ABSTRACT
Herpes zoster (HZ) is a debilitating vaccine-preventable disease. Impairment of cell-mediated immunity, as observed with aging and immunosuppressive disorders and therapies, increases risk. Recombinant zoster vaccine (RZV) is efficacious against HZ in adults aged ≥50 years in different settings, and in immunocompromised adults aged ≥18 years who are at increased risk of developing HZ. RZV is the first and only HZ vaccine approved for use in immunocompromised adults globally, including in Europe and the US. RZV has a clinically acceptable safety profile and elicits robust immune responses in adults aged ≥50 years, and in immunocompromised adults aged ≥18 years who are at increased risk of HZ. Additionally, RZV is efficacious against HZ complications such as post-herpetic neuralgia and HZ-related pain. This review updates knowledge from a randomized controlled trial setting on the efficacy, safety, immunogenicity, and impact on quality of life of RZV.
Plain Language Summary
What is the context?
The varicella zoster virus, which causes chickenpox in childhood, can reactivate in adults and trigger a painful rash called herpes zoster (HZ) or shingles. Almost all adults are at risk of developing HZ as they age or develop risk factors for HZ. Two key studies published in 2015 and 2016 (ZOE-50 and ZOE-70) compared the recombinant zoster vaccine (RZV) with placebo and showed that RZV could effectively prevent HZ in adults aged ≥50 years and ≥70 years, respectively. Several clinical studies were carried out in subsequent years, assessing how effective and safe RZV is compared with a placebo/control in different populations. Based on these studies, RZV was approved for use in adults aged ≥50 years and those aged ≥18 years at increased risk of HZ (European Union) due to immunodeficiency or immunosuppression caused by known disease or therapy (United States).
What is new?
We reviewed clinical studies of RZV published between 1 January, 2015 and 31 October, 2022. The evidence shows that RZV is effective and does not cause safety concerns across the studied populations, including adults aged ≥50 years and immunocompromised adults aged ≥18 years who are at increased risk of HZ.
What is the impact?
The growing amount of knowledge on the efficacy, safety, immunogenicity, and impact on quality of life of RZV should assist in deciding to vaccinate and in ensuring that the individuals who could benefit the most from RZV have access to vaccination.
Introduction
Primary infection with varicella zoster virus (VZV), or chickenpox, is a self-limiting disease that manifests as a diffuse vesicular rash.Citation1 During primary infection, VZV may enter sensory ganglia and become latent.Citation1 It has been hypothesized that latency is maintained by cell-mediated immunity (CMI), with CMI impairment resulting in VZV reactivation and eruption of herpes zoster (HZ), or shingles. HZ typically presents as a unilateral vesicular rash with a dermatomal distribution, pain (post-herpetic neuralgia [PHN]), neurological complications, and, occasionally, vasculopathy.Citation1–3 More than 95% of adults are seropositive for VZV and are at risk of developing HZ.Citation4
Major risk factors for impairment of CMI and reactivation of latent VZV include age-related decline in immunity and immune suppression due to immune diseases and/or immunosuppressive treatments.Citation2 Age is the most important risk factor for HZ, with a marked increase in age-specific incidence after the age of 50 years.Citation5 Additionally, immunocompromised populations are at increased risk of HZ, including hematopoietic stem cell transplant (HSCT) recipients, solid organ transplant recipients, and patients with hematological malignancies, solid tumors, or human immunodeficiency virus (HIV). These individuals are also at increased risk of HZ complications, such as PHN, disseminated HZ, HZ ophthalmicus, and HZ requiring hospitalization;Citation6 notably, the risk among some immunocompromised younger adults exceeds that of immunocompetent adults aged ≥50 years.Citation6–8 Chronic conditions or comorbidities such as asthma, chronic heart disease, chronic obstructive pulmonary disorder, depression, and rheumatoid arthritis also increase the risk of developing HZ.Citation9
Antiviral therapy is recommended for patients with HZ, particularly those aged ≥50 years, immunocompromised, or developing complications such as severe rash or lesions involving the face or eye.Citation10 Effective vaccines are available globally for the prevention of HZ.Citation11 These include the live-attenuated HZ vaccine (ZVL), and the recombinant zoster vaccine (RZV), a recombinant VZV glycoprotein E (gE), with a liposome-based AS01B adjuvant system.Citation11–15 ZVL is indicated in most regions for the immunization of individuals ≥50 years of age; however, immunosuppression and immunodeficiency are contraindications for ZVL and, as of November 2020, the vaccine is no longer available in the US.Citation12,Citation15
RZV received its first marketing authorization for HZ prevention in adults aged ≥50 years in Canada in 2017, and was then approved in the US later in 2017.Citation16 In the US, the FDA approval became an official Centers for Disease Control and Prevention (CDC) policy in 2018. The RZV indication was expanded in 2021 to adults aged ≥18 years who are or will be at increased risk of HZ due to immunodeficiency or immunosuppression caused by known disease or therapy.Citation14 In the same year, the Advisory Committee on Immunization Practices (ACIP) updated its guidelines, recommending two doses of RZV for the prevention of HZ and related complications in adults with immunodeficiency or immunosuppression aged ≥19 years.Citation12
RZV was licensed in the European Union in 2018, where it was initially indicated for the prevention of HZ and PHN in adults aged ≥50 years; the indication was subsequently expanded (August 2020) to include adults aged ≥18 years at increased risk of HZ.Citation16,Citation17 RZV is now approved in many countries worldwide, and is the first and only HZ vaccine approved for use in immunocompromised adults, which fulfilled an unmet need for vaccination against HZ in these patients.Citation12
The efficacy and safety of RZV in older adults were originally evaluated in two pivotal randomized, placebo-controlled, phase III trials in adults aged ≥50 years (ZOE-50) and ≥70 years (ZOE-70).Citation18,Citation19 Collectively, these trials demonstrated >90% efficacy in preventing HZ in immunocompetent adults aged ≥50 years. Since the publication of these trials, the wealth of data regarding RZV has expanded greatly to include long-term follow-up data, health-related patient-reported quality of life (QoL) outcomes, clinical data in other at-risk patient populations, including immunocompromised and frail individuals, and information about co-administration with other vaccines. The aim of this literature review was to summarize published clinical data on the efficacy, safety, immunogenicity, and impact on health-related QoL of RZV in immunocompetent and immunocompromised adults. In parallel, we conducted a separate literature review summarizing published real-world evidence (RWE) studies of RZV (available at: doi 10.1080/21645515.2023.2263979).
Methods
A non-systematic literature review was performed in September 2022, using PubMed to find relevant articles published between 1 January, 2015 and 31 October, 2022. The search string used was: “vaccine” AND “zoster” AND (“efficacy” OR “immunogenicity”) AND (“adjuvant” OR “recombinant”) AND (“study” OR “trial”). Human clinical studies on the efficacy, safety, immunogenicity, and impact on health-related QoL of RZV in adults were included. Reviews, real-world evidence studies, public health impact modeling studies, cost-effectiveness studies, and preclinical studies were excluded. This article is based on previously conducted studies and does not contain any new statistical analyses.
Results
In total, 152 articles were identified in PubMed. Following the exclusion of 101 articles deemed not relevant or not matching the review objectives, 51 clinical studies were included in this review. The studies evaluated the efficacy, safety, immunogenicity, and impact on health-related QoL of RZV in a range of adult patient populations and with different administration regimens.
Efficacy
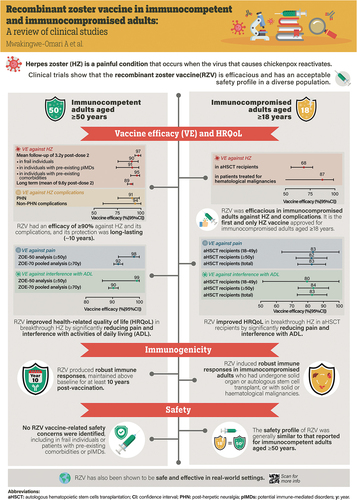
The efficacy of RZV in preventing HZ, PHN, and non-PHN complications has been shown in adults aged ≥50 years and in a range of at-risk patient populations aged ≥18 years ().
