ABSTRACT
Vaccination plays a key role in preventing morbidity and mortality caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We aimed to evaluate the safety and immunogenicity of a SARS-CoV-2 messenger ribonucleic acid (mRNA) vaccine SYS6006. In the two randomized, observer-blinded, placebo-controlled phase 1 trials, 40 adult participants aged 18–59 years and 40 elderly participants aged 60 years or more were randomized to receive two doses of SYS6006 or placebo (saline). Adverse events (AEs) were collected through 30 days post the second vaccination. Immunogenicity was assessed by live-virus neutralizing antibody (Nab), spike protein (S1) binding antibody (S1-IgG), and cellular immunity. The result showed that 7/15, 9/15 and 4/10 adult participants, and 9/15, 8/15 and 4/10 elderly participants reported at least one AE in the 20-µg, 30-µg and placebo groups, respectively. Most AEs were grade 1. Injection-site pain was the most common AE. Two adults and one elder reported fever. No vaccination-related serious AE was reported. SYS6006 elicited wild-type Nab response with a peak geometric mean titer of 232.1 and 130.6 (adults), and 48.7 and 66.7 (elders), in the 20-µg and 30-µg groups, respectively. SYS6006 induced moderate-to-robust Nab response against Delta, and slight Nab response against Omicron BA.2 and BA.5. Robust IgG response against wild type and BA.2 was observed. Cellular immune response was induced. In conclusion, two-dose primary vaccination with SYS6006 demonstrated good safety and immunogenicity during a follow-up period of 51 days in immunologically naive population aged 18 years or more. (Trial registry: Chictr.org.cn ChiCTR2200059103 and ChiCTR2200059104)
Introduction
As of 13 Aug 2023, coronavirus disease 2019 (COVID-19) has caused over 769 million confirmed cases and over 6.9 million deaths globally, according to the World Health Organization (WHO).Citation1 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has evolved a lot of variants with increased transmissibility, immune escape or increased pathogenicity.Citation2,Citation3 Eight SARS-CoV-2 mRNA vaccines have been approved for marketing or emergent usage authorization (EUA) worldwide, and 43 candidate vaccines are in clinical development.Citation4
The vaccines BNT162b1, BNT162b2 and ARCoV were among the earliest SARS-CoV-2 mRNA vaccines entering into clinical development in Chinese immunologically naïve population.Citation5–7 These studies showed that the injection-site adverse reactions (pain, swelling, redness, and induration) and systemic adverse reactions (fever, headache, fatigue, chills, joint pain, and muscle pain) were very common. Although the adverse reactions were more frequent for mRNA vaccine in comparison with inactivated vaccine or recombinant protein vaccine,Citation8,Citation9 the excellent immunogenicity, efficacy and effectiveness of mRNA vaccine make it worthy of more studies and continuous development.
SYS6006 is a SARS-CoV-2 mRNA vaccine containing mRNA molecules coated in lipid nanoparticles (LNP). The vaccine was designed based on the full-length spike (S) protein of the prototype SARS-CoV-2. Different from previous mRNA vaccines, several key mutation sites presented in Delta, Omicron BA.4, BA.5, and BF.7 strains were introduced into SYS6006, which made it able to induce cross-variants neutralizing antibodies in mice and non-human primates.Citation10 This vaccine had been studied in the phase 1, 2 and 3 trials and received EUA in China in March 2023, and was the first approved SARS-CoV-2 mRNA vaccine in China.
The two phase 1 trials aimed to evaluate the safety and immunogenicity of two doses of SYS6006 in Chinese healthy adults and elders who had not received any of SARS-CoV-2 vaccination.
Materials and methods
Study design and participants
The two randomized, observer-blinded, placebo-controlled, dose-escalation phase 1 clinical trials were conducted in the adult participants aged 18–59 and elderly participants aged 60 years or more. The adult trial was performed in the Shulan (Hangzhou) Hospital, Zhejiang Province, China, starting on April 27, 2022. The elder trial was performed in the Shulan (Hangzhou) Hospital and Sir Run Run Hospital of Nanjing Medical University, Jiangsu Province, China, starting on June 1, 2022.
Eligible participants were healthy people aged 18 years or more who had not received any SARS-CoV-2 vaccine before the enrollment. Key exclusion criteria included: axillary temperature ≥37.3°C on the day of enrollment, history of SARS-CoV-2 infection before the enrollment, positive for SARS-CoV-2 antibody, history of hypersensitivity to any component of the investigational vaccine or history of severe allergic reactions to vaccines or drugs, active malignant tumors, severe or uncontrollable medical conditions, history of treatment with immunosuppressive or immunomodulatory agents, blood products or immunoglobulins. All the history was inquired by qualified investigators. Pregnant or breastfeeding women were excluded. A complete list of the inclusion and exclusion criteria is provided in the supplementary materials.
