ABSTRACT
The WHO pre-qualified rotavirus vaccine, ROTAVAC®, is derived naturally from the neonatal 116E rotavirus strain, and stored at −20°C. As refrigerator storage is preferable, immunogenicity and safety of liquid formulations kept at 2–8°C, having excipients to stabilize the rotavirus, with or without buffers, were compared with ROTAVAC® in different clinical studies. Study-1, the pivotal trial for this entire product development work, was a randomized, single-blind trial with two operationally seamless phases: (i) an exploratory phase involving 675 infants in which two formulations, ROTAVAC 5C (LnHRV-1.5 mL and LnHRV-2.0 mL) containing buffer and excipients to stabilize the virus against gastric acidity and temperature, were compared with ROTAVAC®. As the immune response of ROTAVAC 5C (LnHRV-2.0 mL) was non-inferior to ROTAVAC®, it was selected for (ii) confirmatory phase, involving 1,302 infants randomized 1:1:1:1 to receive three lots of LnHRV-2.0 mL, or ROTAVAC®. Primary objectives were the evaluation of non-inferiority and lot-to-lot consistency. The secondary objectives were to assess the safety and interference with the concomitant pentavalent vaccine. As it was separately established that buffers are not required for ROTAVAC®, in Study-2, the safety and immunogenicity of ROTAVAC 5D® (with excipients) were compared with ROTAVAC® and lot-to-lot consistency was assessed in another study. All lots elicited consistent immune responses, did not interfere with UIP vaccines, and had reactogenicity similar to ROTAVAC®. ROTAVAC 5C and ROTAVAC 5D® were immunogenic and well tolerated as ROTAVAC®. ROTAVAC 5D® had comparable immunogenicity and safety profiles with ROTAVAC® and can be stored at 2–8°C, leading to WHO pre-qualification.
Clinical Trials Registration: Clinical Trials Registry of India (CTRI): CTRI/2015/02/005577CTRI/2016/11/007481 and CTRI/2019/03/017934.
Introduction
The World Health Organization (WHO) recommends the inclusion of rotavirus (RV) vaccines in all national immunization programs and as a priority intervention in countries in southeastern Asia and sub-Saharan Africa with high mortality rates to rotavirus gastroenteritis (RVGE).Citation1 The three-dose oral vaccine, ROTAVAC® (Bharat Biotech, Hyderabad, India), is a neonatal human RV vaccine (nHRV) derived from the naturally attenuated and reassorted RV strain 116E, which includes a bovine rotavirus VP4 gene (G9P[11]).Citation2–4 ROTAVAC® was licensed in India in 2015 and introduced in four early adopter states in 2016; it is now implemented in all states as part of a national scale-up for rotavirus immunization.Citation5 ROTAVAC® was prequalified by the WHO in 2018,Citation6 enabling the procurement of the vaccine by the Global Alliance for Vaccines and Immunization (Gavi) and United Nations International Children’s Emergency Fund (UNICEF), and around 450 million doses of the vaccine have been administered.
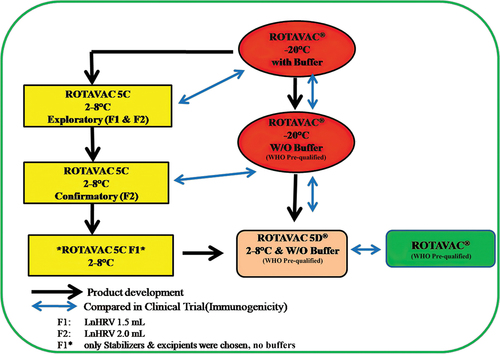
ROTAVAC® (stored at −20°C) has the smallest cold chain footprint among several licensed RV vaccines with a low dose volume (0.5 mL),Citation7,Citation8 although storage at 2–8°C is the preferred characteristic for the vaccines. It has been an endeavor to develop a 2–8°C stable rotavirus vaccine with a similar cold chain footprint. This work has been successful, and its development, carried out in two stages, is elaborated below. As with all rotavirus vaccines, the rotavirus strain was believed to be acid labile, so incorporation of a buffer component is a common feature in all rotavirus vaccines,Citation9 and the original ROTAVAC® formulation consists of a 0.5 mL vaccine component together with 2.5 mL of bicarbonate buffer administered before the vaccine.Citation3 To facilitate the storage and administration of ROTAVAC®, we embarked on a program to develop a ready-to-use liquid formulation (ROTAVAC 5C/LnHRV) containing stabilizers to make the vaccine stable at a refrigerator temperature range (2–8°C) for at least 24 months and also with buffers to ensure that the formulations have adequate acid neutralization capacity (ANC), to counter the destabilizing effect of gastric acidity on the vaccine virus, thereby eliminating the need for the additional external buffer component. All manufacturing steps during the drug substance stage remained the same between ROTAVAC® (PCT/IN2007/000190) and these new liquid formulations containing various sugars and stabilizers. Initially, over 100 different formulations were tested for stability at real-time storage temperature and at accelerated temperatures, as well as for acid neutralizing capacity, from which we first down-selected around 10 vaccine formulations and ultimately two liquid formulations (ROTAVAC 5C, LnHRV-1.5 mL and LnHRV-2.0 mL) were selected for clinical evaluation post extensive safety assessment in different animal toxicity studies (Rotavirus vaccine compositions PCT/IN2010/000041 and PCT/IN2013/000272). In the first clinical study (CTRI/2015/02/005577), we evaluated the immunogenicity and safety of ROTAVAC 5C (LnHRV-1.5 mL) and LnHRV-2.0 mL in comparison with ROTAVAC® with two operationally seamless phases: (i) an exploratory phase and (ii) a confirmatory phase.
Then, after establishing that 116E, the rotavirus strain used for ROTAVAC®, is not acid labile,Citation2 we developed and assessed a third formulation, ROTAVAC 5D®, which has a dose volume of 0.5 mL and contains stabilizers but no buffering components (PCT/IN2017/050237) (). This vaccine formulation (ROTAVAC 5D®) was again tested against ROTAVAC® for its immune performance (Study2- CTRI/2016/11/007481) and immunological lot-to-lot consistency among three different lots (CTRI/2019/03/017934). The overall development pathway is represented in .