Figure 1. Efficacy of RZV in adults aged ≥50 years and in immunocompromised patients aged ≥18 years.
Adults aged ≥50 years
The efficacy of RZV in preventing HZ was first evaluated in ZOE-50 and ZOE-70, two pivotal randomized, placebo-controlled, triple-blinded, phase III trials conducted across 18 countries.Citation18,Citation19 Participants received two doses of either RZV or placebo (0.9% saline solution), administered by intramuscular (IM) injection into the deltoid muscle, 2 months apart. Participants were followed for at least 30 months after administration of the second dose. The primary objective of the two studies was to evaluate overall vaccine efficacy in reducing the risk of HZ in the modified total vaccinated cohort (mTVC), which included all participants who received both doses of study vaccine and did not receive a diagnosis of HZ within 1 month of receiving the second dose. Suspected cases were defined as those with a new unilateral rash with pain that had no alternative diagnosis. The diagnosis of HZ was confirmed by real-time polymerase-chain reaction assay targeting VZV ORF62 or by an adjudication committee following a specific algorithm.Citation18 Participants were followed for at least 90 days after the onset of the episode or until the rash resolved and the participant had been pain-free for 4 weeks.
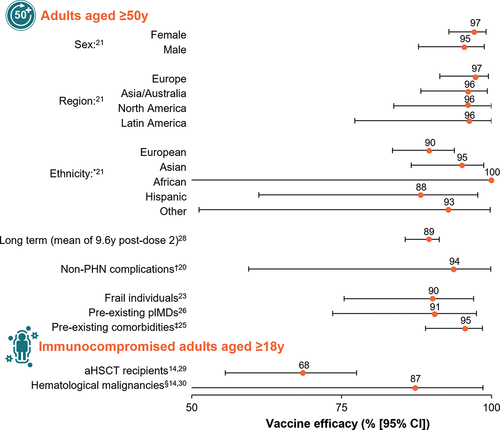
In ZOE-50, 15,411 participants aged ≥50 years (mean age 62.3 years) were randomized to receive either RZV (n = 7698) or placebo (n = 7713).Citation18 During a mean follow-up of 3.2 years, HZ was confirmed in six RZV recipients and 210 placebo recipients (HZ incidence rate 0.3 vs. 9.1 per 1000 person-years); thus, overall vaccine efficacy against HZ was 97.2% (95% confidence interval [CI]: 93.7–99.0; p < .001). In ZOE-70, 13,900 participants aged ≥70 years (mean age 75.6 years) were randomized to receive either RZV (n = 6950) or placebo (n = 6950).Citation19 During a mean follow-up period of 3.7 years, HZ was confirmed in 23 RZV recipients and 223 placebo recipients (HZ incidence rate 0.9 vs. 9.2 per 1000 person-years); thus, vaccine efficacy against HZ was 89.8% (95% CI: 84.2–93.7; p < .001). In the pooled analysis of participants aged ≥70 years from the ZOE trials (subsequently referred to as the ZOE-70 pooled analysis; 16,596 participants in the mTVC), the overall efficacy of RZV (i.e., the primary objective of the pooled analysis) was 91.3% (95% CI: 86.8–94.5) against HZ and 88.8% (95% CI: 68.7–97.1) against PHN.Citation19 Furthermore, a pooled analysis of the ZOE trials showed that RZV was efficacious at preventing HZ-related complications other than PHN, including HZ vasculitis, disseminated HZ, ophthalmic, neurologic, or visceral disease, and stroke.Citation20
The large sizes of the ZOE trials allowed for several post hoc analyses, including a study evaluating the efficacy of RZV against HZ by sex, geographic region, and ethnicity.Citation21 The study reported comparable efficacy of RZV against HZ in males vs. females (>90% over the entire age range).Citation21 Across all geographic regions, efficacy against HZ ranged from 95.7% (95% CI: 83.7–100.0) to 97.2% (95% CI: 91.4–99.5) in adults aged ≥50 years and from 87.3% (95% CI: 58.1–97.6) to 95.1% (95% CI: 87.0–98.7) in adults aged ≥70 years.Citation21 In adults aged ≥50 years, efficacy against HZ was consistently over 95% in participating countries from Europe, Asia/Australia, North America, and Latin America.Citation21 In addition, in adults aged ≥70 years, efficacy against HZ was 90.1% in Europe (95% CI: 82.0–95.1), 95.1% in Asia/Australia (95% CI: 87.0–98.7), 90.1% in North America (95% CI: 77.0–96.5), and 87.3% (95% CI: 58.1–97.6) in Latin America.Citation21
The efficacy of RZV was also evaluated in specific subsets of participants in the ZOE trials. The impact of frailty on RZV clinical outcomes was assessed in a pooled analysis, for which a frailty index was developed and validated.Citation22–24 The proportion of frail individuals ranged from 5.7% in participants aged 50–59 years to 22.7% in those aged ≥80 years.Citation23 Vaccine efficacy against HZ was consistently >90% across all frailty subgroups in the mTVC and was 90.2% (95% CI: 75.4–97.0), 90.4% (95% CI: 84.4–94.4), and 95.8% (95% CI: 91.6–98.2) in frail, pre-frail, and non-frail individuals, respectively.Citation23 Additionally, vaccine efficacy against HZ was evaluated in individuals with comorbidities in a post hoc, pooled analysis of the ZOE trials.Citation25 A total of 82.3% of RZV recipients and 82.7% of placebo recipients in the mTVC had at least one of 15 selected comorbidities at enrollment. Vaccine efficacy against HZ was 95.4% (95% CI: 89.0–98.5) in RZV recipients with one comorbidity and >90% in participants with two to six comorbidities.Citation25 In the ZOE trials, 983 of 14,645 RZV recipients (6.7%) had at least one pre-existing potential immune-mediated disease (pIMD); i.e., autoimmune diseases and other inflammatory and/or neurologic disorders of interest that may or may not have an autoimmune cause. In these individuals, the efficacy of RZV against HZ was 90.5% (95% CI: 73.5–97.5) overall.Citation26
Persistence of efficacy in adults aged ≥50 years
The persistence of efficacy was evaluated in a long-term follow-up study of participants in the ZOE trials who received at least one dose of RZV and were followed for up to 10 years post vaccination (the ongoing ZOE-LTFU study).Citation27,Citation28 An interim analysis of this study showed that the efficacy of RZV in preventing HZ was 81.6% (95% CI: 75.2–86.6) during the ≥4-year period from a mean 5.6 to 9.6 years after the primary vaccination.Citation28 Vaccine efficacy against HZ, evaluated from 1 month post dose 2 to a mean of approximately 9.6 years after the primary vaccination, was reported as 89.0% (95% CI: 85.6–91.3).Citation28
Immunocompromised adults aged ≥18 years
The efficacy of RZV has been evaluated for two immunocompromised patient populations: autologous HSCT (aHSCT) recipients (ZOE-HSCT)Citation29 and patients receiving immunosuppressive cancer treatment for hematological malignancies (ZOSTER-039).Citation30
In the ZOE-HSCT study, 1846 participants received a two-dose schedule of either RZV (n = 922) or placebo (n = 924), with the first dose administered 50 to 70 days post transplantation and the second dose given 1 to 2 months later.Citation29 After a median of 21 months of follow-up post vaccine administration, vaccine efficacy against HZ was estimated to be 68.2% (95% CI: 55.6–77.5). Furthermore, significant reductions in the incidence of PHN and prespecified HZ-related complications were observed in recipients of RZV.Citation29
In the ZOSTER-039 study of patients receiving immunosuppressive cancer treatment for hematological malignancies, participants received two doses of either RZV (n = 283) or placebo (n = 279), 1 to 2 months apart, either during or after receiving immunosuppressive cancer treatment.Citation30 In a post hoc analysis, vaccine efficacy against HZ was estimated to be 87.2% (95% CI: 44.3–98.6) during a median follow-up of 11.1 months from 30 days after dose 2.Citation30
Immunogenicity
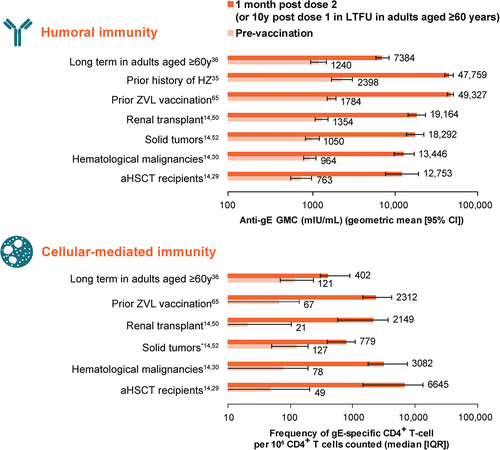
Immunogenicity of RZV 1 month after administration of the second dose of vaccine or placebo has been evaluated in a wide range of participants enrolled in clinical trials (). In these studies, humoral immunogenicity was quantified by measuring anti-gE antibody titers and CMI was quantified by evaluating the frequency of gE-specific CD4+ T cells. RZV elicited significantly higher anti-gE titers and CD4+ T cell counts compared with baseline in a range of patient populations.
