ABSTRACT
With recent advances in U.S. clinical trials for norovirus vaccines, it is an opportune time to examine what is known about the public receptivity to this novel vaccine. From October 2016–September 2017, we surveyed Kaiser Permanente Northwest members in Portland, Oregon, to ask their level of agreement on a 5-point scale with statements about the need for and willingness to get a potential norovirus vaccine for themselves or their child and analyzed their responses according to age, occupational status, prior vaccine uptake, and history of prior norovirus diagnoses. The survey response rate was 13.5% (n = 3,894); 807 (21%) responded as legal guardians, on behalf of a child <18 y of age and 3,087 (79%) were adults aged 18+ y. The majority of respondents were in agreement about getting the norovirus vaccine, if available (60% of legal guardians, 52% of adults aged 18–64 y, and 55% of adults aged 65+ y). Prior vaccination for influenza and rotavirus (among children) was the only correlate significantly associated with more positive attitudes toward receiving norovirus vaccine. Pre-pandemic attitudes in our all-ages study population reveal generally positive attitudes toward willingness to get a norovirus vaccine, particularly among those who previously received influenza or rotavirus vaccines.
Background
Norovirus is the leading cause of acute gastroenteritis in the United States, estimated to cause between 19 and 21 million illnesses and 570–800 deaths each year.Citation1,Citation2 Outbreaks of norovirus are particularly severe among pediatric and elderly populations, with pediatric populations serving a key role in the transmission of norovirus to others in the community.Citation3 The impact of norovirus on communities and healthcare systems, whose providers manage between 1.7 and 1.9 million norovirus-attributed outpatient visits annually in the United States,Citation1 represents a significant burden and has led to efforts to develop multiple norovirus vaccine candidates, two of which are currently in phase 2/3 of clinical trials, targeting populations of adultsCitation4–6 and children.Citation7
Even as vaccine candidates are progressing, there is limited information on public attitudes toward a potential norovirus vaccine. One Guatemalan study, conducted in 2017, assessed attitudes toward a potential norovirus vaccine and found respondent attitudes to be generally positive, except among those with prior history of vaccine refusal.Citation8 With advances in both pediatric,Citation9 and adult clinical trials,Citation10 it is an opportune time to explore attitudes about a potential norovirus vaccine in the United States.
In 2016–2017, we conducted a study to better understand the prevalence of acute gastroenteritis (AGE) pathogens, including norovirus, in the greater Portland, Oregon population.Citation11 We used this opportunity to administer a survey to assess attitudes and beliefs toward a potential norovirus vaccine and examine correlates of positive attitudes toward a norovirus vaccine for adults and children, in anticipation that a potential vaccine could be introduced for either age group. In consideration of prior studies evaluating factors associated with vaccination intent, we examined demographics, individual risk perception toward AGE, as well as prior vaccination uptake (for influenza and rotavirus vaccine), as past vaccination has been found to be a possible predictor of vaccine uptake and may serve as a way to assess attitudes toward future novel vaccines.Citation12
Now, with the advent of the SARS-CoV-2 pandemic, the topic of vaccine acceptance has received increasing attention.Citation13 While still unknown, it is possible that concerns about COVID-19 vaccines may have an impact on attitudes about other current or future vaccines under development. Because we collected these data in 2017, this study provides a unique opportunity to fill a knowledge gap in describing pre-pandemic patterns of norovirus vaccine attitudes and beliefs in the United States. Better understanding of this baseline will allow for future work in assessing how the COVID-19 pandemic may have impacted the acceptability of other new and developing vaccine candidates.
Methods
An on-line, self-administered 34-question survey was disseminated as part of the Community Acquired Gastroenteritis (CAGE) study through the Kaiser Permanente Northwest (KPNW) integrated health system, focused on members living in the Portland, Oregon area. Sampling strategy involved selecting age-stratified, simple random samples of KPNW members on a weekly basis from September 2016 to September 2017, with the goal of enrolling representative proportions across the following age groups (<5, 5–17, 18–44, 45–64, 65+ y of age). Full methods were published previously.Citation11 Individuals with 6 months of continuous health plan enrollment prior to survey completion were included in this analysis. Both adults and children were sampled; legal guardians completed the survey for sampled members aged <18 y. Respondents are divided into three groups: adults aged 18–64 y, adults aged 65 y and older, and children (<18 y). Demographic data (of all adult and child respondents), occupation status (of adult respondents aged 18–64 y, and of legal guardians), as well as information about prior AGE diagnoses were collected. To assess vaccine attitudes, participants were asked to provide their level of agreement, using a scale of 1 to 5 (1 indicating strong disagreement, 2 indicating somewhat in disagreement, 3 indicating neutrality, 4 indicating somewhat in agreement, and 5 indicating strong agreement) with one statement on vaccines in general, two statements about a potential norovirus vaccine, and two statements about perceived susceptibility to norovirus. For legal guardians, these questions were framed as attitudes about vaccination for their child/dependent. In order to see if other factors modified attitudes to the two key questions, responses were also stratified by the presence of four key indicators: history of norovirus disease, high-risk occupation status, receipt of an influenza vaccine in the prior 3 y, and receipt of at least 1 dose of the rotavirus vaccine (only age-eligible children).
