ABSTRACT
We assessed the non-inferiority of homologous boosting compared with heterologous boosting with the recombinant protein vaccine, SCB-2019, in adults previously immunized with different COVID-19 vaccines. Three equal cohorts (N ~ 420) of Philippino adults (18–80 years) previously immunized with Comirnaty, CoronaVac or Vaxzevria COVID-19 vaccines were randomized 1:1 to receive homologous or heterologous (SCB-2019) boosters. Neutralizing antibodies against prototype SARS-CoV-2 (Wuhan-Hu-1) were measured in all participants and against Delta variant and Omicron sub-lineages in subsets (30‒50 per arm) 15 days after boosting. Participants recorded solicited adverse events for 7 days and unsolicited and serious adverse events until Day 60. Prototype SARS-CoV-2 neutralizing responses on Day 15 after SCB-2019 were statistically non-inferior to homologous Vaxzevria boosters, superior to CoronaVac, but lower than homologous Comirnaty. Neutralizing responses against Delta and Omicron BA.1, BA.2, BA.4 and BA.5 variants after heterologous SCB-2019 were higher than homologous CoronaVac or Vaxzevria, but lower than homologous Comirnaty. Responses against Omicron BF.7, BQ.1.1.3, and XBB1.5 following heterologous SCB-2019 were lower than after homologous Comirnaty booster but significantly higher than after Vaxzevria booster. SCB-2019 reactogenicity was similar to CoronaVac or Vaxzevria, but lower than Comirnaty; most frequent events were mild/moderate injection site pain, headache and fatigue. No vaccine-related serious adverse events were reported. Heterologous SCB-2019 boosting was well tolerated and elicited neutralizing responses against all tested SARS-COV-2 viruses including Omicron BA.1, BA.2, BA.4, BA.5, BF.7, BQ.1.1.3, and XBB1.5 sub-lineages that were non-inferior to homologous boosting with CoronaVac or Vaxzevria, but not homologous Comirnaty booster.
Background
COVID-19 has now become a globally endemic disease with continuing infections due to the continuing emergence of new variants of the SARS-CoV-2 virus.Citation1 These new variants have successively accumulated mutations in the spike protein (S-protein), the primary antigenic target of COVID-19 vaccines, and so are becoming less susceptible to the immunity raised by the first-generation vaccines based on the prototype Wuhan-Hu-1 strain.Citation2–4 Such immune evasion, together with the natural waning of antibodies elicited by primary and booster vaccinations, is decreasing the protective efficacy of the first approved COVID-19 vaccines.Citation5,Citation6 Although new monovalent and bivalent formulation mRNA vaccines have been developed against the newly emerged SARS-CoV-2 variants which have been recommended by the FDA for annual revaccination.Citation7 However, as seen during the pandemic, it is unlikely that adequate supplies of these vaccines will be available to meet global demand. Further, the actual effectiveness of such vaccines has been questioned if they are not matched to the current variant.Citation8 Consequently, new booster regimens using heterologous vaccines are being explored to try to broaden the immune response to combat the new variants.Citation9–15
During the pandemic, a wide variety of various types of COVID-19 vaccines were developed and several were implemented in national campaigns as soon as they achieved WHO approval, meaning that different ones were often used in the same country at the same time.Citation16 This was the case in the Philippines, where WHO-approved vaccines included the mRNA vaccine, Comirnaty® (Pfizer/BioNTech), the whole inactivated virus vaccine, CoronaVac® (SinoVac BioTech), and the adenovirus-vector vaccine, Vaxzevria® (AstraZeneca-Oxford).
Clover Biopharmaceuticals has developed a COVID-19 vaccine, SCB-2019, containing a recombinant, pre-fusion form of the trimeric SARS-CoV-2 spike protein (S-protein) derived from the prototype strain (Wuhan-Hu-1) maintained in its trimeric structure using the proprietary Trimer-Tag® technology.Citation17 In the SPECTRA efficacy study, two priming doses of SCB-2019 were shown to elicit a protective efficacy of 67.2% (95·72% CI: 54.3–76.8) against any COVID-19 disease and 100% (97.86% CI: 25.3–100) against severe COVID-19 including hospitalizations at a time when Beta, Delta and Gamma variants were circulating.Citation18 In a previous study, heterologous SCB-2019 boosting was shown to induce high levels of neutralizing antibodies against both prototype and variant SARS-CoV-2 viruses in Brazilian adults who previously received two doses of Vaxzevria.Citation9 Healthcare decision-makers, faced with rising cases due to circulation of the most recent variants, must now plan booster immunization campaigns against a background of a variety of different vaccines used in primary immunization. To inform those decisions, we have now investigated the impact of heterologous boosting with SCB-2019 compared with homologous boosters in cohorts of Philippino adults who were previously immunized with two doses of either Comirnaty, CoronaVac or Vaxzevria vaccines.
Methods
This multi-center, observer-blind, randomized, controlled, phase 3 study is ongoing in the Philippines to assess SCB-2019 when administered as a heterologous booster dose to adults previously immunized with different licensed COVID-19 vaccines, in comparison with homologous booster doses of those vaccines. The study was registered on ClinicalTrials.gov (NCT05188677), and the protocol was approved by the Philippines Food and Drug Administration and all applicable institutional review boards (Supplementary Table S1). The study is being conducted in accordance with international guidelines including the Declaration of Helsinki and Council for International Organizations of Medical Sciences (CIOMS), and ICH GCP. The primary objective was to demonstrate the non-inferiority of the immune response of a heterologous booster dose of SCB-2019 with a homologous booster dose in adults immunized with two doses of either the mRNA vaccine, Comirnaty®, the inactivated whole virus vaccine, CoronaVac®, or the adenovirus-vector vaccine, Vaxzevria®. This report also presents comparisons of the safety and reactogenicity of a heterologous booster dose of SCB-2019 with homologous boosters up to 60 days following vaccination and the immunogenicity against a panel SARS-CoV-2 variants.
