the effectiveness of mRNA vaccines against the Delta variant.Citation74
As to the relationship between distance and collaboration, our study indicated that geographical distance between countries plays a crucial role in influencing cooperation, especially during the COVID-19 pandemics when the impact of distance on collaboration became more pronounced, which is consistent with results from previous studies.Citation75,Citation76 Previous research has shown that geographical distance has a negative impact on international collaboration, primarily attributable to two principal factors. First, scientists from two distant countries may face relatively high costs associated with travel and communication, which can lead to reduced collaboration.Citation77 Second, cultural differences between countries, especially language and religious beliefs, often affect the convenience of communication and the level of trust among collaborating members, thus diminishing international collaborative endeavors.Citation78–80 That is to say, even during non-pandemic periods, collaboration between distant countries is inherently less convenient. During the COVID-19 pandemics, with the implementation of intensive lockdown measures and travel restrictions, the collaboration between distant countries becomes even more challenging. Therefore, it is important to consider how to maintain and even strengthen cooperation between distant countries. The emergence of new communication methods during the COVID-19 pandemic, such as digital platforms, virtual conferences, and other online tools, offers opportunities for effective communication and collaboration despite physical distance. It is essential to make effective use of these communication tools and further develop them to reduce the negative impact of future pandemics on collaboration between distant countries. Furthermore, distant countries should establish long-term collaborative partnerships through frequent visits and cooperative projects during non-pandemic periods, which can help foster mutual understanding, trust, and connection, thereby laying a solid foundation for international collaboration by overcoming cultural and language differences. This proactive approach can help mitigate the challenges posed by geographical distance and facilitate effective collaboration even in times of crisis.
Our analysis indicated that the mRNA vaccine field has experienced rapid development over the past decades, with significant changes in both research focus and collaborative patterns, as well as obvious shifts in the institutions that are major contributors in the mRNA vaccine development. The success of mRNA vaccines in preventing COVID-19 is an inspiring beginning, which may foreshadow their enormous potential for other infectious diseases and cancers. Based on the co-citation analysis in our previous study and the term clustering analysis in this study, several key issues in mRNA vaccine research that warrant further investigation have been summarized.Citation20 First, even though BNT162b2 and mRNA-1273 have been approved for clinical use, various challenges have emerged in real-world vaccination, including reduced effectiveness against mutant strains, rapid decline in neutralizing antibody titers over time, safety concerns, vaccine shortages in the early stages of new outbreaks and inadequate protection for immunocompromised populations. To better prepare for future infectious disease outbreaks, these issues require further research.Citation20 Second, while mRNA vaccines have shown success in preventing COVID-19, their effectiveness in preventing other infectious diseases needs to be further validated. Ideally, mRNA vaccines capable of simultaneously preventing multiple infectious diseases should also be explored.Citation20 Third, self-amplifying mRNA (SAM) vaccines have become a potential ideal solution for addressing pandemics due to their high immunogenicity and reduced production burden. SAM vaccine-related research has grown considerably in recent years, with clinical trials for COVID-19, influenza, rabies, and herpes simplex virus infections started in recent years. This is expected to be a promising avenue for further development.Citation20 Fourth, considering that currently approved mRNA vaccines require low-temperature transportation and strict storage conditions, which poses a challenge for mass vaccination, especially in impoverished and tropical regions. Therefore, the development of thermostable vaccines will be a promising research direction.Citation20 Fifth, selecting appropriate tumor-specific antigens is crucial for the successful development of mRNA cancer vaccines. Advances in next-generation sequencing and bioinformatics have made it possible to select more ideal neoantigens, and thus developing personalized mRNA cancer vaccines based on neoantigens are expected to be a future research trend.Citation20 Sixth, research into delivery systems for mRNA cancer vaccines is essential to induce strong cytotoxic T-cell responses while minimizing the risk of delivery vehicle accumulation, which is particularly important given the need for repeated dosing in cancer patients.Citation20 Finally, in the development of mRNA cancer vaccines, even though appropriate antigens and effective delivery systems can induce robust immune responses, the suppressive tumor microenvironment can still lead to T-cell exhaustion. Overcoming the suppressive tumor microenvironment through combinations of mRNA cancer vaccines with other therapies holds promise in the future.Citation20 The resolution of these issues awaits the collective efforts of universities, research institutions, hospitals, companies, and regulatory authorities. As research focus shifts, the roles of various institutions will also undergo dynamic changes. As more and more issues become better understood, mRNA vaccines hold the promise of playing a significant role in addressing various infectious diseases and cancer in the future.