Table 1. Composition of ROTAVAC vaccine formulations.
Methods
Clinical trial design and subjects
The report provides data from distinct clinical trials with different liquid formulations (), ROTAVAC 5C at two different dose volumes, 1.5 mL and 2.0 mL, and ROTAVAC 5D® with a dose volume of 0.5 mL, all stable at 2–8°C. All trials were conducted according to Good Clinical Practice, & Schedule Y (Drugs and Cosmetics Act, 2005), and ethical guidelines for biomedical research on human subjects (Indian Council of Medical Research, 2006), although these guidelines were revised subsequently. The protocol for each study was registered with the Clinical Trials Registry of India (CTRI) and approved by the National Regulatory Authority, the Drug Controller General, India, and the respective Ethical Committees of each participating site. A data safety monitoring board periodically reviewed the safety data.
Table 2. Clinical trials of ROTAVAC vaccine formulations.
In each study, eligible participants were healthy full-term infants at 6 weeks of age who were available for the study were not involved in any other study, and had not previously received a rotavirus vaccine. Parents or guardians had the objectives and requirements of the trial explained to them before providing written informed consent and the enrollment of their infant.
Vaccine formulations evaluated
The original ROTAVAC® composition is stored at −20°C but has limited stability for around 6 months when kept at 2–8°C. We prepared a series of liquid formulations to improve the stability and buffering capacity of the vaccine at 2–8°C for at least 24 months. These formulations contained a wide range of sugars, protein stabilizers, and excipients at varying concentrations, along with different types of buffers, all of which were in the GRAS (Generally Recognized as Safe) category. Based on extensive stability data at real-time storage temperature and at accelerated temperatures, as well as acid neutralizing capacity (ANC), and extensive safety evaluation in different animal toxicity studies, we down-selected two formulations for clinical evaluation.
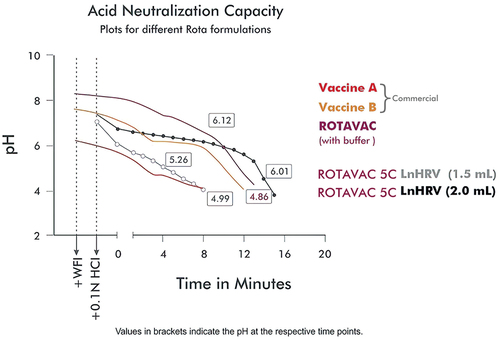
Each dose of the study vaccines, ROTAVAC 5C LnHRV-1.5 mL and LnHRV-2.0 mL, contained at least 105 Focus Forming Units (FFUs) of RV strain 116E in 1.5 mL and 2.0 mL volume per dose, respectively. They both contain stabilizers, excipients (such as sugar excipients), and protein stabilizers to keep the vaccine stable at 2–8°C with almost no decay in vaccine virus concentration over 24 months when stored at 2–8°C, as well as buffer components to vary the ANC. Typical ANC graphs for different formulations are shown in . Stability studies were also carried out at 25°C for 6 months and 37°C for 7 days to enable the use of VVM7 for stability monitoring during programmatic usage. Both were manufactured as per Good Manufacturing Practice standards. The comparator vaccine, ROTAVAC®, containing at least 105 FFU of RV strain 116E in each 0.5 mL dose, was administered orally without a buffering agent. Approximately 30 min before administration, frozen vaccine vials were shifted from −20°C to room temperature for thawing. In Study 1, the exploratory phase, batch numbers used were 61EZ14010, 61AQ14007, and 61DA13001 for the LnHRV-1.5 mL, LnHRV-2 mL, and ROTAVAC® groups, respectively, and the confirmatory phase, batch numbers used were 61AQ16002, 61AQ16003, 61AQ16004, for the LnHRV-2 mL Lots 1, 2, and 3, respectively, and 61DA616011 for the ROTAVAC® group.
In each study, vaccines were administered on Days 0, 28, and 56 concurrently with two other childhood UIP vaccines (an oral polio vaccine, BIOPOLIO®, and a pentavalent combination vaccine, ComVac5®, containing diphtheria, tetanus, pertussis hepatitis B and Haemophilus influenzae type B antigens (both manufactured by Bharat Biotech). There were no specific instructions to mothers regarding breastfeeding before/after vaccination. Peripheral venous blood samples (5 mL) were obtained on Day 0 (before vaccine administration) and Day 84 (28 days after the third dose).
Randomization
Predefined lists using randomly permuted blocks of four and eight were created at the start of each study and uploaded into the eCRF (InForm, Oracle). To ensure that males and females were allocated equally across treatment groups at each site, randomization was stratified by site and sex, with separate randomization lists for males and females at each site. The study staff at all sites were unaware of the randomization sequence. A copy of the randomization list of healthy infant identification numbers and the decoded key were sent to the biostatistician at the end of the study. Vials were labeled to identify the experimental vaccine and lot number or ROTAVAC® with an infant identification number, batch number, expiration date, and protocol number. The study staff member dispensing the vaccines was not blinded to the assignment to the experimental vaccine or the ROTAVAC® group. However, the principal investigator, sponsor, and laboratory staff who performed the serologic assays were blinded to the group allocation.
Study specific details
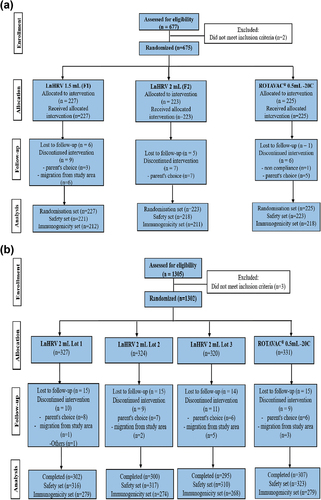
Study 1, comprising the exploratory and confirmatory phases, was registered with the Clinical Trials Registry of India (www.ctri.nic.in) as CTRI/2015/02/005577. The exploratory phase was conducted at nine sites across India. The immune responses to LnHRV-1.5 mL and LnHRV-2 mL were compared with those of ROTAVAC® to identify and select the formulation to be used in the confirmatory phase. The primary objectives were to evaluate and compare the geometric mean concentrations (GMCs) of serum anti-rotavirus immunoglobulin A (anti-RV IgA) four weeks after the administration of the third dose of each vaccine and to assess the safety and reactogenicity of the study vaccines in comparison with the comparator vaccine. A total of 675 healthy infants between 6–7 weeks of age were enrolled and were randomly allocated 1:1:1 to one of the three treatment groups to receive three oral doses of LnHRV-1.5 mL (Group I), LnHRV-2 mL (Group II), or ROTAVAC® (Group III) ().
