ABSTRACT
Biliary tract cancer (BTC) is an aggressive malignancy with few options for advanced-stage treatment. The combination of PD-1/PD-L1 inhibitors with famitinib, a receptor tyrosine kinase (RTK) inhibitor, has demonstrated improved clinical outcomes in several clinical trials. We herein reported a case of a gallbladder cancer (GBC) patient with liver metastases, previously resistant to traditional chemotherapy. Remarkably, the patient achieved a complete response (CR) with a long-lasting survival benefit exceeding 3 years. This was achieved using a novel regimen combining SHR-1701, an anti-PD-L1/TGF-βR fusion protein, and famitinib, even though the patient had proficient mismatch repair (pMMR) and tested negative for PD-L1. Adverse events were limited and manageable. This is the first report of such a treatment regimen being applied in a clinical setting, suggesting that the SHR-1701 and famitinib combination may be a promising immunotherapeutic approach for patients with refractory advanced GBC.
Introduction
Biliary tract cancer (BTC) is an aggressive malignancy with a poor prognosis.Citation1 Chemotherapy stands as the first-line treatment for unresectable BTC, yet effective late-line therapeutics are limited.Citation2 Several trials involving immune checkpoint inhibitors (ICIs) or combinations have observed response rates of around 20%, but resistance remains an issue.Citation3–5 Notably, emerging treatments show promise; for instance, supplementing anti-PD-L1 therapy with LY2109761, a TGF-β receptor type I/II inhibitor, has enhanced tumor eradication from 33% to 77%.Citation6 Ongoing clinical trials are exploring the efficacy of novel bifunctional anti-PD-L1/TGF-βRII agents, including bintrafusp alfa and SHR-1701. Furthermore, paring ICIs with famitinib, a multi-target receptor tyrosine kinase (RTK) inhibitor against VEGFR2/3, PDGFR, Kit, Flt1/3, has outperformed the prevailing standard care in multiple clinical trials.Citation7 Herein, we present a case of a chemotherapy-refractory metastatic gallbladder cancer (GBC) patient, who, with proficient mismatch repair (pMMR) and negative for PD-L1, responded surprisingly well to a novel combine immunotherapy of SHR-1701 plus famitinib.
Case presentation
In June 2020, a previously healthy 55-year-old Chinese woman underwent laparoscopic cholecystectomy for gallstones at a local hospital. Pathological examination of the gallbladder specimen revealed a low to moderately differentiated adenocarcinoma () with serosal and perineural invasion, classified as stage IIIA (T3N0M0, according to the 8th AJCC Cancer Staging System). Immunohistochemistry (IHC) staining showed Ki-67 (+80%), CK20 (-), CK19(+), P53 (-), MLH1 (+), MSH2 (+), MSH6 (+), PMS2 (+), BRAF V600E (-), suggesting pMMR. Notably, her cousin had a history of BTC. One month post-diagnosis, the patient underwent one session of microwave ablation and received two rounds of transarterial chemoembolization (TACE) with gemcitabine (1.0 g/m2 for two liver metastases in the right lobe. Each TACE session was followed by intravenous chemotherapy with oxaliplatin (100 mg/m2). Shortly after the TACE, new lymph node metastases appeared in the hepatic hilum and retroperitoneum, and the original lesions enlarged. Consequently, she was diagnosed with stage IV GBC with liver metastases (T4N1M1). The patient tested negative for PD-L1, as determined by IHC using an antibody from Proteintech (Cat No: 66248–1-Ig), with a Combined Positive Score < 1. Genetic testing from her previous hospital revealed no actionable molecular alterations according to recommended treatment guidelines.
Figure 1. Clinical data of the patient. (a) Histological characteristic of the low-moderately differentiated adenocarcinoma of the gallbladder (hematoxylin-eosin stain; original magnification × 40). (b) Serum CA199 levels before and during treatment with SHR-1701 and famitinib (red arrow indicates the start of combined therapy). (c) Representative CT and MRI images of the abdomen in arterial phase (lesions are marked by triangles; the top row: metastatic lesion 1 in the liver; the middle row: metastatic lesion 2 in the liver; the bottom row: metastatic lesion in the retroperitoneal lymph nodes [red] and lesion previously treated with microwave ablation in the liver [blue]). (d) Timeline of treatment and clinical events.