Limitations
This study is designed to analyze the scientific collaboration in the field of mRNA vaccines, for which purpose, this study has some limitations.
First, the data source used is limited to scientific publications. This approach may ignore certain forms of collaboration that do not result in coauthored publications, and some coauthored publications may not accurately reflect the extent of actual collaboration.Citation34,Citation81 However, scientific publications offer advantages in terms of sample size, analysis efficiency, and result robustness, making them an optimal data source for collaboration analysis.Citation34,Citation81 In future research, additional data sources can be incorporated to support the analysis of scientific publications, such as collaborative patent applications, jointly organized conferences, collaborative clinical trials, and coauthored guidelines.
Second, this study exclusively utilized the WOS database and did not include databases with broader coverage such as Scopus or Dimensions,Citation82,Citation83 nor databases that primarily index local language publications, which may introduce selection bias. However, considering that the WOS database provides comprehensive coverage of high-quality publications and facilitates cross-country comparisons, it adequately meets the requirements of this study.Citation36,Citation83 In future research, incorporating data from additional databases can help assess the consistency and robustness of the results across different sources and gain a more comprehensive understanding of international collaboration patterns.
Third, this study did not differentiate between first authors, corresponding authors, and other authors, which could lead to deviations in the results. For example, in an extreme case, if two countries publish the same set of publications, but one country primarily dominates as the first or corresponding author while the other participates as a follower, our method would yield the same results for both countries when calculating the amount of international collaborative publications. However, the actual level of international collaboration may vary significantly between the two countries. Future research could differentiate authors’ contributions to collaborative publications by assigning weights based on the order of authorships, thereby obtaining more objective results.
Fourth, in this study, when analyzing the evolution of research focuses in the mRNA field over time and the differences in research focuses among different types of collaborations, we employed clustering analysis based on terms in titles, abstracts, and keywords. This method provides relatively broad research directions and may not uncover some smaller categories. To address the limitations of the methodology employed in this study, we also took into consideration the key findings from our previous co-citation analysis in the discussion.
Fifth, when conducting the TOPSIS analysis, although the entropy weighting method used to assign weights to the indicators has its advantages, it also has certain limitations. For instance, it heavily relies on data distribution and does not leverage the expertise and professional knowledge of decision-makers, leading to reduced flexibility when dealing with specific issues. In future research, the entropy weighting method could be combined with subjective weighting. That is, the entropy weighting method could be used to calculate the preliminary weights of each indicator, followed by a final weight assignment by experienced decision-makers, which may lead to more scientific ranking results.
Conclusions
In conclusion, our research has provided valuable insights into the evolution of scientific collaboration in the field of mRNA vaccines, with the following key findings.
First, we observed a significant increase in the proportion of DIIC publications and a significant decrease in the proportion of IC publications in Period 2 and 3 compared to Period 1. Additionally, the average number of authors and institutions increased significantly, indicating larger sizes of teams formed in Period 2 and Period 3.
Second, we found a significant correlation between the impact of publications and the type of collaboration as well as the size of teams. More citations were received by IIC publications, IC publications and publications involving more authors, institutions or countries compared to NIIC publications, NIC publications and those involving fewer authors, institutions or countries. The correlation persisted across Period 1, 2 and 3.
Third, in terms of changes in the inter-country scientific collaboration network, we observed an increased number of countries participating in mRNA research in Period 2 and 3, resulting in improved diversity of collaboration partners and increased enthusiasm for collaboration.