The confirmatory phase of Study 1 was conducted at 15 sites across India. The primary objectives were to evaluate and compare the GMCs of anti-RV IgA four weeks after the administration of the third dose of the selected study vaccine, LnHRV-2 mL (selected from the exploratory phase), and the licensed ROTAVAC® (comparator vaccine) and the consistency in terms of the immune response between three production lots of the study vaccine. The secondary objectives assessed additional immune response measures, including seroconversion as a four-fold rise in anti-RV IgA concentrations post-vaccination and interference with immune responses to other concomitantly administered UIP vaccines, as well as comparisons of the reactogenicity and safety profiles between both groups. A total of 1,302 healthy infants were randomized into four treatment groups in a 1:1:1:1 ratio (three groups received three different production lots (lots 1, 2, and 3) of the selected study vaccine (LnHRV-2 mL), and one group received ROTAVAC®) ().
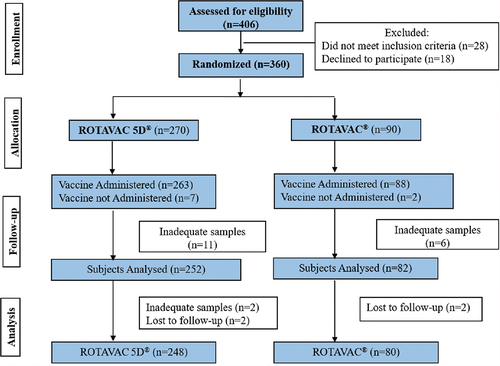
Over four years from the initiation of the original study, further investigations into the role of buffers on the immune performance showed that buffering components are not required for the vaccine to be immunogenic, leading to the development of a new formulation, ROTAVAC 5D®, which is based on LnHRV-1.5 mL but without buffer components. This formulation was investigated in Study 2, registered with the Clinical Trials Registry of India as CTRI/2016/11/007481 and conducted at 6 sites across India. The primary objective was to compare the geometric mean concentrations (GMTs) of serum anti-rotavirus immunoglobulin A (anti-RV IgA) pre and post-vaccination and the secondary objectives was to assess the immunogenicity as 2, 3 & 4-fold rise in serum anti-rotavirus IgA titers 4–5 weeks after third dose in comparison to baseline and safety and reactogenicity of ROTAVAC 5D® compared to ROTAVAC®. A total of 360 healthy infants were randomized into two treatment groups in a 3:1 ratio (one group received ROTAVAC 5D® and the other group received ROTAVAC® (). Then, to determine lot-to-lot consistency in immune response between three lots of ROTAVAC 5D® formulation, a Phase 4 study was conducted among 380 infants (). The study was registered with the Clinical Trials Registry of India as CTRI/2019/03/017934. The primary objectives were to establish the lot-to-lot consistency and GMTs of serum anti-rotavirus IgA antibody measured on 4–6 weeks after administering the 3rd dose, and the secondary objectives were to assess the 4-fold seroconversions, sero response to UIP childhood vaccines, and safety and reactogenicity across three production lots of ROTAVAC 5D®.
Immunogenicity analysis
Serum anti-RV-specific IgA concentrations were estimated using an enzyme-linked immunosorbent assay (ELISA) at the Wellcome Trust Research Laboratories, Christian Medical College (CMC), Vellore, India.Citation10 Briefly, 96-well plates (Costar, Corning) coated with rabbit hyperimmune serum to RV were incubated with purified cell culture lysates (WC3) or mock-infected MA104 cells. Serial dilutions of the standard pool of human serum and test sera were added, followed by biotinylated rabbit anti-human IgA (Jackson ImmunoResearch Laboratories, West Grove, PA), which were subsequently developed, and the absorbance was read at 492 nm. Background-corrected optical density values from sample wells were compared with the standard curve, and the anti-RV IgA concentration was determined based on derived units of IgA arbitrarily assigned to the standard curve. Seropositivity was defined as an anti-rotavirus IgA concentration ≥20 U/ml. A titer is the reciprocal of the highest dilution showing a response, whereas concentration is a calculated value (as we did in our assay). Similar to our previous studies, throughout this manuscript, we use the term concentration for anti-RV IgA, determined by comparison of the net optical density from sample wells to a standard curve generated by a human plasma standardCitation4,Citation11,Citation12 and presented as group geometric mean concentrations (GMC). Seroconversion was defined as a ≥ 4-fold rise in RV-IgA antibody concentration from baseline to 28 days after the last dose.
Reactogenicity and safety assessment
In each study, all infants were observed for 30 min after each vaccination for immediate adverse reactions and signs of vomiting or spitting. Parents/guardians were instructed on how to complete diary cards in which solicited adverse events were recorded for 7 days after each vaccination and any adverse event that occurred from administering the first dose of vaccine until 28 days after the third dose. The clinical trial team regularly contacted parents/guardians by telephone to ensure completion of the cards reviewed at each subsequent study visit. Since all infants received concurrent childhood vaccines, the solicited AEs included local AEs at the site of intramuscular pentavalent vaccine administration (pain, redness, and swelling at the site of injection). General solicited AEs included fever, crying, refusal to feed, diarrhea, and vomiting. AEs were graded for severity and relatedness by the investigators. Any cases of intussusception confirmed by the treating physician were reviewed by an independent case adjudication committee to ascertain if they met the Diagnostic Certainty Level Criteria 1 developed by the Brighton Collaboration Intussusception Working Group.Citation13 Serious AEs were reported to the Drug Controller General of India and the Ethics Committee and reviewed by a Data Safety Monitoring Board within the stipulated timeline. The sponsor covered medical expenses and hospital visits.
