ABSTRACT
COVID-19 vaccination is effective at reducing SARS-CoV-2 complications, but uptake has been low. Our objective in this study was to compare the importance of factors reported to influence the decision to receive a bivalent COVID-19 booster vaccine among health care personnel (HCP) tested for SARS-CoV-2 between October 2022 and April 2023 in a 20-hospital vaccine effectiveness study in the United States (n = 1656). Compared with those who had not received the booster, the factors most likely to be reported to be important were concerns about contracting COVID-19 (84.0% of those who had received the bivalent booster vs. 47.5% of those who had not, difference 36.6% points (PP), 95% confidence interval [CI] 32.1 to 41.1%), spreading infection to family members (89.2% vs. 62.8%, difference 26.3 PP, 95% CI 22.3 to 30.4%), and spreading infection to colleagues at work (85.5% vs. 59.4%, difference 26.1 PP, 95% CI 21.7 to 30.5%). HCP who had received the booster more frequently cited the primary literature (61.7% vs. 31.8%, difference 29.9 PP, 95% CI 24.6 to 35.2%) and employer recommendations (48.3% vs. 29.8%, difference 18.5 PP, 95% CI 13.2 to 23.9%) as influencing their decision. This analysis provides insight into factors for targeting future vaccine messaging.
COVID-19 vaccines have substantially reduced the impact of SARS-CoV-2 infection by preventing death, severe disease, post-COVID complications, and transmission.Citation1–5 In the United States, bivalent mRNA booster vaccines against the ancestral SARS-CoV-2 strain and BA.4/5 Omicron variant were recommended in September 2022. However, hesitancy toward SARS-CoV-2 vaccines has persisted – even among healthcare personnel.Citation6–8
To assess factors considered by U.S. healthcare personnel in deciding whether to receive a bivalent mRNA vaccination, we conducted a cross-sectional survey of participants enrolled in an ongoing multicenter case-control COVID-19 vaccine effectiveness study.Citation9 In the parent study, we enrolled healthcare personnel in 15 states tested for SARS-CoV-2 between 3 October 2022 and 20 April 2023 at the time of a clinically indicated SARS-CoV-2 test, stratified by test-positive cases and test-negative controls. We verified testing and vaccination status with testing and vaccination records. Participants were all healthcare personnel who had testing because of a clinical indication and knew their test results at the time of enrollment, and provided electronic survey data on the extent to which various factors influenced their bivalent vaccination decisions within 60 days of testing (survey questions contained in Appendix 1). The survey was designed be 5 experts, then we field tested in a small group of health care personnel for feedback. We compared the responses between those who had received the bivalent booster versus those who had not by pooling “moderately influenced” and “strongly influenced” categories (compared with “slightly influenced,” “not at all influenced,” and “not applicable” categories), then measuring differences in proportions with confidence intervals using the Yates correction. We provide stratified analyses by education level, job category, and sex. The study was reviewed by the Centers for Disease Control and Prevention (CDC) and conducted as public health surveillance in accordance with applicable federal law and CDC policy.
Among 2397 healthcare personnel enrolled in the parent study during this period, 2373 (99%) received an initial monovalent mRNA booster dose and 1656 (70%) responded that they were eligible for the bivalent booster at the time they completed the survey (according to participant report only). We included 1656 participants, of whom 470 (28%) received a bivalent mRNA dose before their SARS-CoV-2 test date (Figure S1). Those who received a bivalent dose were more likely to be age 50–64 years, male, have a graduate or professional degree, be a physician or advanced practice provider, and have private healthcare insurance, and they were less likely to be Black or Hispanic (Table S1).
Health care personnel who had received the booster, compared with those who had not received the booster, more frequently cited the primary medical literature (61.7% selected “moderately” or “strongly” important among those who had received the bivalent booster vs. 31.8% who has not, difference 29.9% points, 95% confidence interval [CI] 24.6 to 35.2%) and employer recommendations (48.3% vs. 29.8%, difference 18.5% points, 95% CI 13.2 to 23.9%) as influencing their decision. Physicians and advanced practice providers were more likely than nurses and non-clinical staff to cite primary medical literature as important (63.8% vs. 37.1%, difference 26.8% points, 95% CI 19.3 to 34.2%, Figure S2 and Figure S3). Few participants cited personal physicians (18.5%), mass media (15.3%), or social media (6.3%) as being strongly or moderately important, with similar rates between groups. People who received the bivalent vaccine were more likely than those who did not to report that they were influenced by vaccine availability (50.9% vs. 22.9%, difference 28.0% points, 95% CI 22.7 to 33.3%, , Table S2).