Fourth, the US, the UK, and Germany remained the top three countries with the highest frequency of international collaborations throughout all three periods. While some other countries including Italy, Japan, and China experienced significant changes. Italy improved its ranking from 12th in Period 1 to 5th in Period 2 and 4th in Period 3. Japan moved from 15th initially to 5th in Period 3, while China fell from 7th in Period 1 to 11th in Period 3.
Fifth, we found a negative correlation between distance and the number of collaborative publications between countries. This correlation became more pronounced during Period 2 and Period 3, indicating that cooperation between distant countries may have been more affected by the COVID-19 pandemic compared to those with closer proximity.
Sixth, we observed a significant shift in the research focus within the mRNA vaccine field. In Period 1, the primary emphasis was on technical exploration and optimization. In Period 2, the focus shifted to the development of COVID-19 vaccines and testing their efficacy and safety. In Period 3, as research transitioned from product development to assessing long-term vaccine efficacy, evaluating effectiveness against variants, and safety evaluation, more disease-oriented studies were conducted.
Seventh, with the changes in research focuses, there have been shifts in the institutions that are major contributors in the mRNA vaccine development. In Period 1, major contributors were primarily institutions from the US and Germany, including universities and companies like BioNTech, CureVac, and Moderna. In Period 2, both Moderna and BioNTech continued to be major contributors, Pfizer entered this field through its collaboration with BioNTech, and some institutions from countries other than the US and Germany emerged as new major contributors. In Period 3, as research focus changed, the role of companies somewhat diminished. Instead, universities, research institutes, and CDCP from various countries dominated as major contributors.
Contributions
Theoretical, this research contributes to the understanding of the evolution of global scientific collaboration in the field of mRNA vaccines, shedding light on the changing patterns of collaboration types and team sizes and providing insights into the dynamics of scientific research networks. The analysis of the relationship between collaboration indicators and academic impact enhances our knowledge of the factors that influence the quality of scientific publications in this domain. Furthermore, the investigation of the impact of geographical distance on collaboration offers valuable insights into the mechanisms shaping international research networks.
Practically, the findings of this study have implications for policymakers and researchers in the field of mRNA vaccine research. Firstly, the identification of major countries and institutions and shifts in their importance can guide policymakers in prioritizing resources and partnerships, as well as help them strengthen existing collaborations and foster new ones with emerging research leaders in this field. Secondly, the proved impact of geographical distance on collaborations highlights the importance of innovative approaches, such as the use of digital platforms and virtual conferences, in facilitating remote collaboration and overcoming physical barriers imposed by geographical distance. Thirdly, Understanding the dynamics of international collaboration and research focuses in this specific area can facilitate the identification of potential gaps, opportunities, and areas for future cooperation, guiding researchers in establishing effective collaborations, fostering knowledge exchange, and promoting scientific advancements in mRNA vaccine research. Finally, the analysis of the correlation between academic impact and collaboration indicators can assist researchers in assessing the effectiveness and productivity of collaborations, enabling them to make informed decisions about collaboration strategies.
Authors’ contributions
The design of the study was put forwarded by Zhaolian Ouyang and Juan Chen. The data collection was conducted by Juan Chen, Lizi Pan and Dongzi Xu. The analysis and interpretation of data were conducted by Juan Chen, Yan Lu, Ting Zhang, Lizi Pan and Shu Yan. The writing and revision of the manuscript were performed by Juan Chen and Lizi Pan. All authors have read and approved the final manuscript.
Ethics approval
The literature-based study was not conducted in animals or humans, therefore no ethical approval is required.