Figure 2. Immunogenicity of RZV in adults aged ≥50 years and in immunocompromised patients aged ≥18 years.
Adults aged ≥50 years
Administration of RZV by IM or subcutaneous injection at 0 and 2 months produced similar gE-specific humoral immune responses 1 month after the second dose in 60 Japanese adults aged ≥50 years; however, the subcutaneous route was more reactogenic than the IM route.Citation31
In the ZOE trials, humoral immunogenicity was assessed in a random subset of 3293 ZOE-50 and ZOE-70 participants from all 18 study countries.Citation32 For gE-specific CMI responses, 466 ZOE-50 participants were randomly selected. Two doses of RZV induced robust humoral and CMI responses in all age groups (particularly in people aged ≥70 years), which remained substantially above baseline 3 years after vaccination.Citation32 In a further phase III study in adults aged ≥50 years (n = 346), administration of the two-dose regimen of RZV at 0 and 6 months was noninferior to administration at 0 and 2 months on the basis of gE-specific humoral immune responses.Citation33 In contrast, administration on a 0- and 12-month schedule did not meet the noninferiority criterion.Citation33
Following the initial ZOE trials, immunogenicity was also evaluated in adults who had previously received ZVL and those with a prior history of HZ. Specifically, in a study involving adults aged ≥65 years (n = 215) who had received ZVL ≥5 years earlier, and group-matched, ZVL-naïve adults (n = 215), RZV induced strong humoral and CMI responses that remained above pre-vaccination levels 1 year after receipt of the second dose.Citation34 Additionally, high anti-gE antibody concentrations were observed 1 month after receipt of the second dose of RZV in participants aged ≥50 years with a prior history of HZ, in a phase III, open-label, non-randomized, multicenter study.Citation35
A long-term follow-up study in adults aged ≥60 years evaluated humoral and CMI responses 10 years after administration of the first dose of RZV or placebo in a phase II dose-ranging trial.Citation36 Among recipients of RZV, humoral and CMI responses remained approximately 6-fold and 3.5-fold higher, respectively, compared with levels observed prior to vaccination.Citation36 Persistence of antibody titers and CD4+ T cell frequencies above pre-vaccination levels for ≥20 years after initial vaccination was predicted by three different models.Citation36 Of the 70 adults enrolled in the study, 62 individuals received additional dosing 10 years after the initial two-dose course (mean age 82.6 years at first additional dose administration), which induced strong anamnestic humoral and CMI responses.Citation36
Comparative immunogenicity versus ZVL vaccine
Two doses of RZV generated significantly greater humoral and CMI responses compared with ZVL (one dose followed by a placebo dose) after 1 year in a randomized, double-blind, placebo-controlled trial in adults aged 50–85 years.Citation37 The immune responses generated by each vaccine were distinguishable: RZV led to higher gE- and VZV-specific memory Type 1 T helper responses (including memory and effector-memory CD4+ peak responses, VZV-specific interleukin-2 [IL-2]+, and gE-specific IL-2) compared with ZVL, whereas ZVL generated higher effector CD4+ responses.Citation37
Long-term follow-up of participants enrolled in this study showed that recipients of RZV, but not ZVL, maintained post-vaccination immune responses for 5 years.Citation38 In another comparative study that evaluated humoral immune responses in middle-aged and older adults, RZV boosted both gE avidity and VZV neutralizing antibodies significantly more than ZVL.Citation39 One year post vaccination, peak avidity was retained in 81% of RZV recipients compared with 18% of ZVL recipients. In adults aged 50–85 years, peak memory T cell responses post vaccination (day 30 in recipients of ZVL and after day 30 post dose 2 of RZV) were 10-fold greater in recipients of RZV than in recipients of ZVL.Citation40
Co-administration of RZV with other vaccines or medications
The impact of co-administration of two doses of RZV with seasonal influenza vaccine,Citation41 13-valent pneumococcal conjugate vaccine (PCV13),Citation42 23-valent pneumococcal polysaccharide vaccine (PPSV23),Citation43 or the reduced-antigen content diphtheria-tetanus-acellular pertussis vaccine (Tdap)Citation44 has been evaluated in a series of randomized controlled trials in adults aged ≥50 years. Co-administration did not interfere with immune responses (anti-gE antibody concentrations) to RZV or to any of the co-administered vaccines, with the exception of a noninferiority criterion not met for one Tdap antigen (pertactin); the clinical significance of this is unclear.Citation44 Across these four studies, vaccine response rates (anti-gE antibody) to RZV ranged from 95.8% to 99.1% in the co-administration groups, and from 97.9% to 99.1% in the sequential administration groups.Citation41–44
Immunocompromised adults aged ≥18 years
In addition to adults aged ≥50 years, the immunogenicity of RZV has been shown in a range of immunocompromised patient populations aged ≥18 years, including HIV-positive adults (participants received three doses of RZV, with increases in gE-specific humoral and CMI responses seen after doses 2 and 3),Citation45 solid organ transplant recipients,Citation46–49 patients with hematological malignancies,Citation30 patients with solid tumors,Citation50 patients with clonal B cell disorders,Citation51 and autologous and allogeneic HSCT recipients.Citation29,Citation52 For example, in a study of renal transplant recipients aged ≥45 years, vaccination with RZV significantly augmented cellular VZV gE-specific immunity.Citation47 Several factors correlated with significantly higher VZV-specific CMI, including male sex, better kidney function, as indicated by a higher glomerular filtration rate, shorter interval between transplantation and vaccination, and treatment with tacrolimus or mycophenolate, whereas diabetes mellitus was associated with impaired responses.Citation47 RZV was also reported to elicit significant humoral and CMI responses in VZV-seropositive lung transplant recipients aged ≥50 yearsCitation48 and VZV-seronegative solid organ (kidney, liver, heart, lung, pancreas, and/or intestine) transplant recipients aged ≥18 years.Citation49
In a study of patients with clonal B cell disorders (monoclonal B cell lymphocytosis or chronic lymphocytic leukemia [CLL]), an antibody response (≥4 times anti-gE antibody concentration post vaccination vs. pre-vaccination) was observed in 45% of patients after two doses of RZV.Citation51 In addition, the T cell response to RZV vaccination was significantly lower in study participants compared with historical controls, primarily because of lower responses in patients receiving Bruton tyrosine kinase inhibitors (BTKis). The results of a pilot study suggested that RZV is less immunogenic (as measured by post vaccination VZV IgG levels) in recipients of allogeneic HSCT than in recipients of aHSCT.Citation53 In a study of allogeneic HSCT recipients, vaccination with two doses of RZV augmented CMI, and patients with a prior history of HZ had approximately 2-fold higher VZV-specific T cell responses prior to and post vaccination compared with patients without prior HZ.Citation52 In line with the study of renal transplant recipients,Citation47 male sex was identified as an independent factor leading to increased VZV-specific immunity in multivariate analyses.Citation52 None of the remaining studies statistically evaluated the effect of sex on immune responses.