The trial protocols were reviewed and approved by the Institutional Ethics Committees of the two hospitals. Written informed consent was obtained from each participant before screening for eligibility. The trials were done in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice.
Randomization and masking
Each trial included two random cohorts (20-μg and 30-μg random cohorts). In each random cohort, the participants (N = 20) were randomized to receive SYS6006 (N = 15) of the indicated dosage or the placebo (N = 5) in a 3:1 ratio. The randomization list was generated by an independent statistician using SAS 9.4 (or above) by blocked randomization method.
The two trials were designed in an observer-blinded manner. SYS6006 and the placebo were different in appearance. To keep the trials in blinded, an unblinded team handled and administered the vaccine and placebo, but did not participate in other study activities. Disclosure of treatment allocation to other study personnel was not allowed to ensure safety observation and laboratory testing in a blinded manner.
Procedures
Each dose of SYS6006 contained 30 μg mRNA in 0.3 mL suspension which was developed by CSPC Zhongqi Pharmaceutical Co. Ltd and manufactured by CSPC Megalith Biopharmaceutical Co. Ltd. (Shijiazhuang, China). The placebo (saline) was manufactured by Shanghai Sine Jinzhu Pharmacy Co., Ltd. (Shanghai, China). All participants received two doses of SYS6006 or placebo on Days 0 and 21 by intramuscular injection.
The enrollment was done in a dose-escalation manner. The 20-μg random cohort was enrolled first, followed by the 30-μg random cohort. The first eight participants in each random cohort served as sentinels, who were enrolled on the first, second and third day (n = 1, 3 and 4, respectively). If the preliminary safety profile in the sentinels within 4 days post vaccination did not trigger study suspension, the other 12 participants would be enrolled. If the preliminary safety profile in the 20 participants in the 20-μg random cohort within 7 days post vaccination did not trigger study suspension, the participants in the 30-μg random cohort would be enrolled. The enrollment of elderly participants would start if the preliminary safety profile in the adult participants in the same dosage cohort was deemed acceptable.
The participants were observed for one hour after each vaccination for any immediate reaction, and received diary cards to record solicited injection-site and systemic adverse events (AEs) within 14 days and unsolicited AEs within 30 days after each vaccination. All the participants were hospitalized to observe any potential AEs for 4 days after each vaccination. Blood biochemistry, hematology, blood coagulation function, thyroid function, and urinalysis were tested before vaccination and 4 days after each vaccination.
Blood samples were taken to test humoral and cellular immunity before the first vaccination, 14 and 21 days after the first vaccination, and 7, 14, and 30 days after the second vaccination. The live-virus neutralizing antibodies (Nabs) against wild type, Delta, Omicron BA.2 and BA.5 were tested by virus microneutralizing assay. SARS-CoV-2 spike protein (S1)-specific binding antibodies (S1-IgG) against wild type and Omicron BA.2 were tested by enzyme-linked immunosorbent assay (ELISA). Undetectable antibody titers were assigned half the detection limit for calculation. Cellular immune response was assayed by ex-vivo interferon-γ (IFN-γ) and interleukin 2 (IL-2) enzyme-linked immunospot (ELISpot), and IFN-γ, IL-2 and IL-4 intracellular stain (ICS). The peptide pool (spanning 1271 amino acids, 15 in each peptide with 11 overlapping) was derived from Delta S protein.
Outcomes
Safety outcomes include solicited AEs within 14 days after vaccination, unsolicited AEs within 30 days after vaccination, and serious adverse events (SAEs) and adverse events of special interest (AESIs, see Table S1 for more details) within 12 months after vaccination. AE was graded according to the scales (Table S2) issued by the China National Medical Products Administration.Citation11
Humoral immunity outcomes included serum geometric mean titers (GMTs), seroconversion rates (SCRs), and geometric mean fold increase (GMFI) of Nab and S1-IgG. Seroconversion was defined as 4-fold or more increase in antibody titers after vaccination compared to pre-vaccination. Cellular immunity outcomes included frequency of peripheral blood mononuclear cells (PBMC) expressing specific cytokines (IFN-γ, IL-2), and proportion of CD4+ and CD8+ T cell subsets expressing IFN-γ, IL-2 and IL-4.