Participants were male or female adults ≥18 years of age with a documented history of two immunizations with one of the indicated licensed COVID-19 vaccines, with the last dose received at least three months previously. Eligibility criteria included being healthy or having a stable preexisting medical condition, being willing and able to comply with all study requirements and visits, and provision of informed consent. Major exclusion criteria were any fever (axillary temperature ≥37.5°C) or acute illness at the first visit, a laboratory-confirmed SARS-CoV-2 infection detected by rapid antigen testing (RAT) or RT-PCR at the first visit, previous receipt of a COVID-19 vaccine other than Comirnaty, CoronaVac or Vaxzevria, any history of adverse events associated with vaccination or known allergy to any vaccine component, or receipt of any other investigational product within 30 days of study start. Prior confirmed or suspected SARS-CoV-2 infection was not an exclusion criterion.
Vaccines
Each 0.5 mL dose of the vaccine, SCB-2019 (Clover Biopharmaceuticals, Changxing, China), contains 30 μg SCB-2019 recombinant protein adjuvanted with 1.50 mg of the toll-like receptor agonist, CpG-1018 (Dynavax Technologies, Emeryville, CA, USA), and 0.75 mg aluminum hydroxide (Thousand Oaks Biopharmaceuticals, USA). Each 0.3 mL dose of Comirnaty® (BioNTech-Pfizer, Mainz, Germany) contains 30 μg of tozinameran, a COVID-19 mRNA vaccine embedded in lipid nanoparticles. CoronaVac® (SinoVac Life Sciences Co. Ltd., Beijing, China) contains 600 SU inactivated SARS-CoV-2 virus (CZ02 strain) and 0.225 mg aluminum hydroxide in 0.5 mL phosphate-buffered saline per dose. Each 0.5 mL dose of Vaxzevria® (AstraZeneca-Oxford, UK) contains at least 2.5 × 108 infectious units of Chimpanzee adenovirus encoding SARS-CoV-2 spike glycoprotein (ChAdOx1S). Vaccines were administered by intramuscular injection in the deltoid of the non-dominant arm.
Procedures
The study was conducted in three parts with volunteers previously vaccinated with two doses of one of three different vaccines; part 1, Comirnaty; part 2, CoronaVac; part 3, Vaxzevria. All three parts were conducted concomitantly, but because of these different immunization backgrounds each part was recruited and randomized separately. On Day 1 enrolled participants in each cohort were randomly allocated 1:1 to two equal treatment groups (six groups in total) to receive either a homologous booster dose of Comirnaty, CoronaVac or Vaxzevria accordingly, or a heterologous booster dose of SCB-2019. After monitoring for 30 minutes, participants then used an electronic study diary to record any solicited local reactions or systemic adverse events (AE) with severity (mild, moderate, severe) and their axillary temperature daily for 7 days (Supplementary Table S1). Unsolicited adverse events were recorded up to a telephone follow-up on Day 43. Any serious adverse event (SAE), defined as death, or an AE that was life-threatening or led to hospitalization or persistent incapacity, or adverse events of special interest (AESI), defined as an AE potentially associated with COVID-19 or an autoimmune disorder, was to be reported immediately to the investigator throughout the study. Safety follow-up is ongoing for up to six months after vaccination, but this interim report only covers the first 60 days.
Immunogenicity analyses
Sera prepared immediately from blood samples drawn before vaccination on Day 1 and at a second visit on Day 15 were stored at −80°C for shipping to VisMederi srl (Siena, Italy) for the immunogenicity analyses. Immunogenicity was measured in all sera from Days 1 and 15 as virus neutralizing antibodies in a microneutralization assay using prototype Wuhan-Hu-1 SARS-CoV-2,Citation9 and titers were converted into International Units by calibration with the WHO standard 21/234. Neutralizing responses were also measured in subsets of each vaccine group (n = 50 per subset) against the SARS-CoV-2 variants, Delta (B.1.617.2) and Omicron sub-lineages BA.1, BA.2, BA.4, and BA.5. Subsets (n = 30) of the Comirnaty- and Vaxzevria-primed groups were also tested for neutralizing titers against Omicron BF.7, BQ1.1.3 and XBB1.5 variants after homologous and heterologous boosting. Responses against these most recent variants have been further investigated in a separate cohort of CoronaVac-primed and boosted participants which has been reported separately.Citation19 Responses against variants were expressed as microneutralization titers (1/dil), the reciprocal of the lowest dilution protecting 80% of the cells and presented as geometric mean titers (GMTs) at Days 1 and 15 and geometric mean-fold rises (GMFR) from Day 1 to Day 15.
Statistics
The primary immunogenicity objectives were to demonstrate non-inferiority of a heterologous SCB-2019 boost compared with either a homologous Comirnaty, CoronaVac or Vaxzevria booster dose when measured as neutralizing response against prototype Wuhan-Hu-1 SARS-CoV-2. Non-inferiority would be met if the lower limit of the two-sided 95% confidence interval (CI) for the ratio of geometric mean titers (GMT) between any of the three pairs of groups (SCB-2019 GMT to homologous GMT) was above 0.667 calculated using an ANCOVA model. Assuming a 5% drop-out, 212 enrolled subjects in each of the six treatment groups would provide 95.6% power to claim such non-inferiority of SCB-2019 booster to each homologous booster. When non-inferiority was confirmed, superiority was observed if the lower limit of the two-sided 95% CI for the ratio of GMT in the two groups (SCB-2019 GMT to homologous GMT) was 1.5 or greater. The primary immunogenicity analysis was performed on all those who complied with the protocol and provided Day 15 blood samples within the prescribed time windows.