Seroconversion after receipt of two doses of RZV was not impaired in patients with CLL who had been receiving a BTKi (ibrutinib, n = 9; or acalabrutinib, n = 8) for at least 6 months in a non-randomized phase II study.Citation54 Seroconversion (defined as ≥4-fold greater anti-gE titers 6 months after the last vaccine administration compared with pre-vaccination) was detected in 41.5% of patients receiving a BTKi and in 59.1% of treatment-naïve patients with CLL. Notwithstanding these findings, the authors of this study subsequently reported that BTKis did inhibit humoral and CMI responses to RZV in this trial.Citation55
Safety and reactogenicity
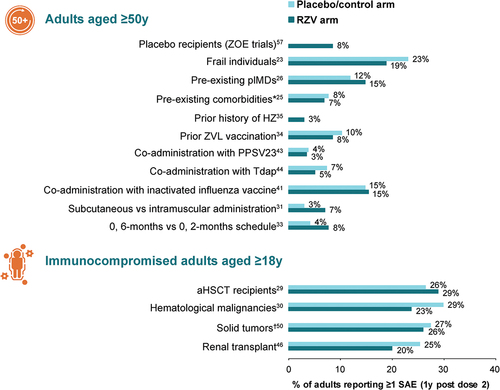
Several studies reported a clinically acceptable safety profile of RZV in adults aged ≥50 years in different settings and in a range of at-risk patient populations aged ≥18 years. There were methodological differences in collection and reporting of safety and reactogenicity data across studies. presents data on the incidence of all serious adverse events (SAEs; treatment related and unrelated) across the reviewed studies.Citation23,Citation25,Citation26,Citation29–31,Citation33–35,Citation41,Citation43,Citation44,Citation46,Citation50,Citation56,Citation57
Figure 3. Incidence of all serious adverse events (treatment related and unrelated) reported in diverse groups of adults aged ≥50 years, and in immunocompromised adults aged ≥18 years.
Adults aged ≥50 years
In the ZOE-50 and ZOE-70 trials, solicited adverse events (AEs) were collected for 7 days after each dose of RZV or placebo in a subcohort of study participants. A pooled analysis of the ZOE trials showed that injection site pain was the most frequent solicited local symptom, reported after 68.1% of RZV doses and 6.9% of placebo doses.Citation58 Grade 3 pain was reported after 3.8% and 0.2% of doses, respectively. The most frequently reported solicited general symptoms were myalgia and fatigue, reported after 32.9% and 32.2% of RZV doses, respectively, compared with ≤10% of placebo doses. Grade 3 myalgia and fatigue were reported after 3.0% of RZV doses and 0.5% of placebo doses.Citation58 The incidence of pIMD (new onset and possible exacerbations) was similar in recipients of RZV and placebo (0.2% up to 30 days post last dose in each group, and 0.6% and 0.7% up to 1 year post last dose in the RZV and placebo groups, respectively).Citation58 The incidence of all SAEs (treatment related and unrelated) was well balanced between RZV and placebo/control groups for all time periods analyzed. Within 30 days of receiving the last dose, 2.3% of RZV recipients and 2.2% of placebo recipients reported at least one SAE; within 1 year of receiving the last dose, these proportions were 10.1% and 10.4%, respectively.Citation58
A post hoc analysis of the total vaccinated cohort (TVC) reactogenicity subset evaluated the frequency of solicited AEs post dose 2 graded by severity (none, mild, moderate, or severe) in participants reporting the same event’s intensity post dose 1.Citation59 Solicited injection site AEs (collected for 7 days after each vaccine dose) were reported after both RZV doses by 94.1% of participants in the TVC reactogenicity subcohort. The most frequently reported solicited AEs in this group were injection site pain (reported in 70.3% and 65.7% of recipients after dose 1 and dose 2, respectively), redness (approximately 29% after each dose), and swelling (approximately 19% after each dose).Citation59 Solicited general AEs were recorded after both RZV doses by 93.9% of participants in the TVC reactogenicity cohort. The most frequently reported solicited AEs in this category were myalgia (in 32.0% and 33.8% of recipients after dose 1 and dose 2, respectively) and fatigue (in 30.9% and 33.6% of recipients after dose 1 and dose 2, respectively).Citation59 Another post hoc analysis did not identify a significant correlation between the intensity of pain reported by RZV recipients in the ZOE trials and the antigen-specific immune responses, with the authors concluding that reactogenicity cannot be used as a surrogate marker for efficacy after vaccination.Citation56
In an interim analysis of the long-term follow-up study of the ZOE participants (5.1–7.1 years [mean] post vaccination), two participants with confirmed HZ subsequently reported HZ-related complications (disseminated HZ, which resolved in 40–55 days; however, one of these cases was complicated by PHN that was ongoing at data lock point, although slowly resolving).Citation27 In the second interim analysis (up to 10 years post vaccination), three additional participants reported PHN or disseminated HZ ≥9 years post vaccination.Citation28 In both analyses, no SAEs were considered causally related to vaccination.Citation27,Citation28
No vaccine-related safety concerns were identified in subsets of the ZOE trials, including frail individualsCitation23 and patients with preexisting comorbiditiesCitation25 or pIMDs.Citation26 Similarly, no safety concerns were reported in other studies of adults aged ≥50 years with a prior history of HZCitation35 or adults aged ≥65 years previously vaccinated with ZVL.Citation34
Immunocompromised adults aged ≥18 years
Several studies have shown that the AE profile of RZV in immunocompromised adults is generally similar to that reported in adults aged ≥50 years. Like the ZOE-50 and ZOE-70 trials, solicited AEs were collected for 7 days after vaccination; the incidence of solicited AEs (local and general) was consistently higher in adults who received RZV than in those who received placebo, and injection site pain was the most common solicited AE.Citation29,Citation30,Citation46,Citation50 Solicited local and general AEs were transient, mild or moderate in intensity, and had a median duration of ≤4 days.Citation46,Citation50 In the ZOE-HSCT study of aHSCT recipients, the frequency of solicited local AEs (injection site pain, redness, and swelling) and solicited general AEs (fatigue, fever, gastrointestinal symptoms, headache, myalgia, and shivering) in the TVC (i.e., participants who received at least one dose of study vaccine) was collectively higher in RZV versus placebo recipients (90% vs. 53%).Citation29 Local AEs were the most common solicited AEs, occurring in 86% of RZV recipients and 10% of placebo recipients. Grade 3 local AEs were reported by 14.2% and 0.2% of RZV and placebo recipients, respectively; grade 3 solicited general AEs were reported by 13.2% and 6.0% of RZV and placebo recipients, respectively. Solicited local symptoms and general symptoms were transient, with a median duration of ≤3 days (grade 3 ≤2 days).Citation29
In the ZOSTER-028 study in patients with solid tumors who received RZV before or during chemotherapy, the incidence of solicited local AEs was 83.9% (RZV) versus 6.4% (placebo); and the incidence of solicited general AEs was 81.3% (RZV) versus 66.4% (placebo).Citation50 In the ZOSTER-039 study in adults with hematological malignancies, the incidence of solicited local AEs was 83.8% (RZV) versus 17.5% (placebo) and the incidence of solicited general AEs was 74.1% (RZV) versus 48.9% (placebo).Citation30 In the ZOSTER-041 trial in immunosuppressed adults after renal transplantation, solicited local AEs occurred in 87.8% of RZV recipients and 9.1% of placebo recipients; the corresponding percentages of solicited general AEs were 68.7% (RZV) and 55.3% (placebo).Citation46 Pain was the most frequently reported solicited local AE (RZV 87.0% vs. placebo 8.3%) and myalgia was the most common solicited general AE (RZV 49.6% vs. placebo 23.5%).Citation46
The incidence of SAEs in a range of immunocompromised adult populations is presented in .Citation29,Citation30,Citation46,Citation50
Impact on patient-reported health-related QoL outcomes
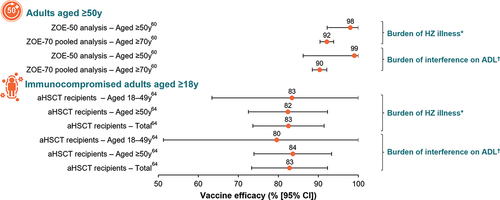
Evidence is emerging of the positive impact of RZV on patient-reported health-related QoL outcomes in both adults aged ≥50 years and at-risk patients aged ≥18 years ().