Statistical analysis
The sample size was not determined based on statistical power calculation, but followed the general recommendation of regulatory authority. The safety analysis was based on the safety set (SS), which included all participants who had been randomized and received at least one dose of vaccination. The number and proportion of participants experiencing adverse events in each group were presented.
Immunogenicity analysis was primarily based on the per protocol set (PPS), which included all participants who did not violate inclusion/exclusion criteria, had been randomized, received the first dose of vaccination as specified in the protocol, and provided pre- and post-vaccination blood samples for immunogenicity evaluation with valid antibody titer values. Neutralizing antibody and S1-IgG titers were log-transformed to calculate GMT and GMFI. The frequency of PBMCs expressing IFN-γ and IL-2 based on ELISpot, and proportion of CD4+ and CD8+ T cell subsets expressing IFN-γ, IL-2 and IL-4 based on ICS were statistically described.
All statistical analyses were performed using SAS 9.4 (or above). The two trials were registered with Chictr.org.cn ChiCTR2200059103 and ChiCTR2200059104 on April 25, 2022.
Results
Participants
From April 27 to July 9, 2022, 84 adults aged 18–59 years were screened. From June 1 to September 5, 2022, 98 elders aged 60 years or more were screened. For each age group, 40 participants were enrolled and randomized to 20-μg SYS6006 group (N = 15), 30-μg SYS6006 group (N = 15) or the placebo group (N = 10). All the participants received two doses of vaccination except that one elderly participant (30-μg SYS6006 group) received only one dose ().
Figure 1. Trial profile. This participant did not receive the second vaccination due to SAE (multiple fractures), but did not withdraw from the study (**).
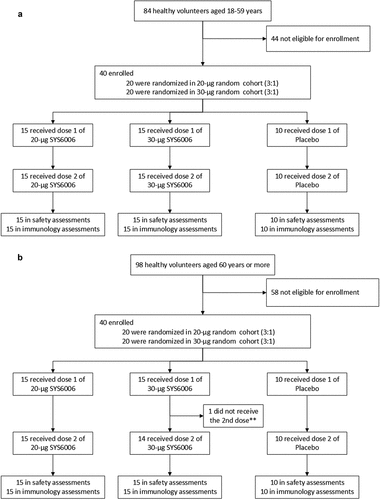
The baseline demographic characteristics were generally similar among the 20-μg SYS6006, 30-μg SYS6006 and the placebo groups within each age group, except that the adult placebo group had higher proportion of male participants. The mean age was 31.1 (range: 20 to 42), 31.1 (range: 21 to 49) and 27.2 (range: 22 to 32) years in the three adult groups, and 65.4 (range: 60 to 86), 63.9 (range: 60 to 67) and 65.9 (range: 60 to 73) years in the three elderly groups. All the participants were Han Chinese in ethnicity except for two adult participants ().
Table 1. Demographics of the participants.
Safety
The safety analysis included all the participants. Most AEs were grade 1 in intensity. In the adult participants, vaccination-related AEs were reported by 7/15, 7/15, and 2/10 participants in the 20-µg, 30-µg, and placebo groups, respectively. Pain was the only solicited injection-site AE reported by 4 and 3 participants in the 20-µg and 30-µg groups, respectively. Two participants in the 30-µg group reported fever. In the elderly participants, vaccination-related AEs were reported by 9/15, 6/15, and 2/10 participants in the 20-µg, 30-µg, and placebo groups, respectively. In the 20-µg group, 5 participants reported injection-site pain, and one participant reported fever. The AE incidences following the first and second doses were similar (Supplementary Figure S1, Supplementary Table S3).
In the two trials, one elderly participant in the 30-μg group reported a SAE (multiple fracture) caused by traffic accident. No AESI or death were observed within 30 days following vaccination. No participant withdrew from the study due to vaccination-associated AEs.
Immunogenicity
All the participants were included in the PPS immunogenicity analysis. One elderly participant in the 30-µg group did not donate blood after Day 21. All participants were seronegative for Nab and S1-IgG before vaccination, except that one elderly participant in the 20-µg group was seropositive (≥1:100) for S1-IgG.