Abbreviation
| CC | = | Clustering Coefficient |
| COVID-19 | = | Coronavirus Disease 2019 |
| CWTS | = | Centre for Science and Technology Studies |
| DDA | = | Derwent Data Analyzer |
| DIIC | = | Domestic Inter-institutional Cooperation |
| IC | = | International Cooperation |
| IIC | = | Inter-Institutional Cooperation |
| M | = | Mean |
| N | = | Number |
| NIAID | = | National Institute of Allergy and Infectious Diseases |
| NIC | = | No International Cooperation |
| NIIC | = | No Inter-institutional Cooperation |
| PHEIC | = | Public Health Emergency of International Concern |
| SAM vaccine | = | Self-amplifying mRNA vaccine |
| SD | = | Standard Deviation |
| TOPSIS | = | Technique for Order Preference by Similarity to Ideal Solution |
| US | = | United States |
| US CDCP | = | US Centers for Disease Control and Prevention |
| US FDA | = | US Food and Drug Administration |
| WD | = | Weighted Degree |
| WHO | = | World Health Organization |
| WOS | = | Web of Science |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability statement
The datasets analyzed during the current study are available from the corresponding author on a reasonable request.
Additional information
Funding
References
- Armenteras D. Guidelines for healthy global scientific collaborations. Nat Ecol Evol. 2021;5(9):1193–23. doi:10.1038/s41559-021-01496-y.
- Shen H, Xie J, Li J, Cheng Y. The correlation between scientific collaboration and citation count at the paper level: a meta-analysis. Scientometrics. 2021;126(4):443–70. doi:10.1007/s11192-021-03888-0.
- Lu W, Ren Y, Huang Y, Bu Y, Zhang Y. Scientific collaboration and career stages: an ego-centric perspective. J Informetr. 2021;15(4):101207. doi:10.1016/j.joi.2021.101207.
- Druedahl LC, Minssen T, Price WN. Collaboration in times of crisis: a study on COVID-19 vaccine R&D partnerships. Vaccine. 2021;39(42):6291–5. doi:10.1016/j.vaccine.2021.08.101.
- Fry CV, Cai X, Zhang Y, Wagner CS. Consolidation in a crisis: patterns of international collaboration in early COVID-19 research. PloS One. 2020;15(7):e36307. doi:10.1371/journal.pone.0236307.
- Wang J, Hong N. The COVID-19 research landscape measuring topics and collaborations using scientific literature. Medicine. 2020;99(43):e22849. doi:10.1097/MD.0000000000022849.
- Chernysh Y, Roubik H. International collaboration in the field of environmental protection: trend analysis and COVID-19 implications. Sustain Basel. 2020;12(24):1–18. doi:10.3390/su122410384.
- Zaer H, Fan W, Orlowski D, Glud AN, Andersen ASM, Schneider MB, Adler JR, Stroh A, Sørensen JCH. A perspective of international collaboration through Web-based telecommunication–inspired by COVID-19 crisis. Front Hum Neurosci. 2020;14:577465. doi:10.3389/fnhum.2020.577465.
- Cordato DJJ, Shad KF, Soubra W, Beran RGG. Health research and education during and after the COVID-19 pandemic: an Australian clinician and researcher perspective. Diagnostics. 2023;13(2):289–300. doi:10.3390/diagnostics13020289.
- Guimon J, Narula R. Ending the COVID-19 pandemic requires more international collaboration. Res Technol Manage. 2020;63(5):438–41. doi:10.1080/08956308.2020.1790239.
- Kim K, Cho KT. A review of global collaboration on COVID-19 research during the pandemic in 2020. Sustain Basel. 2021;13(14):7618. doi:10.3390/su13147618.
- Cai X, Fry CV, Wagner CS. International collaboration during the COVID-19 crisis: autumn 2020 developments. Scientometrics. 2021;126(4):3683–92. doi:10.1007/s11192-021-03873-7.
- Vervoort D, Ma X, Luc JGY. COVID-19 pandemic: a time for collaboration and a unified global health front. Int J Qual Health Care. 2021;33(1):mzaa65. doi:10.1093/intqhc/mzaa065.
- Malekpour M, Abbasi-Kangevari M, Azadnajafabad S, Ghamari S-H, Rezaei N, Rezazadeh-Khadem S, Rezaei N, Aminorroaya A, Abdolhamidi E, Mohammadi Fateh S, et al. How the scientific community responded to the COVID-19 pandemic: a subject-level time-trend bibliometric analysis. PloS One. 2021;16(9):e258064. doi:10.1371/journal.pone.0258064.