Occupations that place individuals at higher risk of norovirus exposure and transmission included working-aged adults (defined as those aged 18 to 64 y) who self-reported any of the following: being a healthcare worker with patient contact, working in food preparation, or working as an educator in contact with children. The rationale for singling out these occupation groups is that a vaccine may be specifically prioritized for groups at increased risk for either acquiring or transmitting virus, similar to Michigan’s vaccination strategy during the 2017 Hepatitis A outbreak.Citation14 Electronic medical records, which include data from Oregon and Washington Immunization Information Systems, were used to assess vaccination history for influenza vaccine (at least 1 dose in the past 3 y for anyone 6 months of age or older) and rotavirus vaccine (at least 1 dose for children aged 6 months to 10 y).
In the analysis, we calculated mean scores (range 1 to 5) for level of agreement across the three respondent age groups. Scores were calculated for each of the survey statements (“I think a vaccine is needed to prevent stomach illness caused by norovirus,” and “If a norovirus vaccine is made available, I would get the vaccine”). Scores were also stratified by occupational status, history of norovirus disease, vaccination history, and level of agreement with statements about vaccines (generally) and norovirus susceptibility. Individuals who chose not to respond to the survey question were removed from the denominator for that analysis ().
Table 1. Demographic characteristics of the community acquired gastroenteritis (CAGE) population, by age group, 2016–2017.
Table 2. Mean scores for attitudes toward the need for a norovirus vaccine† among community acquired gastroenteritis (CAGE) participants 2016–2017.
Table 3. Attitudes toward getting a norovirus vaccine† among community acquired gastroenteritis (CAGE) participants, 2016–2017.
Results
Descriptive data on self-reported demographics are presented for the three respondent groups in . Of 28,889 members sampled for recruitment, 3,894 surveys were completed, for an overall survey response rate of 13.5%. Among respondents, 807 (21%) responded as legal guardians, on behalf of a child <18 y of age; 2,234 (58%) were adults aged 18–64 y; and 853 (22%) were adults aged 65+ y (). In comparison to the overall KPNW membership population, respondents included a higher proportion of females (60% vs. 52%, respectively), people of white race (84% vs. 78%, respectively) and >65 y of age (22% vs. 17% respectively) (data not shown). Response rates differed by age group, with the highest response rates among legal guardians (38%) and adults 65 y of age and older (21%). When asked about specific occupation, 18% of respondents, overall, reported that they worked in healthcare (71% of whom had direct patient contact); 4% reported jobs that involved food handling; and 18% reported jobs that involved contact with children, placing 40% of respondents, across all age-groups, in high-risk occupations.
When comparing respondent groups, adults aged 18–64 y were significantly more likely to be female compared to adults >65 y old and children (67% vs. 57% vs. 45%, respectively). Child respondents were more likely to be Hispanic than adults aged 18 to 64 y or 65 y and older (10% vs. 6% vs. 2%, respectively), and less likely to be white (77% vs. 83% vs. 95%, ().
Norovirus vaccine attitudes: “a norovirus vaccine is needed”
When asked about the need for a norovirus vaccine, respondents were largely neutral; 39% of legal guardians, 51% of adults aged 18–64 y, and 59% of adults aged 65 y or older indicated a score of 3. Legal guardians were more favorable, with 43% somewhat or strongly in agreement that a vaccine was needed, compared to 34% of younger adults, and 35% of older adults. Favorability was the same for Hispanics and non-Hispanics, alike. The proportion who somewhat or strongly disagreed a vaccine was needed ranged from 7% of adults 65 y or older, followed by 15% of adults aged 18 to 64 y, and 18% of legal guardians (). A total of 106 people (3%) did not answer the question. As shown in , those who self-reported ever having a prior diagnosis of norovirus demonstrated the strongest overall agreement with the need for a norovirus vaccine (67% among legal guardians, 38% among adults 18–64 y, and 46% among adults ≥65 y), in comparison to the overall participants in their respective age group (43%, 34%, and 35%, respectively). Presence of other conditions (receipt of an influenza vaccine in the past three y, receipt of at least 1 dose of rotavirus vaccine among those up to age 10 y and having a high-risk occupation) only minimally increased favorable attitudes about the need for a vaccine, compared to overall participants of the same age.