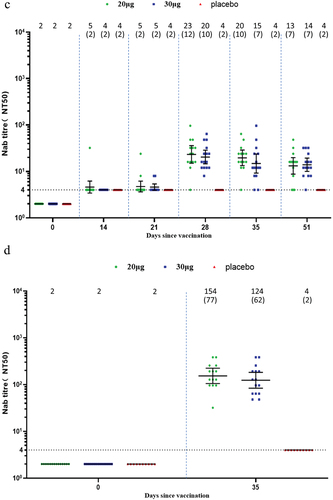
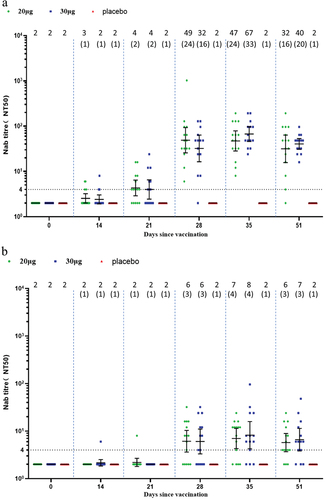
In the adult participants, one dose of SYS6006 induced slightly elevated wild-type Nab titers. Two doses of SYS6006 elicited robust wild-type Nab response which mounted a peak GMT of 232.1 in the 20-µg group, and 130.6 in the 30-µg group, corresponding to 116.0- and 65.3-fold increase, respectively (). One dose of SYS6006 induced wild-type Nab SCRs of 60.0% and 86.7% in the 20-µg and 30-µg groups on Day 14, respectively. All participants seroconverted for wild-type Nab after receiving two doses of SYS6006. SYS6006 induced robust cross-variant Nab against Delta with GMTs of 153.6 and 123.7 in the two vaccine groups on Day 35. SYS6006 also induced slight cross-variant Nab against Omicron BA.2 and BA.5. The peak GMTs were 29.6 for BA.2 on Day 35, and 23.2 for BA.5 on Day 28, in the 20-µg group ().
Figure 2. Neutralizing antibody responses in the adult group. (a) Against wild type; (b) against Omicron BA.2; (c) against Omicron BA.5; (d) against Delta. Interleaved scatter plot: geometric mean titer with 95% CI. The numbers above the scatter plots are the geometric mean titers. The numbers in the parentheses are the geometric mean fold increases versus baseline. The horizontal dot line indicates the starting dilution fold (1:4) for pre-vaccination serum. The starting dilution fold for post-vaccination serum was 1:8.
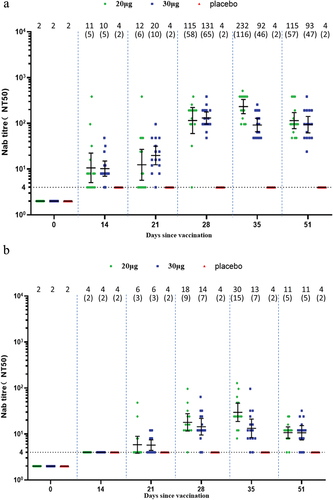
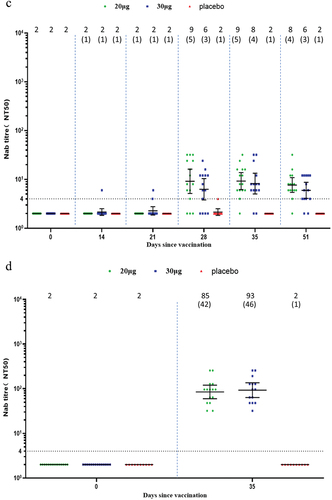
In the elderly participants, one dose of SYS6006 did not induce a significant Nab response. Two doses of SYS6006 elicited moderate wild-type Nab response which mounted a peak GMT of 48.7 in the 20-µg group, and 66.7 in the 30-µg group, corresponding to 24.4- and 33.4-fold increase, respectively (). All participants seroconverted for wild-type Nab after receiving two doses of SYS6006. SYS6006 induced moderate cross-variant Nab against Delta with GMTs of 84.8 and 92.8 in the two vaccine groups on Day 35. SYS6006 also induced slight cross-variant Nab against Omicron BA.2 (peak GMT of 8.2 in the 30-µg group) and BA.5 (peak GMT of 9.2 in the 20-µg group) on Day 35 ().
Figure 3. Neutralizing antibody responses in the elderly group. (a) Against wild type; (b) against Omicron BA.2; (c) against Omicron BA.5; (d) against Delta. Interleaved scatter plot: Geometric mean titer with 95% CI. The numbers above the scatter plots are the geometric mean titers. The numbers in the parentheses are the geometric mean fold increases versus baseline. The horizontal dot line indicates the starting dilution fold (1:4) for pre-vaccination serum. The starting dilution fold for post-vaccination serum was 1:8.