Secondary immunogenicity objectives were to evaluate the neutralizing responses against SARS-CoV-2 variants in subsets of participants from the homologous and heterologous groups. GMT ratios were assessed using an analysis of covariance (ANCOVA) model, including baseline antibody results and age as covariates, and vaccine treatment group as a fixed variable. Two-sided 95% CIs for GMT ratio were obtained by anti-log of the confidence limits for the mean difference of the logarithmically transformed assay results which is calculated using t-distribution for the primary and the secondary immunogenicity objective analyses.
The reactogenicity and safety objectives were descriptive comparisons of incidence rates of solicited local reactions and systemic AEs through Day 7, unsolicited AEs up to Day 29, and SAEs and AESIs up to Day 60 in the six study groups. All participants who received their assigned dose of vaccine (Safety Set) were included in the reactogenicity and safety analyses.
Results
Participants for the three parts of the study were enrolled and randomized to the six different treatment groups and vaccinated accordingly from June 13, 2022, to October 31, 2022. A total of 1331 volunteers were screened and 1330 enrolled and assigned to the six groups as shown in . The demographic characteristics were similar across treatment groups (). Following checking of inclusion/exclusion criteria 1309 vaccinated participants were eligible for the Per Protocol immunogenicity analyses: 206, 211 and 211 after homologous boosting with Comirnaty, CoronaVac and Vaxzevria, respectively, and 214, 214 and 212 for heterologous boosting with SCB-2019, respectively ().
Figure 1. Disposition of participants in Safety Set and per protocol immunogenicity sets of the three study phases.
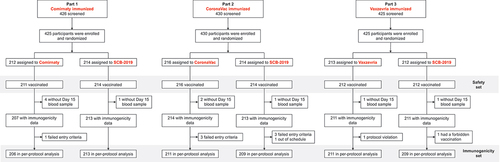
Table 1. Demographics of the exposed study populations (Safety Set).
Immunogenicity
Baseline immunogenicity varied between the three parts of the study due to the different vaccines used for primary immunization but was comparable in the respective homologous and heterologous groups (). Thus, the baseline GMTs against prototype SARS-CoV-2 were 363 and 374 IU/mL following priming with Comirnaty, 247 and 237 IU/mL in the Vaxzevria-immunized groups and 131 and 125 IU/mL in the CoronaVac-primed groups.
Table 2. Viral neutralizing antibody (VNA) titers expressed as international units per mL (95% CI) at Day 15 in the study groups (Per Protocol Sets).
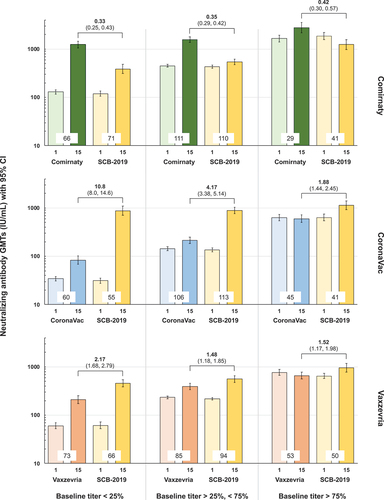
The study was successful as the primary immunogenicity objective for non-inferiority of the response against the prototype Wuhan-Hu-1 virus at Day 15 following heterologous boosting with SCB-2019 was met in parts 2 and 3, for individuals primed with CoronaVac or Vaxzevria. Non-inferiority was not met in part 1 in participants primed with Comirnaty (). Day 15 neutralizing GMTs against prototype SARS-CoV-2 were 203 IU/mL (182–227) after homologous CoronaVac and 939 IU/mL (95% CI: 841–1049) after heterologous SCB-2019. The GMT ratio (GMT SCB-2019/GMT CoronaVac) was 4.63 (95% CI: 3.96–5.41), so meeting the non-inferiority criterion as the lower 95% limit was greater than 0.667 (). Further, as the lower 95% limit of the GMT ratio was greater than 1.5 the response to SCB-2019 was superior to that observed with the CoronaVac booster. In the Vaxzevria cohort Day 15 GMTs were 448 IU/mL (95% CI: 405–494) after a homologous booster and 751 IU/mL (95% CI: 680–829) after heterologous SCB-2019, with a GMT ratio (GMT SCB-2019/GMT Vaxzevria) of 1.68 (95% CI: 1.46–1.93). The lower 95% limit was greater than 0.667 but marginally less than 1.5 showing non-inferiority of SCB-2019 as booster compared with Vaxzevria, but not superiority. Finally, Day 15 GMTs were 1586 IU/mL (95% CI: 1346–1750) following homologous Comirnaty and 569 IU/mL (516–627) after heterologous SCB-2019, with a homologous/heterologous GMT ratio of 0.36 (95% CI: 0.31, 0.41) so failing to meet the non-inferiority criterion (). Although lower than the homologous response to Comirnaty the heterologous response to SCB-2019 represented a 1.53-fold increase in GMT and achieved a higher GMT than observed following homologous booster doses in CoronaVac and Vaxzevria-primed cohorts.
Sensitivity analysis
As there was a wide range in baseline immunity in and between groups due to the different priming vaccines and also due to natural exposure we performed a sensitivity assessment to observe the impact in participants stratified into three groups with low, medium and high baseline titers, illustrated in : 25% with the lowest baseline neutralizing antibody titer, 50% with intermediate titers and 25% with the highest titers. As shown, heterologous SCB-2019 increased titers to a lesser extent that the homologous Comirnaty booster irrespective of baseline immunity. On the other hand, the post-booster GMT was over tenfold higher after heterologous boosting of CoronaVac-primed participants with low baseline immunity who are of higher risk of reinfection and over fourfold higher in the intermediate group. This difference between heterologous and homologous boosting was greater in those with low baseline immunity following either CoronaVac or Vaxzevria priming.