Figure 4. Impact of RZV on health-related quality of life in adults aged ≥50 years and in immunocompromised patients aged ≥18 years.
Adults aged ≥50 years
The impact of RZV on patient-reported health-related QoL outcomes was evaluated among participants enrolled in the ZOE-50 and ZOE-70 studies.Citation60 In the ZOE trials, participants with suspected HZ attended assessment visits, and completed the Zoster Brief Pain Inventory (ZBPI) daily for 28 days after the onset of rash then weekly until either they had been pain-free for 4 consecutive weeks or 90 days had elapsed since the onset of rash (whichever came last). The ZBPI asked patients to rate pain on a 0–10 scale, with 10 signifying the worst imaginable pain; the “worst pain” over the last 24 hours was considered as the most reliable indicator for estimating vaccine efficacy in reducing pain-related burden of illness.Citation60,Citation61 Additionally, the ZBPI questionnaire assessed the degree to which HZ pain interferes with activities of daily living (ADLs; i.e., general activity, mood, walking ability, work, relation with others, sleep, and enjoyment of life).Citation60,Citation61 In individuals who developed confirmed HZ, RZV significantly reduced maximal ZBPI worst pain score in the ZOE-70 pooled analysis and significantly reduced maximal ZBPI average pain scores in an analysis of the ZOE-50 data and in the ZOE-70 pooled analysis. In both the ZOE-50 analysis and the ZOE-70 pooled analysis, RZV was associated with >90.0% reductions in the burden of illness and the burden of interference related to HZ.Citation60
In addition to the inclusion of patient-reported outcomes in randomized controlled trials, the impact of reactogenicity on health-related QoL was evaluated after one and two doses of RZV in an open-label phase IIIb trial in adults aged ≥50 years.Citation62,Citation63 No clinically meaningful reductions in mean Short Form Survey-36 (SF-36) physical functioning scale scores were observed between pre-dose and post RZV dose 1 and dose 2 evaluations. A transient decrease in SF-36 physical functioning score was associated with grade 3 reactogenicity after both dose 1 and dose 2.Citation62,Citation63
Immunocompromised adults aged ≥18 years
In addition to having a positive impact on patient-reported health-related QoL in adults aged ≥50 years, RZV was associated with >80.0% reductions in the burden of illness and the burden of interference related to HZ in aHSCT recipients aged ≥18 years with confirmed HZ.Citation64 The vaccine also reduced the impact of HZ on QoL in breakthrough cases in both adults aged ≥50 years and immunocompromised patients.Citation60,Citation64 In particular, in aHSCT recipients at 1 week post HZ rash onset, statistically significant differences in favor of RZV were observed for the SF-36 domains for bodily pain, social functioning, emotional role, mental health, and mental component scores.Citation64
Discussion
This non-systematic literature review demonstrated that there is a growing wealth of data on the efficacy, immunogenicity, safety, and impact on health-related QoL of RZV in adults aged ≥50 years and in a range of at-risk immunocompromised populations aged ≥18 years. Across all the studies in various populations, RZV was efficacious (in preventing HZ, PHN, and non-PHN complications), immunogenic, and well tolerated. RZV demonstrated persistent efficacy and immunogenicity for up to 10 years after the primary vaccination in long-term follow-up studies,Citation27,Citation28,Citation36 and it retained its efficacy when co-administered with other vaccines (i.e., seasonal influenza vaccine, PCV13, PPSV23, Tdap) in several randomized controlled trials in adults aged ≥50 years.Citation41–44 The clinical profile of RZV is similar, regardless of covariates such as age, race, and sex,Citation21 and currently, RZV is the only zoster vaccine approved for use in immunocompromised adults worldwide.Citation12
None of the reviewed studies reported safety concerns related to RZV administration. In the ZOE trials, the most frequently reported solicited local AE was injection site pain, which was typically mild or moderate in intensity and of short duration; the most frequently reported solicited general AEs were myalgia, fatigue, and headache.Citation58 All SAEs (treatment related and unrelated) were well balanced between RZV and placebo/control groups in the patient populations assessed. No SAEs were considered causally related to RZV in the long-term follow-up study up to 10 years post vaccination.Citation28 No safety concerns were identified in subsets of the ZOE studies, including frail individuals,Citation23 those with preexisting comorbidities,Citation25 and those with preexisting pIMDs.Citation26 Consistently, the safety profile of RZV was comparable in individuals with a prior history of HZCitation35 and those previously vaccinated with ZVL.Citation34 The clinically acceptable safety profile of RZV has also been shown for a range of immunocompromised patient populations aged ≥18 years, including HIV-positive patients,Citation45 solid organ transplant recipients,Citation46–49 patients with hematological malignancies,Citation30 patients with solid tumors,Citation50 patients with clonal B cell disorders,Citation51 and autologous and allogeneic HSCT recipients.Citation29,Citation52 Studies evaluating alternate administration regimens reported no safety concerns with multiple dosing intervalsCitation33 or following co-administration with the seasonal influenza vaccine,Citation41 PCV13,Citation42 PPSV23,Citation43 or Tdap,Citation44 all of which are generally recommended vaccines in the populations where RZV is indicated.
The immunogenicity of RZV has been widely shown in adults aged ≥50 years in different settings: i.e., in participants in the original ZOE trials,Citation32 including the subset of patients who had previously received ZVL,Citation34,Citation65 and patients with a prior history of HZ.Citation35 Importantly, immune responses persist in the long term in adults aged ≥60 years;Citation36 such persistence has been reported for up to 10 years in clinical trials and 20 years in modeling studies,Citation36 and these immunogenicity findings appear to be consistent with the high vaccine efficacy reported for RZV for up to 10 years in long-term follow-up studies.Citation27,Citation28
RZV also elicits strong humoral and CMI responses in a range of at-risk patient populations aged ≥18 years, including HIV-positive adults (participants received three doses of RZV, with increases seen after dose 2),Citation45 solid organ transplant recipients,Citation46–49 patients with hematological malignancies,Citation30 patients with solid tumors,Citation50 patients with clonal B cell disorders,Citation51 and autologous and allogeneic HSCT recipients.Citation29,Citation52 Long-term follow-up studies are warranted in at-risk patient populations.
Studies evaluating alternative administration regimens showed that immune responses were noninferior with a 0, 6-month dosing interval instead of a 0, 2-month dosing interval.Citation33 Furthermore, there was no evidence that co-administration resulted in clinically meaningful interference with the immune responses to RZV or co-administered vaccines.Citation41–44 The use of different schedules and co-administered vaccines has the potential to improve overall vaccine coverage.