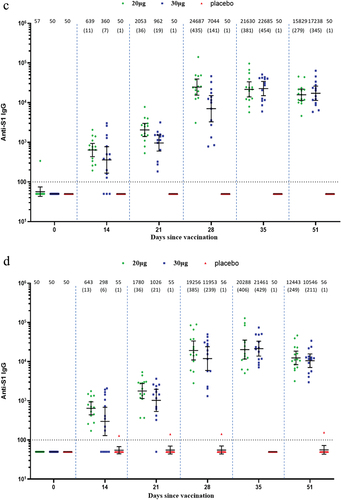
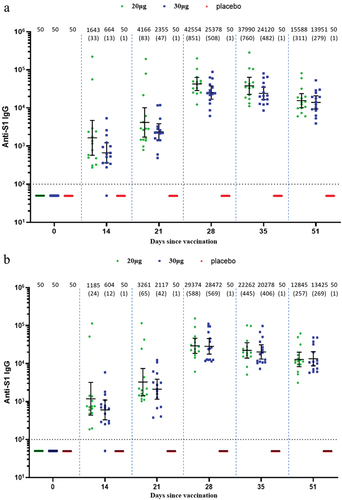
Robust binding antibody S1-IgG against both wild type and BA.2 was induced by SYS6006 in both adult and elderly participants. In the adult participants, the peak GMTs in the 20-µg and 30-µg groups were 42,554.1 and 25,377.9 against wild type, and 29,374.2 and 28,471.7 against BA.2 on Day 28, respectively (). In the elderly participants, the peak GMTs in the 20-µg and 30-µg groups were 24,687.1 and 22,684.7 against wild type, and 20,287.5 and 21,460.5 against BA.2, respectively ().
Figure 4. IgG binding antibodies against both wild type and Omicron BA.2 in the adult and elderly groups. (a) Against wild type in the adult participants; (b) against Omicron BA.2 in the adult participants; (c) against wild type in the elderly participants; (d) against Omicron BA.2 in the elderly participants. Interleaved scatter plot: geometric mean titer with 95% CI. The numbers above the scatter plots are the geometric mean titers. The numbers in the parentheses are the geometric mean fold increases versus baseline. The horizontal dot line indicates the starting dilution fold (1:100).
As expected, Nab and S1-IgG responses were not induced in the placebo group in both adult and elderly participants.
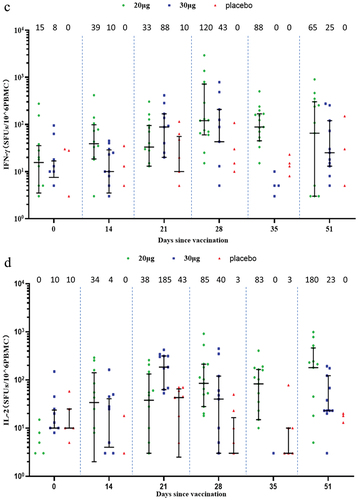
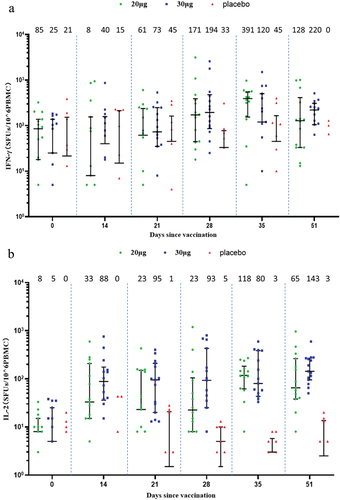
Two-dose vaccination with SYS6006 induced cellular immune response in the ELISpot assay. In the adult participants, the median of spots forming units (SFUs) per 10Citation6 PBMCs were elevated from 85 and 25 at baseline to 391 and 120 on Day 35 for IFN-γ-ELISpot, and from 8 and 5 at baseline to 118 and 80 on Day 35 for IL-2-ELISpot, in the 20-μg and 30-μg groups, respectively. In the elderly participants, the SFUs per 10Citation6 PBMCs were elevated from 15 and 8 at baseline to 120 and 43 on Day 28 for IFN-γ-ELISpot, and from 0 and 10 at baseline to 85 and 40 on Day 28 for IL-2-ELISpot, in the 20-μg and 30-μg groups, respectively. In comparison, cellular response was almost not observed in the placebo group (). ICS assay did not show obvious IFN-γ, IL-2 or IL-4 responses (Supplementary Figures S2 and S3).