![Figure 1. Clinical data of the patient. (a) Histological characteristic of the low-moderately differentiated adenocarcinoma of the gallbladder (hematoxylin-eosin stain; original magnification × 40). (b) Serum CA199 levels before and during treatment with SHR-1701 and famitinib (red arrow indicates the start of combined therapy). (c) Representative CT and MRI images of the abdomen in arterial phase (lesions are marked by triangles; the top row: metastatic lesion 1 in the liver; the middle row: metastatic lesion 2 in the liver; the bottom row: metastatic lesion in the retroperitoneal lymph nodes [red] and lesion previously treated with microwave ablation in the liver [blue]). (d) Timeline of treatment and clinical events.](/cms/asset/0c53f5d3-5e20-4a03-bea4-8dd38fad8723/khvi_a_2294575_f0001_oc.jpg)
On 21 October 2020, the patient was enrolled in a phase II trial (ChiCTR200003792), and began a combined immunotherapy regimen of SHR-1701 (30 mg/kg, q3w, IV) and famitinib (20 mg, qd, PO) for three-week cycles, upon signing informed consent. Tumor evaluations were conducted every 2 cycles. To enhance tolerance, the famitinib dosage was reduced to 15 mg starting from the third cycle. The patient’s serum CA199 levels, which had been rising throughout ablation and TACE, returned to normal upon the medication of SHR-1701 and famitinib, indicating a favorable response (). An abdomen CT after 3 months showed a partial response (PR) in all intra-hepatic lesions by RECIST v1.1 and she reached a complete response (CR) after 11 months (). After completing 24 cycles, and in line with the patient’s preference to take a break from intravenous therapy, SHR-1701 was discontinued and she transitioned to oral famitinib monotherapy for maintenance. Regarding adverse reactions (AEs), anemia was the only grade 3 toxicity observed (CTCAE v5.0), which resolved without specific treatment. Other recorded grade 2 AEs included thrombocytopenia, rash, proteinuria, and elevated AST/ALT levels, and a grade 1 hypoalbuminemia was also noted. All these events resolved following symptomatic management.
As of the latest follow-up in October 2023, the patient is still on famitinib and has sustained remission with an encouraging performance status. The patient has experienced a survival benefit of more than 3 years since initiating the combination immunotherapy ().
In post hoc analysis, formalin-fixed paraffin-embedded (FFEP) tissues of the primary tumor from this patient were used for whole-exome sequencing (WES; Supplementary Table S1) and RNA sequencing (Supplementary Table S2). Compared with the data from matched blood samples, a total of 2589 somatic coding mutations were identified. Putative RTK-associated mutations included intronic alternations of FGFR1 and FGFR2, exonic alternations of ABL2, MERTK, EPHA6, KIT, ROS1, EPHA1 and FLT3, and UTR3 mutations of EGFR. Additionally, we found mutations in TP53, KRAS, SMAD4, ARID2, BRAF, FAT4, KMT2D, NCOR1, NF1, ARID1B, GNAS, and RNF43, which are potentially relevant drivers in GBC.
Single-sample gene set enrichment analysis (ssGSEA) was performed to analyze Hallmark pathways from the MSigDB. We further use an R package “singscore” to visualize the pathway activity of a single pathway. Enrichment scores of the Hallmark pathways were evaluated, and the top-upregulated pathways were primarily enriched in androgen response, TGF-β signaling, and myc targets (, Supplementary Table S3). The rank distribution of genes in each of the three pathways was illustrated (). By the way, it was revealed that another patient with pancreatic cancer who achieved a CR in this trial also exhibited similar pathway analysis results at the transcriptional level (Supplementary Figure S1).
Figure 2. Hallmark pathway profile and changes of NLR for the patient. (a) The heatmap of Hallmark pathway score of this patient. The pathways highlighted in red represent the top common upregulated pathways. (b) The density plot of the three pathways for our patient, with barcodes indicating the gene distribution. (c) The boxplot displays the average value, while the error bar shows the standard error. Data were compared using paired samples t-test. NLR, neutrophil-to-lymphocyte ratio; *, p < 0.05.
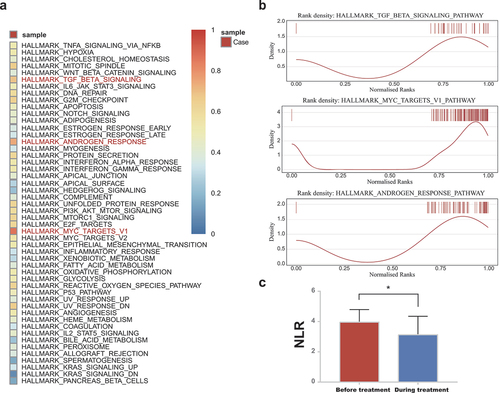
The neutrophil-to-lymphocyte ratio (NLR) is obtained by dividing the absolute neutrophil count by the lymphocyte count. The mean neutrophil count was 4.07 (SD, 2.96) *109/L and 3.23 (SD, 0.84) *109/L, the mean lymphocyte count was 1.07 (SD, 0.18) and 0.97 (SD, 0.12) *109/L, and the mean NLR was 3.96 (SD, 0.48) and 3.61 (SD, 1.29) before and during the trial ().