- Thavorn J, Gowanit C, Muangsin V, Muangsin N. Collaboration network and trends of global coronavirus disease research: a scientometric analysis. IEEE Access. 2021;9:45001–16. doi:10.1109/ACCESS.2021.3066450.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. New Engl J Med. 2020;383(27):2603–15. doi:10.1056/NEJMoa2034577.
- Hajissa K, Mussa A. Positive aspects of the mRNA platform for SARS-CoV-2 vaccines. Hum Vacc Immunother. 2021;17(8):2445–7. doi:10.1080/21645515.2021.1900713.
- Baden LR, El HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New Engl J Med. 2021;384(5):403–16. doi:10.1056/NEJMoa2035389.
- Chen P, Shi X, He W, Zhong G, Tang Y, Wang H, Zhang P. mRNA vaccine-a desirable therapeutic strategy for surmounting COVID-19 pandemic. Hum Vaccin Immunother. 2022;18(1):2040330. doi:10.1080/21645515.2022.2040330.
- Chen J, Zhang T, Lu Y, Yang X, Ouyang Z. Emerging trends of research on mRNA vaccines: a co-citation analysis. Hum Vacc Immunother. 2022;18(6):2110409. doi:10.1080/21645515.2022.2110409.
- Kariko K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, Weissman D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16(11):1833–40. doi:10.1038/mt.2008.200.
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949 Pt 1):1465–8. doi:10.1126/science.1690918.
- Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–79. doi:10.1038/nrd.2017.243.
- Sahin U, Kariko K, Tureci O. mRNA-based therapeutics–developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759–80. doi:10.1038/nrd4278.
- Pardi N, Tuyishime S, Muramatsu H, Kariko K, Mui BL, Tam YK, Madden TD, Hope MJ, Weissman D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release. 2015;217:345–51. doi:10.1016/j.jconrel.2015.08.007.
- Vogel AB, Lambert L, Kinnear E, Busse D, Erbar S, Reuter KC, Wicke L, Perkovic M, Beissert T, Haas H, et al. Self-amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses. Mol Ther. 2018;26(2):446–55. doi:10.1016/j.ymthe.2017.11.017.
- Hekele A, Bertholet S, Archer J, Gibson DG, Palladino G, Brito LA, Otten GR, Brazzoli M, Buccato S, Bonci A, et al. Rapidly produced SAM ® vaccine against H7N9 influenza is immunogenic in mice. Emerg Microbes Infec. 2013;2(1):1–7. doi:10.1038/emi.2013.54.
- Geall AJ, Verma A, Otten GR, Shaw CA, Hekele A, Banerjee K, Cu Y, Beard CW, Brito LA, Krucker T, et al. Nonviral delivery of self-amplifying RNA vaccines. Proc Natl Acad Sci USA. 2012;109(36):14604–9. doi:10.1073/pnas.1209367109.
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, et al. An mRNA vaccine against SARS-CoV-2 – preliminary report. N Engl J Med. 2020;383(20):1920–31. doi:10.1056/NEJMoa2022483.
- Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. New Engl J Med. 2020;383(25):2439–50. doi:10.1056/NEJMoa2027906.
- Abramo G, D’Angelo CA, Di Costa F. How the COVID-19 crisis shaped research collaboration behaviour. Scientometrics. 2022;127(8):5053–71. doi:10.1007/s11192-022-04450-2.
- Li Y, Li H, Liu N, Liu X. Important institutions of inter institutional scientific collaboration networks in materials science. Scientometrics. 2018;117(1):85–103. doi:10.1007/s11192-018-2837-0.
- Fu YC, Marques M, Tseng Y, Powell JJW, Baker DP. An evolving international research collaboration network: spatial and thematic developments in co-authored higher education research, 1998–2018. Scientometrics. 2022;127(3):1403–29. doi:10.1007/s11192-021-04200-w.
- Aksnes DW, Piro FN, Rorstad K. Gender gaps in international research collaboration: a bibliometric approach. Scientometrics. 2019;120(2):747–74. doi:10.1007/s11192-019-03155-3.