Figure 2. Anti-prototype SARS-CoV-2 neutralizing GMTs (IU/mL) with 95% CI at Days 1 and 15 in the three parts of the study according to baseline immunity (Per Protocol Set). Participants are grouped as low (25% with lowest titers), medium (50% with medium titers) and high (25% with highest titers) baseline. Heterologous to homologous GMT ratios (95% CI) at Day 15 are shown above columns; values at bases of columns show numbers of participants per group.
Immunogenicity against SARS-CoV-2 variants
Sera from subsets (n = 49 or 50) of participants from all groups were assessed for neutralizing activity against five major circulating SARS-CoV-2 variants, the Delta (B.1.617.2) variant and Omicron sub-lineages BA.1, BA.2, BA.4, and BA.5. In addition, immune responses against the three most recent variants, Omicron BF.7, BQ.1.1.3 and XBB1.5, were assessed in subsets (n = 30) of participants previously immunized with Comirnaty or Vaxzevria. Results for these recent Omicron variants have been assessed in another cohort of adults who had previously received three doses of CoronaVac, two primary and one booster, which have been reported separately.Citation19
As illustrated in , there is a trend for baseline neutralizing activity to be progressively lower against the sequence of Omicron variants with initially high baseline titers against the first Omicron variant, BA.1, to very low levels against the most recent variant, XBB.1.5. Against this background the profile of immune responses was similar to that observed against the prototype SARS-CoV-2 for both homologous and heterologous boosters. Pre- and post-booster GMTs against the early pandemic variant, Delta, were generally consistent with those against prototype virus in all three parts, as were the GMFR post-boosting (). Notably, the responses to homologous Comirnaty boosters compared with the heterologous SCB-2019 booster were higher, illustrated by GMTs of 266 (95% CI: 213–332), 339 (273–442) and 184 (148–248) for Omicron BF.7, BQ.1.1.3, and XBB1.5 sub-lineages after Comirnaty compared with 73.8 (95% CI: 52.6–103), 80.9 (60.6–108) and 45.9 (30.6–68.8) after the heterologous booster (Supplementary Table S2b). Conversely, SCB-2019 elicited higher responses [113 (95% CI: 80.5–159), and 63.5 (41.6–96.5)] than homologous Vaxzevria [71.3 (95% CI: 46.6–109)) and 22.7 (16.4–31.5)] for the Omicron BF.7 and XBB.1.5 sub-lineages, with similar results for Omicron BQ.1.1.3 [133 (96.4–184) and 130 (93.2–181)], respectively.
Figure 3. neutralizing GMTs (95% CI) against prototype SARS-CoV-2 and variants at Days 1 and 15 in subsets from the three parts of the study (Omicron BF.7, BQ.1.1.3 and XBB1.5 were not tested in the CoronaVac cohort).
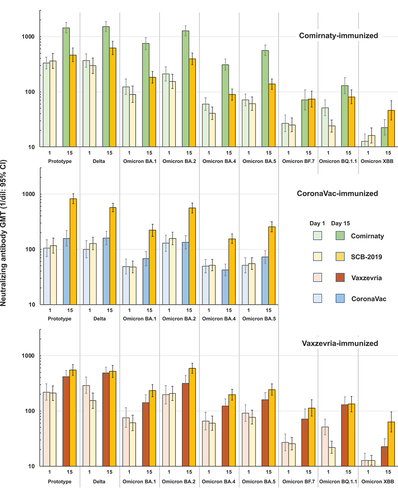
Table 3. Geometric mean-fold rises (95% CI) of neutralizing antibodies against prototype and SARS-CoV-2 variants from Day 1 to Day 15.
Safety and reactogenicity
There were no deaths or immediate vaccine reactions, or any withdrawals or early study terminations due to an adverse event or AESI up to Day 60 () and long-term safety follow-up is ongoing. One SAE reported in the CoronaVac group, an acute kidney injury, was not considered to be related to the vaccination by the investigator. Medically attended adverse events were infrequent and were balanced across groups.
Table 4. Incidences of solicited and unsolicited adverse events, SAEs, MAAEs and AESIs (Safety Set).
Rates and severity of solicited local reactions and systemic adverse events in the 7 days post-vaccination are illustrated in for parts 1, 2 and 3. Generally, SCB-2019 as heterologous booster displayed a consistent reactogenicity profile in the three cohorts primed with Comirnaty, CoronaVac or Vaxzevria; 18.7–22% of the SCB-2019 groups reported a local reaction. In contrast, local reactions were reported after 36.5%, 10.6% and 20.3% of homologous booster doses of Comirnaty, CoronaVac and Vaxzevria, respectively (). Except for one case of mild swelling reported after SCB-2019 in the CoronaVac cohort all reported local reactions against each of the four vaccines were pain at the injection site, mostly mild or moderate in severity. All local reactions were transient and resolved within the reporting period.
Figure 4. Reactogenicity as proportions of each group in the three parts of the study with solicited local reactions and systemic adverse events, shown with severity.
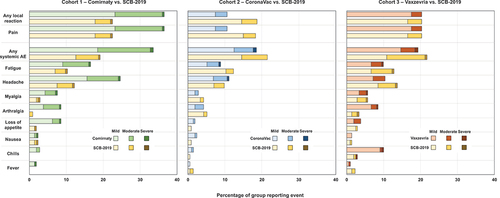
Solicited systemic adverse events were reported by 18.0–21.7% of the heterologous SCB-2019 groups compared with rates of 33.6%, 18.5% and 19.3% after Comirnaty, CoronaVac and Vaxzevria homologous boosters, respectively (). In most cases these were reported as mild or moderate, with single instances of severe cases. The most frequent systemic adverse events were headache and fatigue ().