Figure 1. Responses to questions about how factors influenced vaccination decisions, stratified by whether participants had received the COVID-19 bivalent booster, project PREVENT, October 2022–April 2023. Each bar displays the proportion of responses in each likert category. All bars are indexed to “moderately agree” (e.g., that is where the vertical line is on the graph), so the total length of the bar denotes 100% of responses. The panel on the left shows responses from those who had not received the bivalent booster, while the panel on the right shows responses from those who had received the bivalent booster.
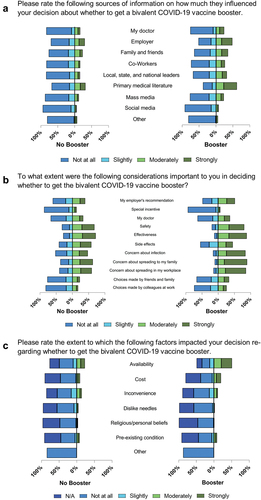
Compared with those who received the booster, the factors most strongly reported to be important in those who had received the bivalent vaccine booster were concerns about (1) contracting COVID-19 (84.0% vs. 47.5%, difference 36.6% points, 95% CI 32.1 to 41.1%; subsequent frequencies reported in a similar fashion), (2) spreading infection to family members (89.2% vs. 62.8%, difference 26.3% points, 95% CI 22.3 to 30.4%), and (3) spreading infection to colleagues at work (85.5% vs. 59.4%, difference 26.1% points, 95% CI 21.7 to 30.5%). Most respondents from both groups indicated that perceptions of vaccine safety and effectiveness influenced their decision, with the relative importance of safety and effectiveness being similar between bivalent boosted and non-boosted groups. We did not specifically elicit participants’ perceptions of how safe or effective they perceived the bivalent booster to be. Choices made by participants’ friends or family (25.8% selected “moderately” or “strongly” influential), colleagues (19.9%), and special incentives (10.9%) did not strongly influence respondents’ decision to receive the bivalent booster (, Table S2).
Overall, our findings suggest that healthcare personnel who had received a bivalent vaccine dose reported that preventing COVID-19 infection and avoiding transmission of infection to friends or family was important in their decision-making. These findings differ from a previous study in the general U.S. population, which found that vaccine awareness, mass media, and social media were reported to influence the decision to receive a bivalent booster.Citation10 Despite very high vaccine uptake with the initial mRNA SARS-CoV-2 series, it is estimated that only 20% of Americans received the bivalent booster, and few have had a recent dose.Citation11 This fact is particularly relevant because waning vaccine-induced immunity makes remaining up-to-date with current vaccination important. Employer-based vaccine campaigns promoted early vaccination, and our results suggest that employers remain a trusted source of information for some healthcare personnel making vaccine booster decisions.Citation12 Finally, healthcare personnel – especially those who received the booster – reported that concerns about the risks of transmission to others influenced their vaccination decisions. This concern is notable since transmission to colleagues might limit the available workforce during a future surge and transmission to patients with extensive comorbidities may lead to more severe disease. Given these insights, future studies on the role of COVID-19 vaccine doses to prevent transmission to others and the effectiveness of targeted messaging to healthcare personnel might positively influence vaccination decisions in this group.
Our report has several limitations. First, we collected information from people at the time of COVID-19 testing, so they may have some recall bias about their motivations for receiving a COVID-19 vaccine. Second, we enrolled people at the time that they had COVID-19 symptoms, so their opinions may have been influenced by a current or recent illness. Finally, our health care personnel cohort was comprised of volunteers in a vaccine effectiveness study, who may have had different perspectives or motivations than those who chose not to enroll.
In conclusion, our population of healthcare personnel provides insights into factors influencing the decision to receive bivalent COVID-19 boosters. Specifically, we found that employer-based messaging was particularly impactful, and the role of vaccination in preventing infection in others was influential in driving vaccination decisions. As future COVID-19 waves and vaccine products become available, these data will be helpful to increase the impact of vaccination campaigns and to remove practical barriers to vaccination.
CDC disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Author contributions
NMM, IDP, and DAT were involved in the conception and design of the study, interpretation of the data, drafting the manuscript, and providing final approval. ESL, KKH were involved in the analysis of the data, drafting the paper, and providing final approval. AK was involved in design of the study, interpretation of the data, critically revising the manuscript for important intellectual content, and providing final approval. UN, KFH, and HAS were involved with data collection, study management, revising the manuscript for important intellectual content, and providing final approval. The PREVENT Investigators were all involved with data collection and conduct of the study, revising the manuscript for intellectual content, and providing final approval. All authors agree to be accountable for all aspects of the work.