Statistics
This comprehensive product development work starting from ROTAVAC 5C to ROTAVAC 5D, comprised two clinical trial studies. Study 1, carried out in two stages, has been a pivotal study to evaluate the impact of stabilizers and buffers on the 116E rotavirus strain. Hence, it has been ensured that study 1 has been powered to the best extent possible to establish the impact, if any. The subsequent study 2 was an extension utilizing the key findings from the study 1. For the exploratory phase in Study 1, sample size calculations for serum anti-rotavirus IgA titers were performed using 0.8 log log10 standard deviation. For each of the two liquid formulations, 178 evaluable healthy infants per arm would provide 90% power if the underlying GMTs were the same for the two treatment arms. Based on a 20% dropout rate, the enrollment target was approximately 675 total healthy infants (225 per arm). For the primary endpoint, non-inferiority compared to ROTAVAC −20C 0.5 mL was assessed with a two-sided 97.5% confidence interval (CI) for the GMT ratio between each of the two ROTAVAC 5C formulations and ROTAVAC −20C 0.5 mL. Non-inferiority of a ROTAVAC 5C formulation was defined as the lower limit of the two-sided 97.5% CI for the GMT ratio (ROTAVAC 5C/ROTAVAC −20C 0.5 mL) being greater than 0.5. For the confirmatory phase in Study 1, a sample size of 260 infants would provide approximately 91% power to demonstrate lot-to-lot consistency if the true difference among the three lots reached a 1.1-fold difference and the log10 standard deviation was 0.8. A sample size of 780 infants for the combined liquid formulations (three lots) and 260 infants for the frozen formulation would provide > 99.9% power to demonstrate non-inferiority if both vaccines had the same true immunogenicity. To account for a 20% drop-out rate, the total target enrollment was 1300 (325 healthy infants per group). Power calculations were performed using SAS for lot-to-lot consistency and CytelSiZ Version 6.2 for non-inferiority.
Immunogenicity and lot consistency were analyzed in all infants who received all three doses of the assigned vaccine. Non-inferiority between LnHRV-2 mL and ROTAVAC® was declared if the lower limit of the one-sided 95% CI for the GMC ratio (combined LnHRV-2 mL: Lots 1, 2, and 3/ROTAVAC®) was greater than 0.5. Immune responses to pentavalent vaccines were assessed by the GMC ratios between groups. Lot consistency was declared if, for all the lot-to-lot comparisons, the two-sided 95% confidence intervals for the GMC ratios were contained within 0.5 to 2.0.
We compared groups by chi-square test for proportions and two-sample t-test on log-transformed titers. We assessed the equivalence of percentages and geometric mean titers (GMTs) using two-sided 95% confidence intervals (CIs). CIs for percentages were calculated using exact binomial calculation, and CIs for differences in percentages were calculated using a likelihood score method. CIs for GMTs and GMT ratios were calculated by taking anti-logarithms of limits of CIs for means of log-transformed titers. For percentages, equivalence margins of 5% (i.e., a CI within (−5%, 5%)) to 15% in absolute difference have generally been used in reported studies; we used 10% and 15%. For GMTs, we used the equivalence criterion of a two-sided 95% CI within the interval (0.5, 2.0), which has been commonly used. We used a two-sided p-value ≤ .05 to indicate statistical significance.
Safety data was analyzed in all infants who received at least one dose of the selected treatment vaccine and had data until 28 days after the third dose. The proportion of healthy infants experiencing at least one adverse event was compared between treatment groups using chi-squared or Fisher’s exact tests. A third-party Contract Research Organization, George Clinical™ conducted all aspects of the trial, including randomization, data management, and analysis using SAS version 9.2. In study 2 ROTAVAC 5D® vs. ROTAVAC®, the Immunogenicity was analyzed in terms of Geometric mean titers, comparison of baseline to post-vaccination titers at 4 (+1) weeks after the third dose and Seroconversions, comparison of two, three & four-fold rise in titers from baseline to post-vaccination. Safety was analyzed as adverse events between the groups by Chi-square or Fischer exact test. The full analysis set (FAS) comprises all randomized healthy subjects. The safety analysis set comprises healthy subjects who received at least one dose of the assigned treatment vaccine and had some post-vaccination data relating to safety (vital signs, laboratory values, adverse events or physical examination). The per protocol (PP) set comprises all healthy subjects who have received all two doses of the assigned vaccine who have no missing values for and blood specimens obtained at subsequent study visits after administration of the vaccine. For the ROTAVAC 5D® lot-to-lot consistency study, the Immunogenicity pre- and post-vaccination serum IgA levels were summarized in terms of mean and standard deviation. For the primary endpoint, geometric mean titers (GMTs) with a 95% confidence interval (CI) were calculated for each treatment group. The ratio of GMTs between the treatment group for baseline and post-vaccination titers were calculated and represented using the Mann-Whitney test. The difference in the proportion of the subjects who seroconverted (with ≥2, ≥3 and ≥ 4 fold increase from baseline) was compared between the three groups using the Chi-square test, and the results were presented as 95% CI and p values. Clinical lot consistency was evaluated using pairwise comparisons of the three consistency lots based on the two-sided 95% CI for the ratio of GMTs. AEs were recorded as solicited and unsolicited AEs. AEs were tabulated and compared across the treatment groups. The proportion of AEs after the vaccine was compared using Chi-square and presented as likelihood ratio and chi-square probability values.
Results
ROTAVAC 5C study 1 -exploratory phase
We enrolled and randomized 675 infants, of whom 641 completed the study according to protocol. The GMC ratio comparing LnHRV-1.5 mL & ROTAVAC® was 0.75 (95% CI: 0.6, 0.95) and LnHRV-2 mL & ROTAVAC® was 0.9 (95% CI: 0.72, 1.14). The lower bound of the 95% CI was not below 0.5, and the upper bound of the 95% CI was below 1, suggesting that LnHRV-1.5 mL was not equivalent to ROTAVAC® (Figure S1). However, LnHRV-2 mL was found to be non-inferior to ROTAVAC® (Figure S1) and was selected for the confirmatory phase. Proportions of infants who seroconverted (4-fold) for anti-RV IgA post-vaccination were 32.08% (25.85, 38.81), 40.48% (33.78, 47.45), and 44.04% (37.34, 50.90) in LnHRV-1.5 mL, LnHRV-2 mL, and ROTAVAC® groups, respectively ().