Unsolicited adverse events in the 43 days after vaccination were reported at similar rates in the different groups, the highest rate of 13.0% was in the CoronaVac group, the lowest rate of 6.6% was in the Comirnaty group (). Few unsolicited AEs were either severe or considered to be related to the vaccination.
Discussion
This study was successful in showing that neutralizing antibody responses to heterologous boosting with SCB-2019 vaccine in adults primed with different COVID-19 vaccines at least three months earlier were non-inferior to those against homologous CoronaVac and Vaxzevria boosters, but not against a homologous Comirnaty booster. Indeed, the response to SCB-2019 was statistically superior to CoronaVac and in the Vaxzevria group only marginally failed to meet the conservative superiority criterion used. If the less conservative but frequently used value of 1.0 for the lower limit of the two-sided 95% CI for the GMT ratio had been selected, then superiority would have been shown for SCB-2019 over both CoronaVac and Vaxzevria. More importantly, in all cases heterologous boosting with SCB-2019 led to high levels of neutralizing activity, not only against prototype (Wuhan-Hu-1) SARS-CoV-2 virus, but also against major variants of SARS-CoV-2 including Delta and Omicron variants, which for Vaxzevria and Comirnaty included the currently predominating Omicron BF.7, BQ.1.1.3, and XBB1.5 sub-lineages. These higher responses to SCB-2019 against these recent Omicron sub-lineages have also been observed in a separately reported part of this study in participants who had previously received three doses of CoronaVac.Citation19 This is an important consideration in view of the current epidemiology of COVID-19, and most notably as the most recent strain tested, Omicron XBB.1.5, has been described by the WHO as the most transmissible and the main cause of current outbreaks.Citation20 Titers post-booster with SCB-2019 were consistent with those following two priming doses of SCB-2019 in the SPECTRA efficacy study.Citation18 These immune responses were achieved with equivalent or better reactogenicity to SCB-2019 than homologous boosting.
As successive variants accumulate mutations in the S-protein, the primary antigenic target of most COVID-19 vaccines,Citation2 they can evade the immunity induced by vaccination.Citation3,Citation4 The S-protein plays a key role in cellular identification, viral fusion and uptake and release of new viral copiesCitation2; it contains the receptor-binding domain (RBD) which interacts with the cellular angiotensin-converting enzyme 2 receptor (ACE2) to allow cell entry.Citation21 Mutations in the RBD region in Omicron variants have been shown to improve binding to ACE2.Citation22 As such, RBD is a key antigen target and RBD mutations lead to Omicron evading vaccine-induced protective antibodies against RBD.Citation23 When exacerbated by waning vaccine-derived immunity, this makes the use of boosters a necessity to maintain high levels of population immunity to try to restrict transmission and circulation of SARS-CoV-2 variants and so contain new outbreaks. There are many potential combinations of different vaccines with heterologous boosting, and the data presented here are part of an ongoing larger project in collaboration with the Coalition for Epidemic Preparedness Innovations (CEPI) to inform on some of the potential combinations using the recombinant protein vaccine, SCB-2019, as a heterologous booster. As noted above, another part of this large project has already been published on the use of SCB-2019 in populations previously immunized with CoronaVac to immediately inform vaccination guidance in countries where primary immunization was with CoronaVacCitation19; some results from that study are reproduced here to allow direct comparison with the mRNA and adenovirus-vector vaccines. Generally, heterologous boosting has been found to be more effective than homologous boosters with the exception of mRNA-primed populations,Citation9–15,Citation20,Citation24–28 and our observations with SCB-2019 are consistent with those studies, showing homologous boosters are superior to heterologous boosters in mRNA-primed populations.Citation12 However, vaccine supplies may vary as original stockpiles are exhausted, some previous vaccines are no longer being manufactured, renewed government tenders result in purchases of different vaccines, and public skepticism about novel technologies as mRNA and vectors have risen due to single but highly publicized reports of adverse events. It is important, therefore, that booster campaigns can be performed with confidence in the knowledge that in a population previously vaccinated with a variety of different vaccines, the available booster vaccine(s) will safely elicit a clinically meaningful increase in immunity for current and future variants, and that the immunized populations have the choice between different vaccine platforms to encourage high coverage.
Faced with the predominance of Omicron as circulating variant and the rapid evolution and emergence of new Omicron sub-lineages, the most important assessment is the impact of heterologous boosting against such variants. So, it is comforting to observe that various heterologous boosting regimens improve neutralizing immunity against the tested Omicron sub-lineages.Citation28–31 This is not necessarily the case with new bivalent variant-matched mRNA boosters.Citation8 Our observations with SCB-2019 are consistent with the use of SCB-2019 as booster in Brazilian adults primed at least 6 months previously with Vaxzevria.Citation9 That study found heterologous SCB-2019 was more immunogenic against Delta and Omicron (B.1.1.529) than homologous Vaxzevria.Citation9 The data we present here confirm this observation for seven sub-lineages of Omicron, including the recent Omicron XBB.1.5 sub-lineage.Citation20 SCB-2019 boosting elicited at least a two-fold increase in GMT for all tested variants in Comirnaty-primed participants, and 3- to 7-fold increases in CoronaVac- and Vaxzevria-primed participants.