Figure 5. Cellular immune response measured by ELISpot. (a) IFN-γ response in the adult group; (b) IL-2 response in the adult group; (c) IFN-γ response in the elderly group; (d.) IL-2 response in the elderly group. Interleaved scatter plot: median with interquartile range. The numbers above the scatter plots are the medians. The peptides derived from Delta S protein were used. The cytokine-secreting PBMCs were corrected for background by subtraction of values obtained with negative (medium-only) control. Negative values were set to zero and are not presented here. IFN: interferon; IL: interleukin; SFUs: spots forming units; PBMC: peripheral blood mononuclear cell.
Discussion
The COVID-19 pandemic constitutes a huge challenge for global public health since the end of 2019. SARS-CoV-2 is characterized by persistent evolution, continuous emergence of new variants, enhanced transmissibility, and significant immune escape. Along with the waning immunity after vaccination over time, these new variants weaken the protection of the original vaccines.Citation12,Citation13 There is a concern that COVID-19 pandemic waves caused by new variants likely recur some months after the last wave. To address the challenge of new variants, a vaccine technology platform able to quickly upgrade the antigen sequence is preferred. Without cultivation of live virus for inactivated vaccines or the construction of recombinant vector for protein vaccines, the mRNA vaccine technology has its merits in making it possible to quickly upgrade vaccines antigens.
As parts of the registration trials, these two phase 1 trials were designed to evaluate the preliminary safety and immunogenicity of the SARS-CoV-2 mRNA vaccine SYS6006. As the first-in-human trials for this vaccine, the participant enrollment was done in a cautiously step-by-step way, i.e., from the sentinels to other participants, from the low-dose cohort to the high-dose cohort, and from adult participants to elderly participants, which aimed to minimize the potential risk posed to the participants. The enrollment did not go forward to the next steps until the preliminary safety profile was assessed against the study suspension criteria and deemed acceptable. In addition, all the participants were hospitalized at the study sites for 4 days after each vaccination, which aimed to closely monitor any AEs. These operational procedures did sufficiently safeguard the participants’ health and benefit.
The participants having stably controlled chronic diseases such as diabetes and hypertension were not excluded from the trials. Diabetes and hypertension are common underlying conditions. The population having such conditions are at increased risk for severe SARS-CoV-2 infection, and the prioritized targeted population for vaccination. Stably controlled conditions pose no additional risk to the participants. Therefore, it has sound rationale to include such conditions.
The preliminary safety results showed that SYS6006 had a good safety profile in both adult and elderly participants. As expected, the AE incidence following SYS6006 vaccination was higher than that following placebo vaccination. However, it was comparable or lower than those with other SARS-CoV-2 mRNA vaccines.Citation5–7 Particularly, the incidence of fever was lower than those observed for other SARS-CoV-2 mRNA vaccines,Citation5–7 and similar to inactivated vaccines or recombinant protein vaccines.Citation8,Citation9
It is noteworthy that fever was defined as axillary temperature ≥ 37.3°C in our study according to the official guideline,Citation11 but defined as oral temperature ≥ 38.0°C in other studies.Citation14–16 A higher dosage up to 30 µg was not associated with a higher AE incidence, which is worthy of further study due to the small sample size in these phase 1 trials. The second dose of SYS6006 did not result in higher AE incidences, which is different from previous reports.Citation6,Citation7,Citation17 Again, this phenomenon should be further studied in a larger sample in order to test the robustness. During the two trials, no study suspension or premature termination were triggered, indicating an acceptable tolerability for SYS6006.
Two-dose primary vaccination with SYS6006 elicited robust wild-type Nab, similar to other mRNA vaccines.Citation5–7 However, only one dose did not induce robust Nab in immunologically naive participants, consistent with previous reports.Citation5,Citation7,Citation18,Citation19 As expected, the elderly participants had lower Nab titers than adult participants did, consistent with other SARS-CoV-2 mRNA vaccines.Citation5,Citation6 SYS6006 induced robust cross-variant Nab against Delta, which was comparable to that against wild type. Although cross-variant Nabs against Omicron BA.2 and BA.5 were also induced, they were less robust. This is consistent with previous reports that Omicron and its subvariants showed significant immune escape from preexisting immunity,Citation20,Citation21 suggesting that booster doses are necessary to combat Omicron and its subvariants.Citation22 By comparison, cross-variant S1-IgG titer against BA.2 was only slightly reduced versus that against wild type, indicating that the mutations in RBD of Omicron and its subvariants exert less impact on the potency of binding antibody.