Table 3. Immunogenicity and seroconversion comparison among treatment groups.
Commonly reported AEs in this study (in order of frequency) were fever (61.2%), crying (11.2%), vomiting (10.4%), pain at the injection site (10.9%), diarrhea (5.9%) and respiratory illness (5.3%)there were no differences in the proportions of infants reporting general solicited AEs among the groups (p = .24). Six infants (four in the LnHRV-1.5 mL group and two in the LnHRV-2 mL group) were reported with a total of 9 serious AEs, none of which were considered to be vaccine-related. There were no cases of intussusception or death.
ROTAVAC 5C study 1- confirmatory phase
A total of 1,302 infants were enrolled and randomized, of whom 1,204 completed the study according to protocol. There were no differences in baseline demographics (age, weight, length, and gender proportion) among groups (). There were 80 protocol deviations reported among 68 enrolled infants, of which 20 were considered major protocol deviations and were removed from the analysis. These included infants being underweight (n = 12), receipt of the incorrect vials (n = 7), incorrect recording of sex into the eCRF (n = 7), and missing blood samples from visit 3 (n = 4). The totals included in the safety set were 1,266, and in the immunogenicity analysis set were 1,100. The most common reasons for discontinuation were loss to follow-up (44.5%), unwillingness by the parent/guardian (22.1%), and migration from the study area (00.8%). There were also 2 (0.2%), 9 (0.7%), and 8 (0.6%) infants who failed to receive their first, second, or third dose of either vaccine. The GMC ratio comparing LnHRV-2 mL to ROTAVAC® was 1.005 (95% CI: 0.85, 1.2), and lot-to-lot consistency between the three consecutive production lots demonstrated non-inferiority (Figure S1). Seroconversion as a four-fold increase in titer is described in . The GMCs of the concomitantly administered pentavalent vaccine at days 0 and 84 between both groups were comparable (Table S1). The GMC ratios between all LnHRV-2 mL lots (combined) and ROTAVAC® for the antibodies to Bordetella pertussis, diphtheria, tetanus, hepatitis B, and Haemophilus influenzae type B were approximately 1, with a lower limit of the 95%CI > 0.50, supporting the hypothesis that LnHRV-2 mL does not interfere with responses to pentavalent vaccine components. Similar GMC ratios were observed between different lots.
Table 4. Demographic characteristics.
Approximately two-fifths of AEs occurring during the 28 days post-vaccination period were reported following the first dose, one-third occurred following the second dose, and the remaining quarter occurred following the third dose. The most commonly reported solicited AEs were fever, injection site pain, crying, cough, and vomiting. All participants had concomitantly been administered UIPUIP vaccines, which are known to be associated with such events. The number of infants reporting at least one AE was 630 (66.8%) and 220 (68.1%) in the LnHRV-2 mL and ROTAVAC® groups, respectively (). Overall, there was no significant difference in the proportion of infants reporting solicited AEs (p = .65).
Table 5. Study 1- confirmatory phase -solicited adverse events post-vaccination.
There were four serious AEs reported in the LnHRV-2 mL group (two after lot 1 and one each after the other lots) and two in the ROTAVAC® group. Serious AEs included bronchiolitis (n = 2), febrile convulsion (n = 1), bronchopneumonia (n = 1), lower respiratory tract infection (n = 1), and excision of a lipoma (n = 1). No serious AEs were considered vaccine-related, and none led to study discontinuation. No cases of intussusception or death were reported.
Study 2- ROTAVAC 5D® vs. ROTAVAC® comparative immunogenicity
A total of 360 infants were enrolled and randomized, of whom 328 completed the study, 248 in the ROTAVAC 5D® group and 80 in the ROTAVAC® group. There were no differences in baseline demographics (age, weight, length, and gender proportion) among groups (). The GMTs for the two vaccine groups were not significantly different, either on Day 0 or Day 84 (). Further, the ratio of GMTs at both Days 0 and 84 was equivalent according to the criterion of a two-sided 95% CI within (0.5, 2.0). They also satisfied the more stringent criterion of a CI within (2/3, 1.5). The safety profile of ROTAVAC 5D® appears to be similar to ROTAVAC® (). Fewer healthy subjects experienced fever and pain with ROTAVAC 5D® compared to ROTAVAC® (). No deaths were reported in any of the study groups during the study.
Table 6. Study 2-ROTAVAC 5D® vs. ROTAVAC®.
ROTAVAC 5D® lot-to-lot consistency
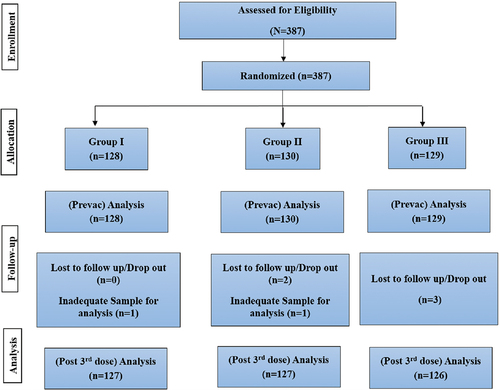
Immunogenicity was analyzed in 380 participants (Lot 1, n = 127; Lot 2, n = 127; and Lot 3, n = 126) who received all three doses of ROTAVAC 5D® and provided baseline and day 84 post-vaccination sera samples. The percentage of subjects demonstrating 4-fold changes in antibody titers are 42.52%, 51.18%, and 49.21% in Lot 1, Lot 2, and Lot 3, respectively. The safety analysis revealed that a majority of the adverse events were mild in nature and comparable across all three lots.
Discussion
This set of studies established that immunogenicity-based clinical trials could be successfully used to differentiate between stable, liquid rotavirus vaccine formulations. After showing non-inferiority to ROTAVAC® in the exploratory study, ROTAVAC 5C LnHRV-2 mL was selected for the confirmatory phase in which LnHRV-2 mL and ROTAVAC® showed comparable immunogenicity and safety profiles and all lot-to-lot comparisons were found to be non-inferior to each other.