Discussion
Given the limited benefits of second-line chemotherapy for advanced BTC, we explored other therapeutic avenues, namely immunotherapy and molecularly targeted drugs, as the disease progressed.Citation8 The patient consequently received SHR-1701 and famitinib as experimental therapies.
To date, PD-L1 stands out as the primary biomarker used to guide patient selection for immunotherapy.Citation9 Nevertheless, PD-L1-negative tumors may also show durable responses to ICIs, as evidenced by multiple studies.Citation10–13 The method of measuring PD-L1 remains a topic of debate.Citation9 Microsatellite instability (MSI) is another marker recognized for predicting the effectiveness of ICIs in solid tumors. However, the selection of patients based solely on the PD-L1 expression and/or MSI profiles may exclude those who might benefit from immunotherapy.Citation14 Despite our patient’s tumor being PD-L1-negative with pMMR, SHR-1701 in combination with famitinib yielded a long-lasting positive outcome. Beyond ICIs, the roles of TGF-β and famitinib cannot be overlooked. The mechanisms underlying a durable CR merit further investigation.Citation15
Historically, difficulties in quantifying TME TGF-β and the levels of circulating latent TGF-β constrained its use as a response indicator to TGF-β inhibitors.Citation16 DNA variation is often emphasized when hunting for biomarkers, whereas information at an RNA level, which occasionally offers crucial hints, has been overlooked. In our study, using the Hallmark gene set for comparison at a single-sample FFEP RNA level, we found that the two CR patients consistently exhibited an upregulation of the TGF-β pathway. The detection of the TGF-β pathway in BTC or other solid tumor patients may pinpoint a group of patients likely to benefit from SHR-1701 and other TGF-β blockades, thus underscoring the potential of RNA sequencing and pathway enrichment scoring as a feasible screening strategy.
TGF-β signaling profoundly influences the tumor microenvironment (TME) by promoting tumor cell invasiveness, migration and metastasis, thereby fostering an immune-suppressive TME.Citation16 Preclinical studies have proved that TGF-β inhibition increased cytotoxic activity of T and NK cells, while reducing M2 macrophage infiltration in several tumor models.Citation17 The TGF inhibitor alone, however, is insufficient to completely eradicate the tumor without the help of the PD-L1 inhibition,Citation18 which highlights the advantage of SHR-1701 as an effective immunotherapy that targets patients with “immune desert” GBC. It makes sense that both infiltrating lymphocytes in TME and circulating lymphocyte counts are emerging as prognostic biomarkers in current anti-cancer immunotherapy. The mean NLR of our patient shifted from 3.96 to 3.61 before and during the trial, indicating the effectiveness of combined immunotherapy. Studies have shown that an NLR of more than 5 is a robust independent predictor of unfavorable outcomes in renal cell carcinoma, melanoma, and non-small cell lung cancer patients under immunotherapy,Citation19 which aligns with our observations.
It’s noteworthy that the patient has maintained a consistent therapeutic response to famitinib over the past 14 months. The durable response might be attributed to the genetic mutations of FGFR1, FGFR2, KIT, and FLT3 aberrations observed in WES data, which are target sites of famitinib. Famitinib has exhibited inhibitory effects on FGFR, most notably against FGFR2 with IC50 values as low as 23 nM.Citation20 Furthermore, intricate interactions exist between pathways across various RTK families. In preclinical models, famitinib also inhibits the proliferation of A431, SK-BR-3, and K562 cells, which highly express EGFR and ERBB.Citation21 Therefore, mutations in RTKs that aren’t direct targets of famitinib, such as the EGFR aberrations identified in our patient, might also enhance its efficacy. Overall, famitinib shows promising antitumor activity in BTC.
BTC presents with more actionable mutations compared to other gastrointestinal cancers.Citation22 Despite being included in the same group called BTC in most clinical trials, GBC and cholangiocarcinoma have unique genome profiles owing to the different anatomical subsites. This case highlights that high-throughput sequencing coupled with bioinformatics analysis may facilitate precision treatment in targeted therapy or immunotherapy for GBC patients.
This is the first report of such a treatment regimen being applied in a clinical setting. When RTK-associated mutations are found at the DNA level and the TGF-β pathway is detected upregulated at the RNA level, SHR-1701 plus famitinib may be a promising choice for refractory advanced GBC patients, which is expected to change the subsequent-line treatment paradigm of GBC and more information will be confirmed by our ongoing phase II clinical trial.