Figure 1. Community acquired gastroenteritis (CAGE) participants level of agreement with the statements: “I think a norovirus is needed” and “if a norovirus vaccine were made available, I (my child) would get it,” by respondent age group, 2016–17.
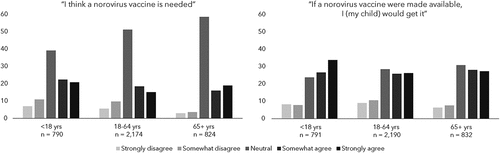
Figure 2. Community acquired gastroenteritis (CAGE) participants level of agreement with the statements: “I think a norovirus is needed” and “if a norovirus vaccine were made available, I (my child) would get it,” by age group and select indicators, 2016–2017.
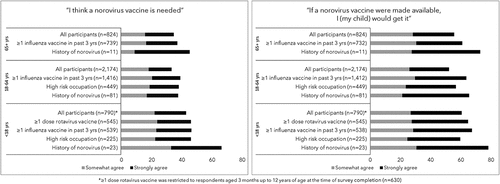
Respondent mean scores toward the need for a norovirus vaccine generally hovered between 3 and 4 (i.e., between neutral and somewhat agree). Factors associated with higher mean scores included receipt of an influenza vaccine anytime in the past 3 y for all respondents (approximately 3.5 across respondent groups vs <3.0 for those with no prior influenza vaccine, p < .01); and receipt of at least 1 dose of rotavirus vaccine for children (3.51 vs. 2.92, p < .01). () Legal guardians whose children had a prior norovirus diagnosis had higher mean scores (3.88) compared to those without a prior diagnosis (3.37, p = .03); among adult respondents, prior norovirus diagnosis was not associated with attitudes about the need for a norovirus vaccine.
Occupational status as a healthcare worker was associated with higher mean scores for legal guardians (3.53 vs. 3.35, p = .05) and working aged adults 3.37 vs. 3.25 (p = .03). Individuals working in food preparation had lower mean scores compared to those not in food preparation (3.29 vs. 4.29, p = .04)
Questions about norovirus susceptibility and risk of severe illness revealed that adults who perceive themselves as getting stomach illness more easily than others had higher mean scores (3.4) compared to those who do not (3.23, p = .01). Legal guardians who disagreed with the statement “I am not at risk of getting seriously ill from norovirus” also had higher mean scores (3.55) compared to those who were neutral about this issue (3.21, p < .001). Agreement with disliking shots was not associated with attitudes about the need for a norovirus vaccine, for any age group.
“If a norovirus vaccine were available, I (my child) would get one”
The majority of respondents were somewhat or strongly in agreement about getting the norovirus vaccine (60% of legal guardians, 52% of younger adults, and 55% of older adults). The proportion who remained neutral was similar across age groups (24% of legal guardians, 29% of younger adults, 31% of older adults). Those who somewhat or strongly disagreed they would get the vaccine for themselves (or their child, for legal guardians) ranged from 14% of adults aged 65 y and older, 16% of legal guardians, and 20% of adults aged 18 to 64 y. () A total of 81 (2%) people did not answer the question. shows that those who reported a history of norovirus showed strongest agreement, with 78% of legal guardians, 65% of young adults, and 73% of older adults somewhat or strongly in favor of getting a norovirus vaccine. Individuals who had received at least one dose of an influenza vaccine in the past 3 y were also more favorable toward getting a vaccine, in comparison to their overall age group, with 67% of legal guardians, 64% of adults aged 18 to 64 y, and 61% of adults aged 65 y or older somewhat or strongly in favor, compared to 60%, 52%, and 55%, respectively.
Mean scores toward willingness to get a norovirus vaccine trended more positive than scores toward the need for a vaccine. Receipt of an influenza vaccine anytime in the past 3 y was significantly associated with higher mean scores (higher agreement) (range: 3.76–3.93, p < .01) for the need for a norovirus vaccine for all respondents; as was receipt of at least 1 dose of rotavirus vaccine for children (3.85, p < .01). () Mean scores for respondents without an influenza vaccine in the prior 3 y ranged from 2.5 to 3.0 for adult age groups, and 3.0 for legal guardians whose children had not received any doses of rotavirus vaccine. Legal guardians whose children have had a prior norovirus diagnosis had higher mean scores (4.26) compared to those without a prior diagnosis (3.68, p = .03); the same was true for adult respondents, with a mean score of 3.79 among those with a prior diagnosis, compared to 3.48 (p = .03) among those without.