The WHO immunization guidelines state that rotavirus vaccines are to be administered concomitantly with UIP vaccines: the oral polio vaccine (OPV) and the DTP-HepB-Hib pentavalent vaccine.Citation14,Citation15 So, we needed to be able to demonstrate that concomitant ROTAVAC 5C LnHRV-2 mL or ROTAVAC® did not have any impact on the immune responses to the concomitantly administered antigens in the pentavalent vaccine when measured as the GMCs of antibodies at days 0 and 84. We were unable to estimate the anti-polio response in these studies due to the closure of the national testing laboratory, but a previous trial showed that ROTAVAC® did not interfere with the immune responses to OPV or the pentavalent vaccine,Citation16 and the lack of interference of ROTAVAC 5C LnHRV-2 mL and ROTAVAC® further corroborates these earlier findings.
The proportion of baseline and post-vaccination seropositivity rates and fold changes were similar to other commercially available vaccines in low-income countries.Citation17–25 There was no meaningful difference in the number of adverse events reported between vaccines. No confirmed cases of intussusception were identified. As the different randomized trials were conducted in over 25 different sites, it ensures the generalizability of the results to heterogeneous subject cohorts, although they are limited to an Indian population.Citation26–30
ROTAVAC 5C LnHRV-2 mL contains stabilizers and excipients, including sugar and protein stabilizers to keep the vaccine stable at 2–8°C. The Study 1 confirmatory phase was designed to demonstrate that LnHRV-2 mL could induce an immune response when presented in a liquid formulation and was found to be comparable to the licensed ROTAVAC®, a WHO Pre-Qualified vaccine. Earlier lyophilized and liquid formulations of another licensed rotavirus vaccine were compared similarly.Citation31 Our previous study on the licensed ROTAVAC® concluded that there was no requirement for a buffer to protect against gastric acidity, allowing a decrease in the dose volume to 0.5 mL.Citation12 If a separate or combined buffer is not required, many important programmatic issues, such as incorrect reconstitution of the vaccine, temporary unavailability of the buffer, reduction of the cold chain footprint, and waste management, can be addressed. In the current study, the chosen formulation, ROTAVAC 5C LnHRV-2 mL, was supplied in 2 mL aliquots, similar to three commercially available rotavirus vaccines. To improve programmatic feasibility, we made further revisions to the existing liquid formulation, which resulted in ROTAVAC 5D® (0.5 mL per dose), which is stored at 2–8°C, does not contain a buffering agent, and is of the same dose volume to ROTAVAC®.
ROTAVAC 5D® was assessed in a multicenter, open-label, randomized controlled trial to evaluate its immunogenicity and safety compared with ROTAVAC®, the first study of its kind to compare the immunogenicity between both formulations (ROTAVAC 5D® with ROTAVAC®), with identical objectives and procedural activities to those of the earlier studies with LnHRV-2 mL. Post-vaccination GMTs (), fold changes, and safety profiles were comparable. In addition, results from an open-label, randomized, controlled trial comparing the immunogenicity and safety of ROTAVAC 5D® with ROTAVAC® in Zambian infants corroborate the results presented here, both ROTAVAC® and ROTAVAC 5D® were well tolerated, and the immunogenicity of ROTAVAC 5D® was non-inferior to ROTAVAC®.Citation32 We have now completed several independent clinical studies to demonstrate comparability across all variants of the stabilized formulations, eventually leading to the licensure of ROTAVAC 5D®.
A main limitation of these studies is the use of immunogenicity as the study outcome since there is no established serological correlate of protection against RV, although serum anti-RV IgA is believed to be the best surrogate marker available.Citation33,Citation34
The data from these studies inform immunization programs which can now be offered the option to choose from a ready-to-use, fully frozen formulation (ROTAVAC®) or a fully liquid formulation (ROTAVAC 5D®), both presented in 0.5 mL dose volumes. Both these rotavirus vaccines, ROTAVAC®Citation6 and ROTAVAC 5D®,Citation35 are now WHO Pre-Qualified and are available for global access through UNICEF.
Conclusion
Based on the clinical studies, ROTAVAC 5C (LnHRV-2.0) mL was found to be as effective as ROTAVAC® (0.5 mL, stored at −20°C) during the exploratory phase. It was then selected for the confirmatory phase where it was shown to be consistent across three production lots and did not interfere with other childhood vaccines recommended by UIP. After further modifications and clinical studies, ROTAVAC 5D® (0.5 mL, stored at 2–8°C) was developed. This liquid formulation has the lowest dose volume and is as safe and effective as ROTAVAC®. It has been WHO Pre-Qualified in 2021.
Author contributions
All authors met the International Committee for Medical Journal Editors criteria for authorship. KMV, VKA, SRC and KKP primarily contributed to the data interpretation and manuscript preparation, while RE and SP reviewed the manuscript. SRC was the study coordinator for the clinical trial and assisted KMV with designing the protocol and generating the interim report. WB was involved with a statistical analysis plan. SB (Late), led the immunogenicity analysis. KMV was responsible for the overall designing, supervision of the project and review of the final paper. All principal investigators [AK, JV, UN, MM, ND, SK, SY, SN, NM, VK, SM, PKP, PS, AK and RKD) were involved in conducting the clinical trial. All authors and the contract research organization had full access to masked data in the study, and all authors had final responsibility for the decision to submit for publication.
Data sharing statement
Individual participant (de-identified) data will be provided upon request with an appropriate research proposal directed to the corresponding author. After the approval of such a proposal, data will be shared through a secure online platform.
Acknowledgments
The authors are grateful to all the principal investigators and their staff who oversaw the conduct of the study at their respective sites and to all the children and their parents/guardians who participated in this study. The authors thank Ms. Sandhya Rani Nandala and Ms. Aparna Bathula for the conduct of the trial, and Ms. Akhila Kunta for preparing the draft manuscript. We specifically thank Dr. Keith Veitch for review of the manuscript. We are grateful to the study staff from the following institutions: Maulana Azad Medical College, New Delhi; Banaras Hindu University, Varanasi; Institute of Child Health, Kolkata; King George Hospital, Visakhapatnam; KLEs Dr Prabhakar Kore Medical College & Hospital, Belgaum; Datta Meghe Institute of Medical Sciences, Wardha; JSS Medical College & Hospital, Mysore; Khalatkar Hospital, Nagpur; SRM Medical College & Research Centre, Kattankulathur; GMERS Medical College, Gujarat; Sir Ganga Ram Hospital, Delhi; Maharshi Hospital & Research Centre, Rajasthan; Kalinga Institute of Medical Sciences, Bhubaneshwar; Meenakshi Mission Hospital, Tamil Nadu; Sant Dnyaneshwar Medical Education Research Center, Pune; and Sree Ramachandra Medical College and Research Centre, Chennai. We thank the late Dr. Sudhir Babji’s team at the Wellcome Trust Research Laboratories, Christian Medical College, Vellore, for sera analysis.