Author contributions
JX and ZQM conceived and designed the study. LXY analyzed the data and drafted the manuscript. XYZ recommended the patient’s participation in the clinical trial at the time of initial diagnosis.
Ethics statement
The study was conducted in compliance with the Declaration of Helsinki and applicable regulatory requirements. The patient’s publication consent was obtained before submitting the manuscript to the journal.
Abbreviations
| BTC | = | Biliary Tract Cancer |
| GBC | = | Gallbladder Cancer |
| ICI | = | Immune Checkpoint Inhibitor |
| PD-L1 | = | Programmed Death-ligand 1 |
| TGF-β | = | Transforming Growth Factor |
| RTK | = | Receptor Tyrosine Kinase |
| CR | = | Complete Response |
| pMMR | = | Proficient Mismatch Repair |
| FFEP | = | Formalin-Fixed Paraffin-Embedded |
| dMMR | = | Deficient Mismatch Repair |
| IHC | = | Immunohistochemistry |
| MSI | = | Microsatellite Instability |
| NCCN | = | National Comprehensive Cancer Network |
| NLR | = | Neutrophil-to-Lymphocyte Ratio |
| ORR | = | Overall Response Rate |
| PD | = | Progression of Disease |
| PFS | = | Progression-Free Survival |
| PR | = | Partial Response |
| TACE | = | Transarterial Chemoembolization |
| TME | = | Tumor Microenvironment |
| WES | = | Whole-Exome Sequencing |
| AJCC | = | American Joint Committee on Cancer |
| RECIST | = | Response Evaluation Criteria in Solid Tumors |
| CTCAE | = | National Cancer Institute Common Terminology Criteria |
Supplementary Figures.pdf
Download PDF (1.8 MB)Supplementary Tables.pdf
Download PDF (2.9 MB)Acknowledgments
The authors would like to thank the patients, their families, and the study personnel involved in this trial.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data needed are presented in the article and the supplementary materials, or can be asked via the leading corresponding author (J. Xie).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2294575.
Additional information
Funding
References
- Valle JW, Kelley RK, Nervi B, Oh D-Y, Zhu AX. Biliary tract cancer. Lancet. 2021;397:428–5. doi: 10.1016/S0140-6736(21)00153-7.
- Rizzo A, Brandi G. First-line chemotherapy in advanced biliary tract cancer Ten years after the ABC-02 trial: “and yet It Moves!”. Cancer Treat Res Commun. 2021;27:100335. doi: 10.1016/j.ctarc.2021.100335.
- Zhou J, Sun Y, Zhang W, Yuan J, Peng Z, Wang W, Gong J, Yang L, Cao Y, Zhao H, et al. Phase b study of anlotinib combined with TQB2450 in pretreated advanced biliary tract cancer and biomarker analysis. Hepatology. 2023;77(1):65–76. doi: 10.1002/hep.32548.
- Cousin S, Cantarel C, Guegan JP, Mazard T, Gomez-Roca C, Metges J-P, Bellera C, Adenis A, Korakis I, Poureau P-G, et al. Regorafenib–avelumab combination in patients with biliary tract cancer (REGOMUNE): a single-arm, open-label, phase II trial. Eur J Cancer. 2022;162:161–9. doi: 10.1016/j.ejca.2021.11.012.
- Lin J, Yang X, Long J, Zhao S, Mao J, Wang D, Bai Y, Bian J, Zhang L, Yang X, et al. Pembrolizumab combined with lenvatinib as non-first-line therapy in patients with refractory biliary tract carcinoma. Hepatobiliary Surg Nutr. 2020;9(4):414–24. doi: 10.21037/hbsn-20-338.
- Lind H, Gameiro SR, Jochems C, Donahue RN, Strauss J, Gulley JL, Palena C, Schlom J. Dual targeting of TGF-β and PD-L1 via a bifunctional anti-PD-L1/TGF-βRII agent: status of preclinical and clinical advances. J Immunother Cancer. 2020;8:e000433. doi: 10.1136/jitc-2019-000433.
- Xia L, Peng J, Lou G, Pan M, Zhou Q, Hu W, Shi H, Wang L, Gao Y, Zhu J, et al. Antitumor activity and safety of camrelizumab plus famitinib in patients with platinum-resistant recurrent ovarian cancer: results from an open-label, multicenter phase 2 basket study. J Immunother Cancer. 2022;10(1):2022. doi: 10.1136/jitc-2021-003831.
- Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, Falk S, Gillmore R, Wadsley J, Patel K, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22(5):690–701. doi: 10.1016/S1470-2045(21)00027-9.