SupplementalMaterials_0925_Final.docx
Download MS Word (355.2 KB)Acknowledgments
The authors would like to acknowledge the health care personnel and health care systems who agreed to participate in this study: Rawan Hassanain, Eva Gonzalez; Jesus Torres, Denise Tritt, Sankan Nyanseor, Matthew McCullough, James Galbraith, Elizabeth Krebs, Fernand Rwamwejo, Emma Shuster, Christine Crider, Silas Bussmann, Kristen Hepfer, Nidhi Kanabar, Lauren Zimmerman, Gideon Avorno, Caitlin Wizda, Nikita Umale, Brandon Lee, Mohammad Adrian Hasdianda, Alicia Edwardson, Karen Hopcia, Laurie Kemble, Danielle Beckham; Miguel Neeley, Catherine Fairfield, Shannon Landers, Jennifer Smith Martine Desulme, Peter Poerzgen, Abigail Lopes, Whitney Covington, Cady Hart, Abigail Lopes, Danielle Ferdinand, Jonathan Jui, Jillian Tozloski, Manar Hamied, Erin Garzella, Marina Griffith, Austin Stevens, Micki Rockett, Natalie LaHote, Jonathan Rufino, Tala Teymour, Maria Davila, Suzette Fernandez, Chloe Namias, Susan Thompson, Yan Phipps, and Christopher Costa.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2284471.
Additional information
Funding
References
- Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22(9):1293–4. doi:10.1016/S1473-3099(22)00320-6.
- Byambasuren O, Stehlik P, Clark J, Alcorn K, Glasziou P. Effect of COVID-19 vaccination on long Covid: systematic review. BMJ Med. 2023;2(1):e000385. doi:10.1136/bmjmed-2022-000385.
- Milman O, Yelin I, Aharony N, Katz R, Herzel E, Ben-Tov A, Kuint J, Gazit S, Chodick G, Patalon T, et al. Community-level evidence for SARS-CoV-2 vaccine protection of unvaccinated individuals. Nat Med. 2021;27(8):1367–9. doi:10.1038/s41591-021-01407-5.
- Bobrovitz N, Ware H, Ma X, Li Z, Hosseini R, Cao C, Selemon A, Whelan M, Premji Z, Issa H, et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 2023;23(5):556–67. doi:10.1016/S1473-3099(22)00801-5.
- Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, Groome MJ, Huppert A, O’Brien KL, Smith PG, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924–44. doi:10.1016/S0140-6736(22)00152-0.
- Li M, Luo Y, Watson R, Zheng Y, Ren J, Tang J, Chen Y. Healthcare workers’ (HCWs) attitudes and related factors towards COVID-19 vaccination: a rapid systematic review. Postgrad Med J. 2021;99:520–8. doi:10.1136/postgradmedj-2021-140195. Epub 20210630; PubMed PMID: 34193545.
- Desye B. Prevalence and determinants of COVID-19 vaccine acceptance among healthcare workers: a systematic review. Front Public Health. 2022;10:941206. doi:10.3389/fpubh.2022.941206. Epub 20220728; PubMed PMID: 35968421; PMCID: PMC9366855.
- Block Ngaybe M, Schmitt HJ, Mallahan S, Sena R, Werts S, Rooney B, Magrath P, Madhivanan P. Qualitative assessment of COVID-19 vaccination acceptance among healthcare workers in Pima County. Hum Vaccines Immunother. 2023;19:2211464. doi:10.1080/21645515.2023.2211464. Epub 20230515; PubMed PMID: 37190772.
- Pilishvili T, Gierke R, Fleming-Dutra KE, Farrar JL, Mohr NM, Talan DA, Krishnadasan A, Harland KK, Smithline HA, Hou PC, et al. Effectiveness of mRNA COVID-19 vaccine among U.S. Health care personnel. N Engl J Med. 2021;385(25):e90. doi: 10.1056/NEJMoa2106599. Epub 20210922; PubMed PMID: 34551224; PMCID: PMC8482809.
- Sinclair AH, Taylor MK, Weitz JS, Beckett SJ, Samanez-Larkin GR. Reasons for receiving or not receiving bivalent COVID-19 booster vaccinations among adults — United States, November 1–December 10, 2022. MMWR. 2023;72(3):73–9. doi:10.15585/mmwr.mm7203a5.
- Allen J, Almukhtar S, Aufrichtig A, Barnard A, Bloch M, Bullock P, Cahalan S, Cai W. Coronavirus in the U.S.: latest map and case count. New York: New York Times; 2023 [accesssed 2023 May 15]. https://www.nytimes.com/interactive/2023/us/covid-cases.html.
- Gualano MR, Santoro PE, Borrelli I, Rossi MF, Amantea C, Tumminello A, Daniele A, Beccia F, Moscato U. Employee participation in workplace vaccination campaigns: a systematic review and meta-analysis. Vaccines (Basel). 2022;10(11):1898. doi: 10.3390/vaccines10111898. Epub 20221110; PubMed PMID: 36366407; PMCID: PMC9698273.