Healthcare worker occupation was associated with higher mean scores for working-aged adults; occupational exposure to children and work in food preparation were both associated with lower mean scores among legal guardians, compared to those who did not work with children or work in food preparation, respectively.
Questions about norovirus susceptibility and risk of severe illness revealed that adults who perceive themselves as getting stomach illness more easily than others had higher willingness to get a norovirus vaccine (mean score 3.67) compared to those who do not (3.42, p < .001). Both legal guardians and adults who disagreed with the statement, “I (my child) am not at risk of getting seriously ill from norovirus,” also had higher mean scores (3.89 and 3.6, respectively) compared to those who agreed with that statement (3.63, p = .01 and 3.44, p = .05, respectively). Agreement with not liking shots was associated with lower mean scores for adults (including adults aged 65 y and older), but not for legal guardians.
Discussion
Respondents were mostly neutral about the need for a norovirus vaccine, which suggests that demand for the vaccine in this random sample of KPNW members was not high at the time of the survey. However, the majority of surveyed individuals expressed intent to get a norovirus vaccine for themselves or their child, if it became available; and a minority (less than 20%) said they would choose not to get a norovirus vaccine. Perceived risk of disease was an important predictor of willingness to get a potential norovirus vaccine among legal guardian and adult respondents but was not associated with favorable attitudes toward the need for a norovirus vaccine. Among legal guardians and adults aged 18–64 y, history of norovirus illness, as well as feelings of susceptibility or the potential for severe disease were uniformly associated with willingness to get a potential vaccine. However, attitudes toward the need for a norovirus vaccine were inconsistent, even among those with the same risk perception. Importantly, this study reveals that even if individuals do not feel a strong urgency around the need for a norovirus vaccine, they may be willing to get the vaccine, once available, particularly those who feel norovirus presents a risk to them or their child. Therefore, helping people understand that norovirus disease is associated with significant morbidity in the United States, remains an important message.
Occupations that place individuals at higher risk of norovirus exposure and transmission were not consistently associated with more positive attitudes about a potential norovirus vaccine. Of the three occupation groups identified, only healthcare workers were generally more favorable about the vaccine, and food care workers were generally less favorable, compared to their peers. Vaccine recommendations often single out individuals who are at highest risk of exposure to disease, as evidenced by recommendations for the COVID-19 vaccine where healthcare workers were among the first recipients. Messaging around risk status should be carefully considered and directly communicated with populations when vaccine recommendations are being made.
Prior vaccine uptake for rotavirus (among children) and influenza was the only correlate that was consistently and significantly associated with more positive attitudes toward the need for and willingness to get the norovirus vaccine across respondents of all ages. Respondents without prior vaccination had the most unfavorable attitudes (i.e., lowest mean scores) toward the norovirus vaccine across all examined correlates. These data confirm that prior vaccination history could be an important predictor of intention to receive other vaccines, even those not yet available or recommended.
The extent to which the COVID-19 pandemic may have shifted vaccine attitudes is still unknown. A longitudinal study in the UK assessed changes in vaccine attitudes among older adults, from March 2020-March 2021, and found both reduced complacency around getting vaccinated but also increased concerns about potential risks of vaccination.Citation15 Another recent study examined changes in influenza vaccine uptake over consecutive seasons prior to and during the COVID-19 pandemic and found that among adults, uptake decreased by about 4% in states in the lowest quartile of COVID-19 coverage and increased about 4% in states in the highest quartile.Citation12 While the study was focused on the population-level (thus, not able to assess changes within individuals over time), the finding supports the idea that the introduction of the COVID-19 vaccine had some impact on willingness to get the influenza vaccine, but that effect was not large and it occurred in equal measure in favor of, and against, influenza vaccine uptake. If that finding prevails, and the SARS-CoV-2 pandemic has in fact solidified pre-pandemic attitudes, and people have remained generally consistent in their intentions to vaccinate, then we could anticipate that about 53% of adults, and 60% of legal guardians in our study would consider getting a norovirus vaccine for themselves or their child, if it becomes available.