Compared with ZVL, RZV generates greater humoral and CMI responses in adults aged ≥50 years.Citation37,Citation39,Citation40 Moreover, recipients of RZV but not ZVL maintained higher immune responses at 2, 4, and 5 years post vaccination compared with pre-vaccination levels.Citation38 The higher IL-2 and other memory responses generated by RZV compared with ZVL have been suggested to contribute to the superior efficacy of RZV.Citation37
In addition to maintaining QoL by preventing HZ episodes and HZ-related complications,Citation19,Citation20 RZV also significantly reduces the impact on HZ-related pain in adults aged ≥50 years and aHSCT recipients aged ≥18 years with confirmed HZ. In aHSCT recipients, RZV significantly reduced the severity of HZ disease, thus attenuating the subsequent impact on health-related QoL outcomes (e.g., bodily pain, social functioning, emotional role, and mental health).Citation60–64
This literature review was limited by its non-systematic nature, which made it inherently less comprehensive than a systematic review. We addressed this limitation by choosing search terms that enabled broad evaluation of the topic, as well as a year range that allowed inclusion of any studies published after the pivotal ZOE trials. While non-systematic reviews are limited by the inability to answer hypothesis-driven research questions by design, this study did not draw any novel conclusions; readers are invited to refer to the original publications for further details regarding the methodologies, results, and limitations of the studies.
In conclusion, although this literature review was limited by a non-systematic approach and exclusive use of the PubMed database, it demonstrates the growing evidence that RZV is immunogenic, effective for the prevention of HZ and HZ-related complications, and has an acceptable safety profile in adults aged ≥50 years in different settings, and in a range of at-risk immunocompromised patient populations aged ≥18 years. Vaccination of immunocompromised adults aged ≥18 years may enable providers to vaccinate adults at a time most appropriate to the immunocompromising condition or therapy.Citation12 Globally, the accumulating evidence should support public health agencies and healthcare providers in evaluating benefits versus risk of RZV vaccination in each individual, in an effort to prevent HZ disease and HZ-related burden of illness through vaccination.
Author contributions
All authors were involved in the planning, discussion, and interpretation of the data. All reviewed and revised the manuscript, and approved the final manuscript as submitted.
Acknowledgments
Medical writing support was provided by Blair Jarvis, a contract writer working on behalf of Apollo, and Silvia Pregnolato of Apollo, OPEN Health Communications, and was funded by GSK, in accordance with Good Publication Practice 3 (GPP) guidelines (www.ismpp.org/gpp-2022).
Disclosure statement
All authors are employees of GSK and hold or may hold stock options. NL holds a patent on the herpes zoster vaccine. AS01 is a trademark owned by or licensed to the GSK group of companies.
Additional information
Funding
References
- Cohen JI, Solomon CG. Herpes zoster. N Engl J Med. 2013;369(3):255–13. doi:10.1056/NEJMcp1302674.
- Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;8(8):731–40. doi:10.1016/S1474-4422(09)70134-6.
- Bahouth MN, Venkatesan A. Acute viral illnesses and ischemic stroke: pathophysiological considerations in the era of the COVID-19 pandemic. Stroke. 2021;52(5):1885–94. doi:10.1161/STROKEAHA.120.030630.
- Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines. 2010;9(3 Suppl):21–6. doi:10.1586/erv.10.30.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833. doi:10.1136/bmjopen-2014-004833.
- McKay SL, Guo A, Pergam SA, Dooling K. Herpes zoster risk in immunocompromised adults in the United States: a systematic review. Clin Infect Dis. 2020;71(7):e125–34. doi:10.1093/cid/ciz1090.
- Centers for Disease Control and Prevention. ACIP evidence to recommendations framework for use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years; 2023 [accessed 2023 Feb]. https://www.cdc.gov/vaccines/acip/recs/grade/recombinant-zoster-immunocompromised-etr.html.
- Yun H, Yang S, Chen L, Xie F, Winthrop K, Baddley JW, Saag KG, Singh J, Curtis JR. Risk of herpes zoster in autoimmune and inflammatory diseases: implications for vaccination. Arthritis Rheumatol. 2016;68(9):2328–37. doi:10.1002/art.39670.
- Batram M, Witte J, Schwarz M, Hain J, Ultsch B, Steinmann M, Bhavsar A, Wutzler P, Criée CP, Hermann C, et al. Burden of herpes zoster in adult patients with underlying conditions: analysis of German claims data, 2007–2018. Dermatol Ther (Heidelb). 2021;11(3):1009–26. doi:10.1007/s13555-021-00535-7.
- Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, Grose C, Hambleton S, Kennedy PG, Oxman MN, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016. doi:10.1038/nrdp.2015.16.
- Mbinta JF, Nguyen BP, Awuni PMA, Paynter J, Simpson CR. Post-licensure zoster vaccine effectiveness against herpes zoster and postherpetic neuralgia in older adults: a systematic review and meta-analysis. Lancet Healthy Longev. 2022;3(4):e263–75. doi:10.1016/s2666-7568(22)00039-3.
- Anderson TC, Masters NB, Guo A, Shepersky L, Leidner AJ, Lee GM, Kotton CN, Dooling KL. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the Advisory Committee on Immunization Practices - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(3):80–4. doi:10.15585/mmwr.mm7103a2.
- Harbecke R, Cohen JI, Oxman MN. Herpes zoster vaccines. J Infect Dis. 2021;224(12 Suppl 2):S429–42. doi:10.1093/infdis/jiab387.
- GlaxoSmithKline Biologicals. Shingrix (zoster vaccine recombinant, adjuvanted) suspension for intramuscular injection - Highlights of Prescribing Information; Revised 2021 Jul [ accessed 2022 Oct]. https://www.fda.gov/media/108597/download.
- Merck Sharp and Dohme B.V. Zostavax (zoster vaccine live, attenuated) suspension for subcutaneous or intramuscular injection - Highlights of Prescribing Information; [ accessed 2022 Oct]. https://www.fda.gov/media/82524/download.
- Tavares-Da-Silva F, Co MM, Dessart C, Hervé C, López-Fauqued M, Mahaux O, Van Holle L, Stegmann JU. Review of the initial post-marketing safety surveillance for the recombinant zoster vaccine. Vaccine. 2020;38(18):3489–500. doi:10.1016/j.vaccine.2019.11.058.
- European Medicines Agency. Shingrix EPAR - Product Information; Last updated 2021 Oct 13 [accessed 2022 Oct]. https://www.ema.europa.eu/documents/product-information/shingrix-epar-product-information_en.pdf.
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–96. doi:10.1056/NEJMoa1501184.
- Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Díez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–32. doi:10.1056/NEJMoa1603800.
- Kovac M, Lal H, Cunningham AL, Levin MJ, Johnson RW, Campora L, Volpi A, Heineman TC. Complications of herpes zoster in immunocompetent older adults: incidence in vaccine and placebo groups in two large phase 3 trials. Vaccine. 2018;36(12):1537–41. doi:10.1016/j.vaccine.2018.02.029.
- Willer DO, Oostvogels L, Cunningham AL, Gervais P, Gorfinkel I, Hyung Kim J, Talarico C, Wascotte V, Zahaf T, Colindres R, et al. Efficacy of the adjuvanted recombinant zoster vaccine (RZV) by sex, geographic region, and geographic ancestry/ethnicity: a post-hoc analysis of the ZOE-50 and ZOE-70 randomized trials. Vaccine. 2019;37(43):6262–7. doi:10.1016/j.vaccine.2019.09.028.
- Curran D, Andrew MK, Levin MJ, Turriani E, Matthews S, Fogarty C, Klein NP, Grupping K, Oostvogels L, Schmader KE. Evaluation of two frailty indices, with practical application in a vaccine clinical trial. Hum Vaccin Immunother. 2019;15(12):2960–8. doi:10.1080/21645515.2019.1622974.
- Curran D, Kim JH, Matthews S, Dessart C, Levin MJ, Oostvogels L, Riley ME, Schmader KE, Cunningham AL, McNeil SA, et al. Recombinant zoster vaccine is efficacious and safe in frail individuals. J Am Geriatr Soc. 2021;69(3):744–52. doi:10.1111/jgs.16917.