Disclosure statement
KMV, KKP, VKA, SRC, KKP, RE, SDP are employees of Bharat Biotech International Limited with no stock options or incentives. WB is an independent statistical development consultant.
Additional information
Funding
References
- Meeting of the Strategic Advisory Group of Experts on Immunization, April 2009: conclusions and recommendations. [accessed 2023 May 17]. https://www.who.int/publications-detail-redirect/WER8423.
- Bhan MK, Lew JF, Sazawal S, Das BK, Gentsch JR, Glass RI. Protection conferred by neonatal rotavirus infection against subsequent rotavirus diarrhea. J Infect Dis. 1993;168(2):282–12. doi:10.1093/infdis/168.2.282.
- Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, Goyal N, Kawade A, Kang G, Rathore SS, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vacciane in Indian infants: a randomised, double-blind, placebo-controlled trial. Lancet. 2014;383(9935):2136–43. doi:10.1016/S0140-6736(13)62630-6.
- Bhandari N, Rongsen-Chandola T, Bavdekar A, John J, Antony K, Taneja S, Goyal N, Kawade A, Kang G, Rathore SS, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian children in the second year of life. Vaccine. 2014;32(Suppl 1):A110–A16. doi:10.1016/j.vaccine.2014.04.079.
- Malik A, Haldar P, Ray A, Shet A, Kapuria B, Bhadana S, Santosham M, Ghosh RS, Steinglass R, Kumar R, et al. Introducing rotavirus vaccine in the universal immunization programme in India: from evidence to policy to implementation. Vaccine. 2019;37(39):5817–24. doi:10.1016/j.vaccine.2019.07.104.
- Rotavac 5D WHO - Prequalification of Medical products (IVDs, medicines, vaccines and immunization devices, vector control). 2020 July 17 [accessed 2023 May 17]. https://extranet.who.int/pqweb/content/rotavac.
- Product information for vaccines and cold chain equipment. [accessed 2023 May 18]. https://www.gavi.org/our-alliance/market-shaping/product-information-vaccines-cold-chain-equipment.
- rotavirus-vaccine-profilespdf.pdf. [accessed 2023 May 18]. https://www.gavi.org/sites/default/files/document/rotavirus-vaccine-profilespdf.pdf.
- Immunogenicity of tetravalent rhesus rotavirus vaccine administered with buffer and oral polio vaccine - PubMed. [accessed 2023 May 17]. https://pubmed.ncbi.nlm.nih.gov/1650128/.
- Ward RL, Bernstein DI, Smith VE, Sander DS, Shaw A, Eiden JJ, Heaton P, Offit PA, Clark HF. Rotavirus immunoglobulin a responses stimulated by each of 3 doses of a quadrivalent human/bovine reassortant rotavirus vaccine. J Infect Dis. 2004;189(12):2290–3. doi:10.1086/421248.
- Ella R, Babji S, Ciarlet M, Blackwelder WC, Vadrevu KM. A randomized, open-labelled, non-inferiority phase 4 clinical trial to evaluate the immunogenicity and safety of the live, attenuated, oral rotavirus vaccine, ROTAVAC® in comparison with a licensed rotavirus vaccine in healthy infants. Vaccine. 2019;37(31):4407–13. doi:10.1016/j.vaccine.2019.05.069.
- Ella R, Bobba R, Muralidhar S, Babji S, Vadrevu KM, Bhan MK. A Phase 4, multicentre, randomized, single-blind clinical trial to evaluate the immunogenicity of the live, attenuated, oral rotavirus vaccine (116E), ROTAVAC®, administered simultaneously with or without the buffering agent in healthy infants in India. Hum Vaccin Immunother. 2018;14(7):1791–9. doi:10.1080/21645515.2018.1450709.
- Bines JE, Kohl KS, Forster J, Zanardi LR, Davis RL, Hansen J, Murphy TM, Music S, Niu M, Varricchio F, et al. Acute intussusception in infants and children as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine. 2004;22(5–6):569–74. doi:10.1016/j.vaccine.2003.09.016.
- Isanaka S, Guindo O, Langendorf C, Matar Seck A, Plikaytis BD, Sayinzoga-Makombe N, McNeal MM, Meyer N, Adehossi E, Djibo A, et al. Efficacy of a low-cost, heat-stable oral rotavirus vaccine in Niger. N Engl J Med. 2017;376(12):1121–30. doi:10.1056/NEJMoa1609462.
- Rongsen-Chandola T, Strand TA, Goyal N, Flem E, Rathore SS, Arya A, Winje BA, Lazarus R, Shanmugasundaram E, Babji S, et al. Effect of withholding breastfeeding on the immune response to a live oral rotavirus vaccine in North Indian infants. Vaccine. 2014;32(Suppl1):A134–139. doi:10.1016/j.vaccine.2014.04.078.
- Chandola TR, Taneja S, Goyal N, Antony K, Bhatia K, More D, Bhandari N, Cho I, Mohan K, Prasad S, et al. ROTAVAC® does not interfere with the immune response to childhood vaccines in Indian infants: a randomized placebo controlled trial. Heliyon. 2017;3(5):e00302. doi:10.1016/j.heliyon.2017.e00302.
- Lawrence J, He S, Martin J, Schödel F, Ciarlet M, Murray AV. Safety and immunogenicity of pentavalent rotavirus vaccine in a randomized, double-blind, placebo-controlled study in healthy elderly subjects. Hum Vaccin Immunother. 2014;10(8):2247–54. doi:10.4161/hv.29107.