- Essers D, Grigoli F, Pugacheva E. Network effects and research collaborations: evidence from IMF working paper co-authorship. Scientometrics. 2022;127(12):357–68. doi:10.1007/s11192-022-04335-4.
- Harzing A, Alakangas S. Google scholar, Scopus and the Web of science: a longitudinal and cross-disciplinary comparison. Scientometrics. 2016;106(2):787–804. doi:10.1007/s11192-015-1798-9.
- Mongeon P, Paul-Hus A. The journal coverage of web of science and Scopus: a comparative analysis. Scientometrics. 2016;106(1):213–28. doi:10.1007/s11192-015-1765-5.
- Niu F, Qiu J. Network structure, distribution and the growth of Chinese international research collaboration. Scientometrics. 2014;98(2):1221–33. doi:10.1007/s11192-013-1170-x.
- Fonkou MDM, Bragazzi NL, Tsinda EK, Bouba Y, Mmbando GS, Kong JD. COVID-19 pandemic related research in Africa: bibliometric analysis of scholarly output, collaborations and scientific leadership. Int J Env Res Pub He. 2021;18(14):7273. doi:10.3390/ijerph18147273.
- Feng X, Martynov I, Suttkus A, Lacher M, Mayer S. Publication trends and global collaborations on esophageal atresia research: a bibliometric study. Eur J Pediatr Surg. 2021;31(2):164–71. doi:10.1055/s-0040-1702223.
- Si Y. Co-authorship in energy justice studies: assessing research collaboration through social network analysis and topic modeling. Energy Strateg Rev. 2022;41:100859. doi:10.1016/j.esr.2022.100859.
- Planas D, Saunders N, Maes P, Guivel-Benhassine F, Planchais C, Buchrieser J, Bolland W-H, Porrot F, Staropoli I, Lemoine F, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602(7898):671. doi:10.1038/s41586-021-04389-z.
- Cao Y, Wang J, Jian F, Xiao T, Song W, Yisimayi A, Huang W, Li Q, Wang P, An R, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602(7898):657. doi:10.1038/s41586-021-04385-3.
- University L. CWTS Leiden Ranking. 2023. [accessed 2023. Oct 12 Jan 1]. https://www.leidenranking.com/information/universities.
- Opricovic S, Tzeng GH. Compromise solution by MCDM methods: a comparative analysis of VIKOR and TOPSIS. Eur J Oper Res. 2004;156(2):445–55. doi:10.1016/S0377-2217(03)00020-1.
- Chen CT. Extensions of the TOPSIS for group decision-making under fuzzy environment. Fuzzy Set Syst. 2000;114(1):1–9. doi:10.1016/S0165-0114(97)00377-1.
- Shih H, Shyur H, Lee ES. An extension of TOPSIS for group decision making. Math Comput Model. 2007;45(7–8):801–13. doi:10.1016/j.mcm.2006.03.023.
- Zhi-Hong Z, Yi Y, Jing-Nan S. Entropy method for determination of weight of evaluating indicators in fuzzy synthetic evaluation for water quality assessment. J Environ Sci. 2006;18(5):1020–3. doi:10.1016/S1001-0742(06)60032-6.
- Meng X, Guo M, Gao Z, Kang L. Interaction between travel restriction policies and the spread of COVID-19. Transp Policy. 2023;136:209–27. doi:10.1016/j.tranpol.2023.04.002.
- Girum T, Lentiro K, Geremew M, Migora B, Shewamare S, Shimbre MS. Optimal strategies for COVID-19 prevention from global evidence achieved through social distancing, stay at home, travel restriction and lockdown: a systematic review. Arch Public Health. 2021;79(1):1–150. doi:10.1186/s13690-021-00663-8.
- Goncalves A, Porto BL, Rodolfo B, Faggion CM Jr, Agostini BA, Sousa-Neto MD, Moraes RR. Brazilian articles in top-tier dental journals and influence of international collaboration on citation rates. Braz Dent J. 2019;30(4):307–16. doi:10.1590/0103-6440201902826.