- Andrew MK, Matthews S, Kim JH, Riley ME, Curran D. An easy-to-implement clinical-trial frailty index based on accumulation of deficits: validation in zoster vaccine clinical trials. Clin Interv Aging. 2022;17:1261–74. doi:10.2147/cia.S364997.
- Oostvogels L, Heineman TC, Johnson RW, Levin MJ, McElhaney JE, Van den Steen P, Zahaf T, Dagnew AF, Chlibek R, Diez-Domingo J, et al. Medical conditions at enrollment do not impact efficacy and safety of the adjuvanted recombinant zoster vaccine: a pooled post-hoc analysis of two parallel randomized trials. Hum Vaccin Immunother. 2019;15(12):2865–72. doi:10.1080/21645515.2019.1627818.
- Dagnew AF, Rausch D, Hervé C, Zahaf T, Levin MJ, Schuind A. Efficacy and serious adverse events profile of the adjuvanted recombinant zoster vaccine in adults with pre-existing potential immune-mediated diseases: a pooled post hoc analysis on two parallel randomized trials. Rheumatology (Oxford). 2021;60(3):1226–33. doi:10.1093/rheumatology/keaa424.
- Boutry C, Hastie A, Diez-Domingo J, Tinoco JC, Yu CJ, Andrews C, Beytout J, Caso C, Cheng HS, Cheong HJ, et al. The adjuvanted recombinant zoster vaccine confers long-term protection against herpes zoster: interim results of an extension study of the pivotal phase 3 clinical trials ZOE-50 and ZOE-70. Clin Infect Dis. 2022;74(8):1459–67. doi:10.1093/cid/ciab629.
- Strezova A, Diez-Domingo J, Al Shawafi K, Tinoco JC, Shi M, Pirrotta P, Mwakingwe-Omari A. Long-term protection against herpes zoster by the adjuvanted recombinant zoster vaccine: interim efficacy, immunogenicity, and safety results up to 10 years after initial vaccination. Open Forum Infect Dis. 2022;9(10):ofac485. doi:10.1093/ofid/ofac485.
- Bastidas A, de la Serna J, El Idrissi M, Oostvogels L, Quittet P, López-Jiménez J, Vural F, Pohlreich D, Zuckerman T, Issa NC, et al. Effect of recombinant zoster vaccine on incidence of herpes zoster after autologous stem cell transplantation: a randomized clinical trial. JAMA. 2019;322(2):123–33. doi:10.1001/jama.2019.9053.
- Dagnew AF, Ilhan O, Lee WS, Woszczyk D, Kwak JY, Bowcock S, Sohn SK, Rodriguez Macías G, Chiou TJ, Quiel D, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019;19(9):988–1000. doi:10.1016/s1473-3099(19)30163-x.
- Vink P, Shiramoto M, Ogawa M, Eda M, Douha M, Heineman T, Lal H. Safety and immunogenicity of a herpes zoster subunit vaccine in Japanese population aged ≥50 years when administered subcutaneously vs. intramuscularly. Hum Vaccin Immunother. 2017;13(3):574–8. doi:10.1080/21645515.2016.1232787.
- Cunningham AL, Heineman TC, Lal H, Godeaux O, Chlibek R, Hwang SJ, McElhaney JE, Vesikari T, Andrews C, Choi WS, et al. Immune responses to a recombinant glycoprotein E herpes zoster vaccine in adults aged 50 years or older. J Infect Dis. 2018;217(11):1750–60. doi:10.1093/infdis/jiy095.
- Lal H, Poder A, Campora L, Geeraerts B, Oostvogels L, Vanden Abeele C, Heineman TC. Immunogenicity, reactogenicity and safety of 2 doses of an adjuvanted herpes zoster subunit vaccine administered 2, 6 or 12 months apart in older adults: results of a phase III, randomized, open-label, multicenter study. Vaccine. 2018;36(1):148–54. doi:10.1016/j.vaccine.2017.11.019.
- Dagnew AF, Klein NP, Hervé C, Kalema G, Di Paolo E, Peterson J, Salaun B, Schuind A. The adjuvanted recombinant zoster vaccine in adults aged ≥65 years previously vaccinated with a live-attenuated herpes zoster vaccine. J Infect Dis. 2021;224(7):1139–46. doi:10.1093/infdis/jiaa083.
- Godeaux O, Kovac M, Shu D, Grupping K, Campora L, Douha M, Heineman TC, Lal H. Immunogenicity and safety of an adjuvanted herpes zoster subunit candidate vaccine in adults ≥ 50 years of age with a prior history of herpes zoster: a phase III, non-randomized, open-label clinical trial. Hum Vaccin Immunother. 2017;13(5):1051–8. doi:10.1080/21645515.2016.1265715.
- Hastie A, Catteau G, Enemuo A, Mrkvan T, Salaun B, Volpe S, Smetana J, Rombo L, Schwarz T, Pauksens K, et al. Immunogenicity of the adjuvanted recombinant zoster vaccine: persistence and anamnestic response to additional doses administered 10 years after primary vaccination. J Infect Dis. 2021;224(12):2025–34. doi:10.1093/infdis/jiaa300.
- Weinberg A, Kroehl ME, Johnson MJ, Hammes A, Reinhold D, Lang N, Levin MJ. Comparative immune responses to licensed herpes zoster vaccines. J Infect Dis. 2018;218(suppl_2):S81–7. doi:10.1093/infdis/jiy383.
- Johnson MJ, Liu C, Ghosh D, Lang N, Levin MJ, Weinberg A. Cell-mediated immune responses after administration of the live or the recombinant zoster vaccine: 5-year persistence. J Infect Dis. 2022;225(8):1477–81. doi:10.1093/infdis/jiab580.
- Schmid DS, Miao C, Leung J, Johnson M, Weinberg A, Levin MJ. Comparative antibody responses to the live-attenuated and recombinant herpes zoster vaccines. J Virol. 2021;95(12):e0024021. doi:10.1128/jvi.00240-21.
- Levin MJ, Kroehl ME, Johnson MJ, Hammes A, Reinhold D, Lang N, Weinberg A. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest. 2018;128(10):4429–40. doi:10.1172/jci121484.
- Schwarz TF, Aggarwal N, Moeckesch B, Schenkenberger I, Claeys C, Douha M, Godeaux O, Grupping K, Heineman TC, Fauqued ML, et al. Immunogenicity and safety of an adjuvanted herpes zoster subunit vaccine coadministered with seasonal influenza vaccine in adults aged 50 years or older. J Infect Dis. 2017;216(11):1352–61. doi:10.1093/infdis/jix481.
- Min JY, Mwakingwe-Omari A, Riley M, Molo LY, Soni J, Girard G, Danier J. The adjuvanted recombinant zoster vaccine co-administered with the 13-valent pneumococcal conjugate vaccine in adults aged ≥50 years: a randomized trial. J Infect. 2022;84(4):490–8. doi:10.1016/j.jinf.2021.12.033.
- Maréchal C, Lal H, Poder A, Ferguson M, Enweonye I, Heineman TC, Hervé C, Rheault P, Talli J, Wauters D, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine co-administered with the 23-valent pneumococcal polysaccharide vaccine in adults ≥50 years of age: a randomized trial. Vaccine. 2018;36(29):4278–86. doi:10.1016/j.vaccine.2018.05.110.
- Strezova A, Lal H, Enweonye I, Campora L, Beukelaers P, Segall N, Heineman TC, Schuind AE, Oostvogels L. The adjuvanted recombinant zoster vaccine co-administered with a tetanus, diphtheria and pertussis vaccine in adults aged ≥50 years: a randomized trial. Vaccine. 2019;37(39):5877–85. doi:10.1016/j.vaccine.2019.08.001.