Trend for a slightly higher Nab response was seen in the adult group after 20-µg vaccination as compared with 30-µg vaccination. The reason is to be elucidated. Because of the small sample size, it did not indicate a statistical significance. Whether this is clinically relevant remains unknown. Further study is needed to verify the robustness.
Two-dose vaccination with SYS6006 induced cellular immune responses in both adult and elderly participants as indicated by IFN-γ- and IL-2-expressed ELISpot assay results, which is consistent with other mRNA vaccines.Citation6,Citation7,Citation23 Since IFN-γ and IL-2 are the cytokines of Th1 type, the results indicated that SYS6006 induced a Th1-biased CD4+ T cell response, which is important in reducing disease severity. Depending on the age and vaccine dosage, ELISpot responses peaked at different timepoints. The reason remains unknown. Taking into account the small sample size and high variety in ELISpot assay, the results should be confirmed in further studies. ICS assay did not show an obvious cellular response. The high ICS response at baseline and in the placebo group might be associated with long stimulating time, high concentration of peptide, and/or gating strategy.
There are some limitations for these trials. First, the sample size for the two phase 1 trials was small. The preliminary safety and immunogenicity observed in the studies might not be robust enough and should be further investigated in a larger sample size. Second, we did not include convalescent or reference plasma/serum in antibody assays for comparison. Third, Nab response against Delta was tested only on Day 14 post the second dose and the antibody titers at other timepoints were not available. Fourth, the long-term follow-up for safety and immunogenicity are still ongoing and will be reported later.
In conclusion, two-dose primary vaccination with SYS6006 demonstrated good safety and immunogenicity during a follow-up period of 51 days in immunologically naïve healthy population aged 18 years or more, warranting further clinical development of this vaccine.
Author contribution
Li LJ, Lu X, Li CL, Chen GL, Su YW, Yang HY, Qiu YZ, and Zou YY participated in the protocol design and data interpretation; Wu KQ, Wu Y, Peng CG, Jin TH, Jiang Q, Guo T, Wang YH, Su C, Ma JH, Xu Y, Huang CC, and Zhang Q participated in data collection; Wang X, and Liu KL participated in data monitoring; Ji L and Ni SN coordinated data management and statistical analysis, respectively, which were done by outside vendors; Qiu YZ and Zou YY prepared the draft manuscript that was reviewed and revised by all authors to create the final draft. The corresponding authors had full access to all the data in the studies and had final responsibility for the decision to submit for publication.
HVI_Supplementary Materials_revised_clean.docx
Download MS Word (2.6 MB)Acknowledgments
The trials were funded by CSPC Megalith Biopharmaceutical Co., Ltd. We thank all the participants in the trials and our external service providers (contract research organizations, laboratories, clinical suppliers, and biostatistics providers) for their assistance in the trials. We also thank Yanan Liu for assistance in the manuscript formatting.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2285089
Additional information
Funding
References
- World Health Organization. Weekly epidemiological update on COVID-19; 2023 Aug 17 [accessed 2023 Aug 21]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19.
- Wang Q, Guo Y, Iketani S, Nair MS, Li Z, Mohri H, Wang M, Yu J, Bowen AD, Chang JY, et al. Antibody evasion by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature. 2022;608(7923):603–14. doi:10.1038/s41586-022-05053-w.
- Carabelli AM, Peacock TP, Thorne LG, Harvey WT, Hughes J, de Silva TI, Peacock SJ, Barclay WS, de Silva TI, Towers GJ, et al. COVID-19 Genomics UK Consortium, SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat Rev Microbiol. 2023;21:162–77. doi:10.1038/s41579-022-00841-7.
- World Health Organization. COVID-19 vaccine tracker and landscape; [accessed 2023 May 19]. www.who.int/teams/blueprint/covid-19/covid-19-vaccine-tracker-and-landscape.
- Li J, Hui A, Zhang X, Yang Y, Tang R, Ye H, Ji R, Lin M, Zhu Z, Türeci Ö, et al. Safety and immunogenicity of the SARS-CoV-2 BNT162b1 mRNA vaccine in younger and older Chinese adults: a randomized, placebo-controlled, double-blind phase 1 study. Nat Med. 2021;27(6):1062–70. doi:10.1038/s41591-021-01330-9.
- Hui AM, Li J, Zhu L, Tang R, Ye H, Lin M, Ge L, Wang X, Peng F, Wu Z, et al. Immunogenicity and safety of BNT162b2 mRNA vaccine in Chinese adults: a phase 2 randomized clinical trial. Lancet Reg Health West Pac. 2022;29:100586. doi:10.1016/j.lanwpc.2022.100586.