- Leydesdorff L, Bornmann L, Wagner CS. The relative influences of government funding and international collaboration on citation impact. J Assoc Inf Sci Tech. 2019;70(2):198–201. doi:10.1002/asi.24109.
- Roncati L, Roncati M. Emergency use authorization (EUA), conditional marketing authorization (CMA), and the precautionary principle at the time of COVID-19 pandemic. J Public Health Pol. 2021;42(3):518–21. doi:10.1057/s41271-021-00299-6.
- Lamb YN. BNT162b2 mRNA COVID-19 vaccine: first approval. Drugs. 2021;81(4):495–501. doi:10.1007/s40265-021-01480-7.
- Peng L, Renauer PA, Okten A, Fang Z, Park JJ, Zhou X, Lin Q, Dong MB, Filler R, Xiong Q, et al. Variant-specific vaccination induces systems immune responses and potent in vivo protection against SARS-CoV-2. Cell Rep Med. 2022;3(5):100634. doi:10.1016/j.xcrm.2022.100634.
- Fang Z, Peng L, Filler R, Suzuki, K, McNamara, A, Lin, Q, Renauer, PA, Yang, L, Menasche, B, Sanchez, A, et al. Omicron-specific mRNA vaccination alone and as a heterologous booster against SARS-CoV-2. Nat Commun. 2022;13(1).
- Fang Z, Monteiro VS, Hahn AM, Grubaugh ND, Lucas C, Chen S. Bivalent mRNA vaccine booster induces robust antibody immunity against Omicron lineages BA.2, BA.2.12.1, BA.2.75 and BA.5. Cell Discov. 2022;8(1):108. doi:10.1038/s41421-022-00473-4.
- Li M, Ren J, Si X, Sun Z, Wang P, Zhang X, Liu K, Wei B. The global mRNA vaccine patent landscape. Hum Vaccin Immunother. 2022;18(6):2095837. doi:10.1080/21645515.2022.2095837.
- Alberer M, Gnad-Vogt U, Hong HS, Mehr KT, Backert L, Finak G, Gottardo R, Bica MA, Garofano A, Koch SD, et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390(10101):1511–20. doi:10.1016/S0140-6736(17)31665-3.
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–78. doi:10.1016/S0140-6736(20)31604-4.
- Voysey M, Clemens S, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi:10.1016/S0140-6736(20)32661-1.
- Wan E, Chui C, Wang Y, Ng VWS, Yan VKC, Lai FTT, Li X, Wong CKH, Chan EWY, Wong CSM, et al. Herpes zoster related hospitalization after inactivated (CoronaVac) and mRNA (BNT162b2) SARS-CoV-2 vaccination: a self-controlled case series and nested case-control study. Lancet Reg Health West Pac. 2022;21:100393. doi:10.1016/j.lanwpc.2022.100393.
- Xiong X, Wong C, Au I, Lai FTT, Li X, Wan EYF, Chui CSL, Chan EWY, Cheng FWT, Lau KTK, et al. Safety of inactivated and mRNA COVID-19 vaccination among patients treated for hypothyroidism: a population-based cohort study. Thyroid. 2022;32(5):505–14. doi:10.1089/thy.2021.0684.
- Lundstrom K, Barh D, Uhal BD, Takayama K, Aljabali AAA, Abd El-Aziz TM, Lal A, Redwan EM, Adadi P, Chauhan G, et al. COVID-19 vaccines and thrombosis—roadblock or dead-end street? Biomolecules. 2021;11(7):1020. doi:10.3390/biom11071020.
- Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, Morales M, Ziv T, Shorer Arbel Y, Scarfò L, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–73. doi:10.1182/blood.2021011568.
- Nakagama Y, Candray K, Kaku N, Komase Y, Rodriguez-Funes M-V, Dominguez R, Tsuchida T, Kunishima H, Nagai E, Adachi E, et al. Antibody avidity maturation following recovery from infection or the booster vaccination grants breadth of SARS-CoV-2 neutralizing capacity. J Infect Dis. 2023;227(6):780–7. doi:10.1093/infdis/jiac492.