- Grossman JE, Vasudevan D, Joyce CE, Hildago M. Is PD-L1 a consistent biomarker for anti-PD-1 therapy? The model of balstilimab in a virally-driven tumor. Oncogene. 2021;40:1393–5. doi: 10.1038/s41388-020-01611-6.
- Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65. doi: 10.1016/S0140-6736(16)32517-X.
- Kefford R, Ribas A, Hamid O, Robert C, Daud A, Wolchok JD, Joshua AM, Hodi FS, Gangadhar TC, Hersey P, et al. Clinical efficacy and correlation with tumor PD-L1 expression in patients (pts) with melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. J Clin Oncol. 2014;32:. doi: 10.1200/jco.2014.32.15_suppl.3005.
- Mehra R, Seiwert TY, Gupta S, Weiss J, Gluck I, Eder JP, Burtness B, Tahara M, Keam B, Kang H, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer. 2018;119(2):153–9. doi: 10.1038/s41416-018-0131-9.
- Naumann RW, Hollebecque A, Meyer T, Devlin M-J, Oaknin A, Kerger J, López-Picazo JM, Machiels J-P, Delord J-P, Evans TRJ, et al. Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: results from the phase I/II CheckMate 358 trial. J Clin Oncol. 2019;37(31):2825–34. doi: 10.1200/JCO.19.00739.
- Dos Santos Fernandes G, da Motta Girardi D, Dib Batista Bugiato Faria L, Giacomini Bernardes JP, de Almeida Coudry R. Impressive response to immunotherapy in a metastatic gastric cancer patient: could somatic copy number alterations help patient selection? J Immunother Cancer. 2017;5:84. doi: 10.1186/s40425-017-0291-9.
- Santoni M, Rizzo A, Kucharz J, Mollica V, Rosellini M, Marchetti A, Tassinari E, Monteiro FSM, Soares A, Molina-Cerrillo J, et al. Complete remissions following immunotherapy or immuno-oncology combinations in cancer patients: the MOUSEION-03 meta-analysis. Cancer Immunol Immunother. 2023;72(6):1365–79. doi: 10.1007/s00262-022-03349-4.
- Tauriello DVF, Sancho E, Batlle E. Overcoming TGFβ-mediated immune evasion in cancer. Nat Rev Cancer. 2022;22(1):25–44. doi: 10.1038/s41568-021-00413-6.
- Panagi M, Voutouri C, Mpekris F, Papageorgis P, Martin MR, Martin JD, Demetriou P, Pierides C, Polydorou C, Stylianou A, et al. TGF-β inhibition combined with cytotoxic nanomedicine normalizes triple negative breast cancer microenvironment towards anti-tumor immunity. Theranostics. 2020;10(4):1910–22. doi: 10.7150/thno.36936.
- Lan Y, Moustafa M, Knoll M, Xu C, Furkel J, Lazorchak A, Yeung T-L, Hasheminasab S-M, Jenkins MH, Meister S, et al. Simultaneous targeting of TGF-β/PD-L1 synergizes with radiotherapy by reprogramming the tumor microenvironment to overcome immune evasion. Cancer Cell. 2021;39(10):1388–403.e10. doi: 10.1016/j.ccell.2021.08.008.
- Capone M, Giannarelli D, Mallardo D, Madonna G, Festino L, Grimaldi AM, Vanella V, Simeone E, Paone M, Palmieri G, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6:74. doi: 10.1186/s40425-018-0383-1.
- Salati M, Caputo F, Baldessari C, Carotenuto, P, Messina, M, Caramaschi, S, Dominici, M, Bonetti, L.R. The evolving iole of FGFR2 inhibitors in intrahepatic Cholangiocarcinoma: from molecular biology to clinical targeting. Cancer Manag Res. 2021;13:7747–57. doi: 10.2147/CMAR.S330710.
- Xie C, Zhou J, Guo Z, Diao X, Gao Z, Zhong D, Jiang H, Zhang L, Chen X. Metabolism and bioactivation of famitinib, a novel inhibitor of receptor tyrosine kinase, in cancer patients. Br J Pharmacol. 2013;168:1687–706. doi: 10.1111/bph.12047.
- Zhang Y, Zuo C, Liu L, Hu Y, Yang B, Qiu S, Li Y, Cao D, Ju Z, Ge J, et al. Single-cell RNA-sequencing atlas reveals an MDK-dependent immunosuppressive environment in ErbB pathway-mutated gallbladder cancer. J Hepatol. 2021;75(5):1128–41. doi: 10.1016/j.jhep.2021.06.023.