- Chen GL, Li XF, Dai XH, Li N, Cheng ML, Huang Z, Shen J, Ge Y-H, Shen Z-W, Deng Y-Q, et al. Safety and immunogenicity of the SARS-CoV-2 ARCoV mRNA vaccine in Chinese adults: a randomized, double-blind, placebo-controlled, phase 1 trial. Lancet Microbe. 2022;3:e193–e202. doi:10.1016/S2666-5247(21)00280-9.
- Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–92. doi:10.1016/S1473-3099(20)30843-4.
- Yang S, Li Y, Dai L, Wang J, He P, Li C, Fang X, Wang C, Zhao X, Huang E, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomized, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021;21:1107–19. doi:10.1016/S1473-3099(21)00127-4.
- Xu K, Lei W, Kang B, Yang H, Wang Y, Lu Y, Lv L, Sun Y, Zhang J, Wang X, et al. A novel mRNA vaccine, SYS6006, against SARS-CoV-2. Front Immunol. 2023;13:1051576. doi:10.3389/fimmu.2022.1051576.
- China National Medical Products Administration. Guidelines for grading adverse events in clinical trials of prophylactic vaccines (2019 edition); [accessed 2023 Mar 24]. https://www.nmpa.gov.cn/yaopin/ypggtg/ypqtgg/20191231111901460.html.
- Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, Groome MJ, Huppert A, O’Brien KL, Smith PG, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924–44. doi:10.1016/S0140-6736(22)00152-0.
- Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O’Connell A-M, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–46. doi:10.1056/NEJMoa2119451.
- Costa Clemens SA, Weckx L, Clemens R, Almeida Mendes AV, Ramos Souza A, Silveira MBV, da Guarda SNF, de Nobrega MM, de Moraes Pinto MI, Gonzalez IGS, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomized study. Lancet. 2022;399:521–9. doi:10.1016/S0140-6736(22)00094-0.
- Liu X, Li Y, Wang Z, Cao S, Huang W, Yuan L, Huang Y-J, Zheng Y, Chen J, Ying B, et al. Safety and superior immunogenicity of heterologous boosting with an RBD-based SARS-CoV-2 mRNA vaccine in Chinese adults. Cell Res. 2022;32(8):777–80. doi:10.1038/s41422-022-00681-3.
- Mallah SI, Alawadhi A, Jawad J, Wasif P, Alsaffar B, Alalawi E, Mohamed AM, Butler AE, Alalawi B, Qayed D, et al. Safety and efficacy of COVID-19 prime-boost vaccinations: homologous BBIBP-CorV versus heterologous BNT162b2 boosters in BBIBP-CorV-primed individuals. Vaccine. 2023;41(12):1925–33. doi:10.1016/j.vaccine.2023.01.032.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi:10.1056/NEJMoa2034577.
- Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Bailey R, Swanson KA, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults [published correction appears in Nature 2021 Feb;590(7844):E26]. Nature. 2020;586:589–93. doi:10.1038/s41586-020-2639-4.
- Li J, Hui AM, Zhang X, Ge L, Qiu Y, Tang R, Ye H, Wang X, Lin M, Zhu Z, et al. Immune persistence and safety after SARS-CoV-2 BNT162b1 mRNA vaccination in Chinese adults: a randomized, placebo-controlled, double-blind phase 1 trial. Adv Ther. 2022;39(8):3789–98. doi:10.1007/s12325-022-02206-1.
- Hachmann NP, Miller J, Collier AY, Ventura JD, Yu J, Rowe M, Bondzie EA, Powers O, Surve N, Hall K, et al. Neutralisation escape by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med. 2022;387:86–8. doi:10.1056/NEJMc2206576.
- Muik A, Lui BG, Wallisch AK, Bacher M, Mühl J, Reinholz J, Ozhelvaci O, Beckmann N, Güimil Garcia RDLC, Poran A, et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine–elicited human sera. Science. 2022;375:678–80. doi:10.1126/science.abn7591.
- Xia H, Zou J, Kurhade C, Cai H, Yang Q, Cutler M, Cooper D, Muik A, Jansen KU, Xie X, et al. Neutralisation and durability of 2 or 3 doses of the BNT162b2 vaccine against Omicron SARS-CoV-2. Cell Host & Microbe. 2022;30:485–8.e3. doi:10.1016/j.chom.2022.02.015.
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, et al. An mRNA vaccine against SARS-CoV-2 – preliminary report. N Engl J Med. 2020;383:1920–31. doi:10.1056/NEJMoa2022483.