- Sugiyama A, Kurisu A, Nagashima S, Hando K, Saipova K, Akhmedova S, Abe K, Imada H, Hussain MRA, Ouoba S, et al. Seroepidemiological study of factors affecting anti-spike IgG antibody titers after a two-dose mRNA COVID-19 vaccination in 3744 healthy Japanese volunteers. Sci Rep-UK. 2022;12(1):16294. doi:10.1038/s41598-022-20747-x.
- Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–92. doi:10.1016/S1473-3099(20)30843-4.
- Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, Li Y, Zhu L, Wang N, Lv Z, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi:10.1126/science.abc1932.
- Kraemer M, Yang CH, Gutierrez B, Wu C-H, Klein B, Pigott DM, du Plessis L, Faria NR, Li R, Hanage WP, et al. The effect of human mobility and control measures on the COVID-19 epidemic in China. Science. 2020;368(6490):493–7. doi:10.1126/science.abb4218.
- ClinicalTrials. Study to describe the safety, tolerability, immunogenicity, and efficacy of RNA vaccine candidates against COVID-19 in healthy individuals. 2023 Feb 28 2020.
- Thomas SJ, Moreira ED, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. New Engl J Med. 2021;385(19):1761–73. doi:10.1056/NEJMoa2110345.
- Meo SA, Bukhari IA, Akram J, Meo AS, Klonoff DC. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna vaccines. Eur Rev Med Pharmaco. 2021;25:1663–9.
- Mlcochova P, Kemp SA, Dhar MS, Papa G, Meng B, Ferreira IATM, Datir R, Collier DA, Albecka A, Singh S, et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599(7883):114. doi:10.1038/s41586-021-03944-y.
- Parreira MR, Machado KB, Logares R, Diniz-Filho JAF, Nabout JC. The roles of geographic distance and socioeconomic factors on international collaboration among ecologists. Scientometrics. 2017;113(3):1539–50. doi:10.1007/s11192-017-2502-z.
- Petruzzelli AM. The impact of technological relatedness, prior ties, and geographical distance on university-industry collaborations: a joint-patent analysis. Technovation. 2011;31(7):309–19. doi:10.1016/j.technovation.2011.01.008.
- Hou L, Pan Y, Zhu JJH. Impact of scientific, economic, geopolitical, and cultural factors on international research collaboration. J Informetr. 2021;15(3):101194. doi:10.1016/j.joi.2021.101194.
- Tenzer H, Pudelko M, Harzing A. The impact of language barriers on trust formation in multinational teams. J Int Bus Stud. 2014;45(5):508–35. doi:10.1057/jibs.2013.64.
- Hwang K. Effects of the language barrier on processes and performance of international scientific collaboration, collaborators’ participation, organizational integrity, and interorganizational relationships. Sci Commun. 2013;35(1):3–1. doi:10.1177/1075547012437442.
- Peltokorpi V, Clausen L. Linguistic and cultural barriers to intercultural communication in foreign subsidiaries. Asian Bus Manag. 2011;10(4):509–28. doi:10.1057/abm.2011.20.
- Yuan L, Hao Y, Li M, Bao C, Li J, Wu D. Who are the international research collaboration partners for China? A novel data perspective based on NSFC grants. Scientometrics. 2018;116(1):401–22. doi:10.1007/s11192-018-2753-3.
- Martin-Martin A, Thelwall M, Orduna-Malea E, Lopez-Cozar ED. Google scholar, Microsoft academic, Scopus, dimensions, Web of science, and open citations’ COCI: a multidisciplinary comparison of coverage via citations. Scientometrics. 2021;126(1):871–906. doi:10.1007/s11192-020-03690-4.
- Singh VK, Singh P, Karmakar M, Leta J, Mayr P. The journal coverage of Web of science, Scopus and dimensions: a comparative analysis. Scientometrics. 2021;126(6):213–28. doi:10.1007/s11192-021-03948-5.