Heterologous boosters have been reported to induce higher rates of some systemic adverse events, notably fever, myalgia, malaise and fatigue,Citation20,Citation25,Citation32 but we found that boosting with SCB-2019 was equally well or better tolerated than homologous boosting, particularly when compared with the Comirnaty booster which induced the highest rates of local pain and headache in this study. However, any differences in the three regimens were transient and with no meaningful clinical significance.
One limitation of our study is the short duration of post-vaccination response assessed, 15 days after the booster dose. It has been reported that immunity begins to wane within one month of completion of a primary seriesCitation5,Citation33,Citation34 although boosting with an adenovirus-vector vaccine in mRNA-vaccine primed adults has been suggested to lead to an initially higher and then potentially longer duration of protective antibody titers.Citation26 We do not yet have any information of the durability of the response to a heterologous SCB-2019 booster which will be provided by the planned long-term follow-up of this study. Another limitation is that responses observed are probably the consequence of hybrid immunity, i.e., a combination of immunity derived from vaccination and natural infection as the participants were exposed to new waves of variants, but we did not striate participants based on their history of SARS-CoV-2 infection – either serological evidence or medical history – nor did we assess potential COVID-19 infection during the study itself. However, data from the United States indicate that 48% of adults have hybrid immunity and 96% have antibodies against SARS-CoV-2 so the real-world use of COVID-19 vaccines must be considered against such a background that vaccination policymakers will face when considering booster policies.Citation35
The SARS-CoV-2 virus represents a moving target and newer variants may have emerged before new vaccines are developed and made ready for distribution. For the 2023–2024 vaccination campaign the WHO,Citation36 European Medicines Agency (EMA),Citation37 and US Food and Drug Administration Vaccines and Related Biological Products Advisory Committee (VRBPAC)Citation38 have all recommended using a monovalent XBB.1 descendent lineage, such as XBB.1.5. As viral mutation continues, further recommendations for strain adapted vaccines will be necessary. Nevertheless, it is important that studies such as ours are performed to provide data on how different vaccines interact to broaden the immune response and cover new variants as they emerge.
Conclusions
Data from this ongoing study confirm that the recombinant protein vaccine, SCB-2019, induces robust neutralizing antibody responses against all tested SARS-CoV-2 variants when administered to cohorts previously immunized with inactivated whole-virus, adenovirus-vector or mRNA COVID-19 vaccines. Responses were particularly notable when measuring immunity against Omicron variants including the XBB.1.5 variant which at time of writing was the predominant strain in circulation globally.
Author contributions
Eric Plennevaux, Igor Smolenov, Branda Hu, Faith Gao, Hannalyn Ilagan contributed to the conception, design, and conduct of the study, analysis and interpretation of data and drafting the article. Camilo C. Roa, Jr., Mari Rose A. de Los Reyes contributed to the acquisition of data and revising the article. Htay Htay Han, Donna Ambrosino, George Siber, and Ralf Clemens participated in the analysis and interpretation of data and revision of the article. All authors gave their final approval of the version to be submitted.
Clover_Heterologous_booster_Supplementary_appendix_.docx
Download MS Word (20.7 KB)Acknowledgments
We are grateful to all the participants who volunteered to be in this study, and to the staff at all the study centers for their invaluable support. Professors Sue Ann Costa Clemens and Emanuele Montomoli, and Drs. Peter Richmond and Anh Wartel are thanked for their expert advice and contributions. Editorial assistance in the preparation of this article was provided by Dr. Keith Veitch of KeithVeitch Communications (Amsterdam, the Netherlands) with the financial support of Clover Biopharmaceuticals.
Disclosure statement
Eric Plennevaux, Igor Smolenov, Branda Hu, Faith Gao, Hannalyn Ilagan and Htay Htay Han are full-time employees of the study sponsor. George Siber has served as a Scientific Advisory Board Member and received consulting fees from Clover Biopharmaceuticals, AdVaccine, CanSino, Everest Medicines, Valneva, Senda and Vaxart and owns equities in Clover, AdVaccine, Vaxxinity and Everest Medicines. Donna Ambrosino has received consulting fees from Clover Biopharmaceuticals, Vaxxinity, Everest Medicines, Senda, and served as a Scientific Advisory Board Member for Clover Biopharmaceuticals, Vaxxinity, Senda, Everest Medicines, and Inventprise, and served as a board member for Clover Biopharmaceuticals and Inventprise and owns stock in Clover, Everest Medicine, and Inventprise. Ralf Clemens has received funding from the Bill & Melinda Gates Foundation, consulting fees from Icosavax, Hillevax, honoraria from AstraZeneca, served as a board member for Clover Biopharmaceuticals, Curevac, IVI, Inventprise and owns stocks in Icosavax, HilleVax, Curevac, Novartis, Roche, GSK, and Clover Biopharmaceuticals. Camilo C. Roa, Jr. and Mari Rose A. de Los Reyes have no competing interests to declare.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2301632.
Additional information
Funding
References
- Callaway E. Are COVID surges becoming more predictable? New Omicron variants offer a hint. Nature. 2022;605(7909):204–11. doi:10.1038/d41586-022-01240-x.
- Candido KL, Eich CR, de Fariña LO, Kadowaki, MK, da Conceição Silva, JL, Maller, A, Simão, RD. Spike protein of SARS-CoV-2 variants: a brief review and practical implications. Braz J Microbiol. 2022;53(3):1133–57. doi:10.1007/s42770-022-00743-z.
- Murano K, Guo Y, Siomi H. The emergence of SARS-CoV-2 variants threatens to decrease the efficacy of neutralizing antibodies and vaccines. Biochem Soc Trans. 2021;49(6):2879–90. doi:10.1042/BST20210859.
- Noor R, Shareen S, Billah M. COVID-19 vaccines: their effectiveness against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its emerging variants. Bull Natl Res Cent. 2022;46:96. doi:10.1186/s42269-022-00787-z.