- Berkowitz EM, Moyle G, Stellbrink HJ, Schürmann D, Kegg S, Stoll M, El Idrissi M, Oostvogels L, Heineman TC, Brockmeyer N, et al. Safety and immunogenicity of an adjuvanted herpes zoster subunit candidate vaccine in HIV-infected adults: a phase 1/2a randomized, placebo-controlled study. J Infect Dis. 2015;211(8):1279–87. doi:10.1093/infdis/jiu606.
- Vink P, Ramon Torrell JM, Sanchez Fructuoso A, Kim SJ, Kim SI, Zaltzman J, Ortiz F, Campistol Plana JM, Fernandez Rodriguez AM, Rebollo Rodrigo H, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in chronically immunosuppressed adults following renal transplant: a phase 3, randomized clinical trial. Clin Infect Dis. 2020;70(2):181–90. doi:10.1093/cid/ciz177.
- Lindemann M, Baumann C, Wilde B, Gäckler A, Meller L, Horn PA, Krawczyk A, Witzke O. Prospective, longitudinal study on specific cellular immune responses after vaccination with an adjuvanted, recombinant zoster vaccine in kidney transplant recipients. Vaccines (Basel). 2022;10(6):844. doi:10.3390/vaccines10060844.
- Hirzel C, L’Huillier AG, Ferreira VH, Marinelli T, Ku T, Ierullo M, Miao C, Schmid DS, Juvet S, Humar A, et al. Safety and immunogenicity of adjuvanted recombinant subunit herpes zoster vaccine in lung transplant recipients. Am J Transplant. 2021;21(6):2246–53. doi:10.1111/ajt.16534.
- L’Huillier AG, Hirzel C, Ferreira VH, Ierullo M, Ku T, Selzner N, Schiff J, Juvet S, Miao C, Schmid DS, et al. Evaluation of recombinant herpes zoster vaccine for primary immunization of varicella-seronegative transplant recipients. Transplantation. 2021;105(10):2316–23. doi:10.1097/tp.0000000000003621.
- Vink P, Delgado Mingorance I, Maximiano Alonso C, Rubio-Viqueira B, Jung KH, Rodriguez Moreno JF, Grande E, Marrupe Gonzalez D, Lowndes S, Puente J, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in patients with solid tumors, vaccinated before or during chemotherapy: a randomized trial. Cancer. 2019;125(8):1301–12. doi:10.1002/cncr.31909.
- Muchtar E, Koehler AB, Johnson MJ, Rabe KG, Ding W, Call TG, Leis JF, Kenderian SS, Hayman SR, Wang Y, et al. Humoral and cellular immune responses to recombinant herpes zoster vaccine in patients with chronic lymphocytic leukemia and monoclonal B cell lymphocytosis. Am J Hematol. 2022;97(1):90–8. doi:10.1002/ajh.26388.
- Koldehoff M, Horn PA, Lindemann M. Cellular immune response after vaccination with an adjuvanted, recombinant zoster vaccine in allogeneic hematopoietic stem cell transplant recipients. Vaccines (Basel). 2022;10(5):809. doi:10.3390/vaccines10050809.
- Camargo JF, Lin RY, Natori Y, Anderson AD, Alencar MC, Wang TP, Morris MI, Komanduri KV. Reduced immunogenicity of the adjuvanted recombinant zoster vaccine after hematopoietic cell transplant: a pilot study. Blood Adv. 2020;4(19):4618–22. doi:10.1182/bloodadvances.2020002269.
- Pleyer C, Ali MA, Cohen JI, Tian X, Soto S, Ahn IE, Gaglione EM, Nierman P, Marti GE, Hesdorffer C, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137(2):185–9. doi:10.1182/blood.2020008758.
- Pleyer C, Laing KJ, Ali MA, McClurkan CL, Soto S, Ahn IE, Nierman P, Maddux E, Lotter J, Superata J, et al. BTK inhibitors impair humoral and cellular responses to recombinant zoster vaccine in CLL. Blood Adv. 2022;6(6):1732–40. doi:10.1182/bloodadvances.2021006574.
- Callegaro A, Burny W, Herve C, Hyung Kim J, Levin MJ, Zahaf T, Cunningham AL, Didierlaurent AM. Association between immunogenicity and reactogenicity: a post hoc analysis of 2 phase 3 studies with the adjuvanted recombinant zoster vaccine. J Infect Dis. 2022;226(11):1943–8. doi:10.1093/infdis/jiab536.
- Ocran-Appiah J, Boutry C, Hervé C, Soni J, Schuind A, Olsson Å, Anitta A, Novo Marta A, Charles A, Mark A, et al. Safety of the adjuvanted recombinant zoster vaccine in adults aged 50 years or older. A phase IIIB, non-randomized, multinational, open-label study in previous ZOE-50 and ZOE-70 placebo recipients. Vaccine. 2021;39(1):6–10. doi:10.1016/j.vaccine.2020.10.029.
- López-Fauqued M, Campora L, Delannois F, El Idrissi M, Oostvogels L, De Looze FJ, Diez-Domingo J, Heineman TC, Lal H, McElhaney JE, et al. Safety profile of the adjuvanted recombinant zoster vaccine: pooled analysis of two large randomised phase 3 trials. Vaccine. 2019;37(18):2482–93. doi:10.1016/j.vaccine.2019.03.043.
- Colindres R, Wascotte V, Brecx A, Clarke C, Hervé C, Kim JH, Levin MJ, Oostvogels L, Zahaf T, Schuind A, et al. Post hoc analysis of reactogenicity trends between dose 1 and dose 2 of the adjuvanted recombinant zoster vaccine in two parallel randomized trials. Hum Vaccin Immunother. 2020;16(11):2628–33. doi:10.1080/21645515.2020.1741312.
- Curran D, Oostvogels L, Heineman T, Matthews S, McElhaney J, McNeil S, Diez-Domingo J, Lal H, Andrews C, Athan E, et al. Quality of life impact of an adjuvanted recombinant zoster vaccine in adults aged 50 years and older. J Gerontol A Biol Sci Med Sci. 2019;74(8):1231–8. doi:10.1093/gerona/gly150.
- Coplan PM, Schmader K, Nikas A, Chan IS, Choo P, Levin MJ, Johnson G, Bauer M, Williams HM, Kaplan KM, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5(6):344–56. doi:10.1016/j.jpain.2004.06.001.
- Schmader KE, Levin MJ, Grupping K, Matthews S, Butuk D, Chen M, Idrissi ME, Fissette LA, Fogarty C, Hartley P, et al. The impact of reactogenicity after the first dose of recombinant zoster vaccine on the physical functioning and quality of life of older adults: an open-label, phase III trial. J Gerontol A Biol Sci Med Sci. 2019;74(8):1217–24. doi:10.1093/gerona/gly218.
- Schmader KE, Levin MJ, Chen M, Matthews S, Riley ME, Woo W, Hervé C, Grupping K, Schuind AE, Oostvogels L, et al. Impact of reactogenicity after two doses of recombinant zoster vaccine upon physical functioning and quality of life: an open phase III trial in older adults. J Gerontol A Biol Sci Med Sci. 2021;76(3):485–90. doi:10.1093/gerona/glaa127.
- Curran D, Matthews S, Rowley SD, Young JH, Bastidas A, Anagnostopoulos A, Barista I, Chandrasekar PH, Dickinson M, El Idrissi M, et al. Recombinant zoster vaccine significantly reduces the impact on quality of life caused by herpes zoster in adult autologous hematopoietic stem cell transplant recipients: a randomized placebo-controlled trial (ZOE-HSCT). Biol Blood Marrow Transplant. 2019;25(12):2474–81. doi:10.1016/j.bbmt.2019.07.036.
- Grupping K, Campora L, Douha M, Heineman TC, Klein NP, Lal H, Peterson J, Vastiau I, Oostvogels L. Immunogenicity and safety of the HZ/su adjuvanted herpes zoster subunit vaccine in adults previously vaccinated with a live attenuated herpes zoster vaccine. J Infect Dis. 2017;216(11):1343–51. doi:10.1093/infdis/jix482.