- Narang A, Bose A, Pandit AN, Dutta P, Kang G, Bhattacharya SK, Datta S, PV S, Delem A, Han HH, et al. Immunogenicity, reactogenicity and safety of human rotavirus vaccine (RIX4414) in Indian infants. Hum Vaccin. 2009;5(6):414–9. doi:10.4161/hv.5.6.8176.
- Armah GE, Breiman RF, Tapia MD, Dallas MJ., Neuzil KM, Binka FN, Sow SO, Ojwando J, Ciarlet M, Steele AD. Immunogenicity of the pentavalent rotavirus vaccine in African infants. Vaccine. 2012;30(Suppl 1):A86–A93. doi:10.1016/j.vaccine.2011.10.006.
- Kompithra RZ, Paul A, Manoharan D, Babji S, Sarkar R, Mathew LG, Kang G. Immunogenicity of a three dose and five dose oral human rotavirus vaccine (RIX4414) schedule in south Indian infants. Vaccine. 2014;32(Suppl 1):A129–A133. doi:10.1016/j.vaccine.2014.03.002.
- Kawade A, Babji S, Kamath V, Raut A, Kumar CM, Kundu R, Venkatramanan P, Lalwani SK, Bavdekar A, Juvekar S, et al. Immunogenicity and lot-to-lot consistency of a ready to use liquid bovine-human reassortant pentavalent rotavirus vaccine (ROTASIIL - Liquid) in Indian infants. Vaccine. 2019;37(19):2554–60. doi:10.1016/j.vaccine.2019.03.067.
- Cunliffe NA, Witte D, Ngwira BM, Todd S, Bostock NJ, Turner AM, Chimpeni P, Victor JC, Steele AD, Bouckenooghe A, et al. Efficacy of human rotavirus vaccine against severe gastroenteritis in Malawian children in the first two years of life: a randomised, double-blind, placebo controlled trial. Vaccine. 2012;30(1):A36–A43. doi:10.1016/j.vaccine.2011.09.120.
- Madhi SA, Kirsten M, Louw C, Bos P, Aspinall S, Bouckenooghe A, Neuzil KM, Steele AD. Efficacy and immunogenicity of two or three dose rotavirus-vaccine regimen in South African children over two consecutive rotavirus-seasons: a randomized, double-blind, placebo-controlled trial. Vaccine. 2012;30(Suppl 1):A44–A51. doi:10.1016/j.vaccine.2011.08.080.
- Steele AD, Reynders J, Scholtz F, Bos P, De Beer MC, Tumbo J, Van der Merwe CF, Delem A, De Vos B. Comparison of 2 different regimens for reactogenicity, safety, and immunogenicity of the live attenuated oral rotavirus vaccine RIX4414 coadministered with oral polio vaccine in South African infants. J Infect Dis. 2010;202(Suppl:):S93–S100. doi:10.1086/653550.
- Mwenda JM, Ntoto KM, Abebe A, Enweronu‐Laryea C, Amina I, Mchomvu J, Kisakye A, Mpabalwani E, Pazvakavambwa I, Armah G, et al. Burden and epidemiology of rotavirus diarrhea in selected African countries: preliminary results from the African rotavirus surveillance network. J Infect Dis. 2010;202(S1):S5–S11. doi:10.1086/653557.
- Ramachandran M, Das BK, Vij A, Kumar R, Bhambal SS, Kesari N, Rawat H, Bahl L, Thakur S, Woods PA, et al. Unusual diversity of human rotavirus G and P genotypes in India. J Clin Microbiol. 1996;34(2):436–9. doi:10.1128/jcm.34.2.436-439.1996.
- Tiku VR, Sharma S, Verma A, Kumar P, Raghavendhar S, Aneja S, Paul VK, Bhan MK, Ray P. Rotavirus diversity among diarrheal children in Delhi, India during 2007–2012. Vaccine. 2014;32(Suppl 1):A62–A67. doi:10.1016/j.vaccine.2014.03.005.
- Mishra V, Awasthi S, Nag VL, Tandon R. Genomic diversity of group a rotavirus strains in patients aged 1–36 months admitted for acute watery diarrhoea in northern India: a hospital-based study. Clin Microbiol Infect. 2010;16(1):45–50. doi:10.1111/j.1469-0691.2009.02772.x.
- Sharma S, Ray P, Gentsch JR, Glass RI, Kalra V, Bhan MK. Emergence of G12 rotavirus strains in Delhi, India, in 2000 to 2007. J Clin Microbiol. 2008;46(4):1343–8. doi:10.1128/JCM.02358-07.
- Jain V, Das BK, Bhan MK, Glass RI, Gentsch JR. Great diversity of group a rotavirus strains and high prevalence of mixed rotavirus infections in India. J Clin Microbiol. 2001;39(10):3524–9. doi:10.1128/JCM.39.10.3524-3529.2001.
- Vesikari T, Karvonen A, Bouckenooghe A, Suryakiran PV, Smolenov I, Han HH. Immunogenicity, reactogenicity and safety of the human rotavirus vaccine RIX4414 oral suspension (liquid formulation) in Finnish infants. Vaccine. 2011;29(11):2079–84. doi:10.1016/j.vaccine.2011.01.004.
- Chilengi R, Mwila- Kazimbaya K, Chirwa M, Sukwa N, Chipeta C, Velu RM, Katanekwa N, Babji S, Kang G, McNeal MM, et al. Immunogenicity and safety of two monovalent rotavirus vaccines, ROTAVAC® and ROTAVAC 5D® in Zambian infants. Vaccine. 2021;39(27):3633–40. doi:10.1016/j.vaccine.2021.04.060.
- Desselberger U. Rotaviruses. Virus Res. 2014;190:75–96. doi:10.1016/j.virusres.2014.06.016.
- Angel J, Franco MA, Greenberg HB. Rotavirus immune responses and correlates of protection. Curr Opin Virol. 2012;2(4):419–25. doi:10.1016/j.coviro.2012.05.003.
- ROTAVAC 5D®. WHO - Prequalification of Medical products (IVDs, medicines, vaccines and immunization devices, Vector Control). 2021 June 21 [accessed 2023 May 19]. https://extranet.who.int/pqweb/content/rotavac-5d.