- Florentino PTV, Millington T, Cerqueira-Silva T, Robertson C, de Araújo Oliveira V, Júnior JBS, Alves FJO, Penna GO, Katikireddi SV, Boaventura VS, et al. Vaccine effectiveness of two-dose BNT162b2 against symptomatic and severe COVID-19 among adolescents in Brazil and Scotland over time: a test-negative case-control study. Lancet Infect Dis. 2022;22(11):1577–86. doi:10.1016/S1473-3099(22)00451-0.
- Shao W, Chen X, CZheng C, Liu H, Wang G, Zhang B, Li Z, Zhang W. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern in real-world: a literature review and meta-analysis. Emerg Microbes Infect. 2022;11(1):2383–92. doi:10.1080/22221751.2022.2122582.
- Hause AM, Marquez P, Zhang B, Myers TR, Gee J, Su JR, Blanc PG, Thomas A, Thompson D, Shimabukuro TT, et al. Safety monitoring of bivalent COVID-19 mRNA vaccine booster doses among persons aged ≥12 years — United States, August 31–October 23, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(44):1401–6. doi:10.15585/mmwr.mm7144a3.
- Kurhade C, Zou J, Xia H, Liu M, Chang HC, Ren P, Xie X, Shi P. Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster. Nat Med. 2023;29(2):344–7. doi:10.1038/s41591-022-02162-x.
- Behrens GMN, Barros-Martins J, Cossmann A, Ramos GM, Stankov MV, Odak I, Dopfer-Jablonka A, Hetzel L, Köhler M, Patzer G, et al. BNT162b2-boosted immune responses six months after heterologous or homologous ChAdOx1nCoV-19/BNT162b2 vaccination against COVID-19. Nat Commun. 2022;13(1):4872. doi:10.1038/s41467-022-32527-2.
- Low EV, Tok PSK, Husin M, Suah JL, Tng BH, Thevananthan T, Appannan MR, Yahaya H, Mohd Zin S, Muhamad Zin F, et al. Assessment of heterologous and homologous boosting with inactivated COVID-19 vaccine at 3 months compared with homologous boosting of BNT162b2 at 6 months. JAMA Netw Open. 2022;5(8):e2226046. doi:10.1001/jamanetworkopen.2022.26046.
- Costa Clemens SA, Milan EP, Sprinz E, Neto JC, Pacciarini F, Li P, Chen HL, Smolenov I, Pollard A, Clemens R. Homologous and heterologous boosting of the Chadox1-S1-S COVID-19 vaccine with the SCB-2019 vaccine candidate: a randomized, controlled, phase 2 study. Open Forum Infect Dis. 2022;9(8):ofac418. doi:10.1093/ofid/ofac418.
- Atmar RL, Lyke KE, Deming ME, Jackson LA, Branche AR, El Sahly HM, Rostad CA, Martin JM, Johnston C, Rupp RE, et al. Homologous and heterologous COVID-19 booster vaccinations. N Engl J Med. 2022;386(11):1046–57. doi:10.1056/NEJMoa2116414.
- Zhang Y, Ma X, Yan G, Wu Y, Chen Y, Zhou Z, Wan N, Su W, Liu F-W, Dai M-X, et al. Immunogenicity, durability, and safety of an mRNA and three platform-based COVID-19 vaccines as a third dose following two doses of CoronaVac in China: a randomised, double-blinded, placebo-controlled, phase 2 trial. EClinicalMedicine. 2022;54:101680. doi:10.1016/j.eclinm.2022.101680.
- Chen C-J, Yang L-Y, Chang W-Y, Huang Y-C, Chiu C-H, Shih S-R, Huang C-G, Huang KYA. A randomized controlled trial of heterologous ChAdOx1 nCoV-19 and recombinant subunit vaccine MVC-COV1901 against COVID-19. Nat Comm. 2022;13:5466. doi:10.1038/s41467-022-33146-7.
- Tabarsi P, Anjidani N, Shahpari R, Roshanzamir K, Fallah N, Andre G, Petrovsky N, Barati S. Immunogenicity and safety of SpikoGen®, an adjuvanted recombinant SARS-CoV-2 spike protein vaccine as a homologous and heterologous booster vaccination: a randomized placebo-controlled trial. Immunology. 2022;167:340–53. doi:10.1111/imm.13540.
- WHO. Status of COVID-19 vaccines within WHO EUL/PQ evaluation process; [accessed 2023 Sep 6]. https://extranet.who.int/pqweb/sites/default/files/documents/Status_COVID_VAX_12January2023.pdf.
- Richmond P, Hatchuel L, Dong M, Ma B, Hu B, Smolenov I, Li P, Liang P, Han HH, Liang J, et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397(10275):682–94. doi:10.1016/S0140-6736(21)00241-5.
- Bravo L, Smolenov I, Han HH, Li P, Hosain R, Rockhold F, Clemens SAC, Roa C, Borja-Tabora C, Quinsaat A, et al. Efficacy of the adjuvanted subunit protein COVID-19 vaccine, SCB-2019: a phase 2 and 3 multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2022;399(10323):461–72. doi:10.1016/S0140-6736(22)00055-1.
- Roa CC Jr, de Los Reyes MRA, Plennevaux E, Smolenov I, Hu B, Gao F, Ilagan H, Ambrosino D, Siber G, Clemens R. Superior boosting of neutralizing titers against Omicron SARS-CoV-2 variants by heterologous SCB-2019 vaccine vs a homologous booster in CoronaVac-primed adults. J Infect Dis. 2023;22:1253–62. doi:10.1093/infdis/jiad262.
- WHO. COVID-19 weekly epidemiological update – edition September 1, 2023; [accessed 2023 Sep 8]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—1-september-2023.
- Shirbhate E, Pandey J, Patel VK, Kamal M, Jawaid T, Gorain B, Kesharwani P, Rajak H. Understanding the role of ACE-2 receptor in pathogenesis of COVID-19 disease: a potential approach for therapeutic intervention. Pharmacol Rep. 2021;73(6):1539–50. doi:10.1007/s43440-021-00303-6.
- Lupala CS, Ye Y, Chen H, Su X-D, Liu H. Mutations on RBD of SARS-CoV-2 Omicron variant result in stronger binding to human ACE2 receptor. Biochem Biophys Res Comm. 2022;590:34–41. doi:10.1016/j.bbrc.2021.12.079.
- Cao Y, Wang J, Jian F, Xiao T, Song W, Yisimayi A, Huang W, Li Q, Wang P, An R, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602(7898):657–63. doi:10.1038/s41586-021-04385-3.
- Nguyen TT, Quach THT, Tran TM, Phuoc HN, Nguyen HT, Vo TK, Vo GV. Reactogenicity and immunogenicity of heterologous prime-boost immunization with COVID-19 vaccine. Biomed Pharmacother. 2022;147:112650. doi:10.1016/j.biopha.2022.112650.
- Garg I, Sheikh AB, Pal S, Shekhar R. Mix-and-match COVID-19 vaccinations (heterologous boost): a review. Infect Dis Rep. 2022;14(4):537–46. doi:10.3390/idr14040057.
- Rashedi R, Samieefar N, Masoumi N, Mohseni S, Rezaei N. COVID-19 vaccines mix-and-match: the concept, the efficacy and the doubts. Rev J Med Virol. 2022;94:1294–9. doi:10.1002/jmv.27463.
- Deng J, Ma Y, Liu Q, Du M, Liu M, Liu J. Comparison of the effectiveness and safety of heterologous booster doses with homologous booster doses for SARS-CoV-2 vaccines: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19(17):10752. doi:10.3390/ijerph191710752.
- Sitaris I, Jacobsen H, Higdon MM, Dowling WE, Bar-Zeev N, Knoll MD. Systemic review of primary and booster COVID-19 sera neutralizing ability against SARS-CoV-2 omicron variant. NPJ Vaccines. 2022;7:147. doi:10.1038/s41541-022-00565-y.
- Khong K-W, Liu D, Leung K-Y, Lu L, Lam H-Y, Chen L, Chan P-C, Lam H-M, Xie X, Zhang R, et al. Antibody response of combination of BNT162b2 and CoronaVac platforms of COVID-19 vaccines against Omicron variant. Vaccines. 2022;10(2):160. doi:10.3390/vaccines10020160.
- Wang X, Zhao X, Song J, Wu J, Zhu Y, Li M, Cui Y, Chen Y, Yang L, Liu J, et al. Homologous or heterologous booster of inactivated vaccine reduces SARS-CoV-2 Omicron variant escape from neutralizing antibodies. Emerg Microb Infect. 2022;11:477–81. doi:10.1080/22221751.2022.2030200.
- Niyomnaitham S, Jongkaewwattana A, Meesing A, Chusri S, Nanthapisal S, Hirankarn N, Siwamogsatham S, Kirdlarp S, Chaiwarith R, Niyom SL, et al. Immune responses of the third dose of AZD1222 vaccine or BNT162b2 mRNA vaccine after two doses of CoronaVac vaccines against Delta and Omicron variants. medRxiv. Preprint posted 2022 Oct 3. doi:10.1101/2022.10.02.22280572.
- Shaw RH, Stuart A, Greenland M, Liu X, Nguyen Van-Tam JS, Snape MD. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397:2043–6. doi:10.1016/S0140-6736(21)01115-6.
- Tan CS, Collier AY, Yu J, Liu J, Chandrashekar A, McMahan K, Jacob-Dolan C, He X, Roy V, Hauser BM, et al. Durability of heterologous and homologous COVID-19 vaccine boosts. JAMA Netw Open. 2022;5(8):e2226335. doi:10.1001/jamanetworkopen.2022.26335.
- Addo IY, Dadzie FA, Okeke SR, Boadi C, Boadu EF. Duration of immunity following full vaccination against SARS-CoV-2: a systematic review. Arch Public Health. 2022;80:200. doi:10.1186/s13690-022-00935-x.
- Jones JM, Manrique IM, Stone MS, Grebe E, Saa P, Germanio CD, Spencer BR, Notari E, Bravo M, Lanteri MC, et al. Estimates of SARS-CoV-2 seroprevalence and incidence of primary SARS-CoV-2 infections among blood donors, by COVID-19 vaccination status — United States, April 2021–September 2022. MMWR Morb Mortal Wkly Rep. 2023;72:601–5. doi:10.15585/mmwr.mm7222a3.
- WHO. Statement on the antigen composition of COVID-19 vaccines; 2023 May 18 [accessed 2023 Sep 8]. https://www.who.int/news/item/18-05-2023-statement-on-the-antigen-composition-of-covid-19-vaccines.
- EMA. ECDC-EMA statement on updating COVID-19 vaccines composition for new SARS-CoV-2 virus variants; 2023 June 6 [accessed 2023 Sep 8]. https://www.ema.europa.eu/en/documents/other/ecdc-ema-statement-updating-covid-19-vaccines-composition-new-sars-cov-2-virus-variants_en.pdf.
- FDA. 182nd meeting of the Vaccines and Related Biological Products Advisory Committee (VRBPAC); 2023 June 15 [accessed 2023 Sep 8]. https://www.fda.gov/media/169804/download.