Another study exploring parental attitudes about a potential monoclonal antibody pediatric immunization for respiratory syncytial virus (RSV) found that 60% of an international cohort of over 5,600 parents expressed intention to accept a (then) hypothetical immunization if it became part of the immunization program in their country.Citation16 Importantly, this study was conducted in 2021 so provides a good measure of parental receptivity in the midst of the COVID-19 pandemic. The fact that we also found that 60% of legal guardians intended to have their child receive an approved and available norovirus vaccine provides some reassurance that our study findings may still be relevant today. With a recently approved and recommended RSV pediatric immunization in the United States,Citation17 there is the opportunity to assess whether vaccine uptake mirrors parental intention, as documented in this recent study.
Study limitations include an overall low response rate and administration of the survey in a specific geographic area, which limit our ability to speak to the generalizability of study findings. Sample sizes limited our capacity to assess differences in attitudes by respondent type (adults vs. legal guardians). Substantial missing data for household income (>50%) also limits our ability to address differences by this measure of socioeconomic status.
The majority of respondents, across ages, were willing to get a norovirus vaccine; risk perception, as well as prior vaccine uptake, were strongly associated with that willingness. Several studies have reported that norovirus has replaced rotavirus as the most commonly detected pathogen among individuals with medically attended acute gastroenteritis in the United States,Citation18 however only 3% of study participants reported having a prior norovirus infection, highlighting how uncommonly the virus is clinically confirmed. Declines in rotavirus, as observed in the years since the introduction of the vaccine in 2006, have been attributed to the increasing vaccine coverage, from 43.9% in 2009, and 72.6% in 2013,Citation19 resulting in 70–80% decreases in rotavirus-associated hospitalizations.Citation20 A similar uptake trajectory for a norovirus vaccine is possible, if the public is made aware of how commonly people are infected with norovirus and are educated on the risks associated with norovirus disease. Continued work to understand how individuals perceive potential risks of norovirus in this post-pandemic era are needed in order to better characterize norovirus vaccine attitudes and intentions.
Author contribution
Holly Groom, Allison Naleway, and Mark Schmidt were involved with the conception and design, as well as the interpretation of the data and drafting of the paper. Claire Mattison, Suzanne Salas and Laura Calderwood were involved with the data analysis, interpretation of the data, drafting and revisions to the manuscript. Judy Donald was involved with the conception and design, and revisions to the manuscript. Sara Mirza was involved in the drafting of the paper and revisions to the content. All authors gave final approval to this version of the manuscript and all agree to be accountable for all aspects of the work.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosure statement
Holly Groom, Mark Schmidt, Suzanne Salas, Judy Donald and Allison Naleway received funding from Takeda Vaccines, Inc. to conduct this research.
Additional information
Funding
References
- Hall AJ, Lopman BA, Payne DC, Patel MM, Gastañaduy PA, Vinjé J, Parashar UD. Norovirus disease in the United States. Emerg Infect Dis. 2013 Aug;19(8):1198–8. doi:10.3201/eid1908.130465.
- Burke RM, Mattison CP, Pindyck T, Dahl RM, Rudd J, Bi D, Curns AT, Parashar U, Hall AJ. Burden of norovirus in the United States, as estimated based on administrative data: updates for medically attended illness and mortality, 2001–2015. Clin Infect Dis. 2021 Jul 1;73(1):1–8. doi:10.1093/cid/ciaa438.
- Steimle LN, Havumaki J, Eisenberg MC, Eisenberg JNS, Prosser LA, Pike J, Ortega-Sanchez IR, Mattison CP, Hall AJ, Steele MK, et al. Cost-effectiveness of pediatric norovirus vaccination in daycare settings. Vaccine. 2021 Apr 8;39(15):2133–2145. doi:10.1016/j.vaccine.2021.02.066.
- Treanor J, Sherwood J, Cramer JP, Le Cam Bouveret N, Lin S, Baehner F, Borkowski A. A phase 2 study of the bivalent VLP norovirus vaccine candidate in older adults; impact of MPL adjuvant or a second dose. Vaccine. 2020 Aug 10;38(36):5842–50. doi:10.1016/j.vaccine.2020.06.011.
- Sherwood J, Mendelman PM, Lloyd E, Liu M, Boslego J, Borkowski A, Jackson A, Faix D. Efficacy of an intramuscular bivalent norovirus GI.1/GII.4 virus-like particle vaccine candidate in healthy US adults. Vaccine. 2020 Sep 22;38(41):6442–9. doi:10.1016/j.vaccine.2020.07.069.
- Vaxart announces positive preliminary topline data from dose-ranging phase 2 study of its bivalent norovirus vaccine candidate; 2023 [accessed 2023 Jul 6]. https://www.globenewswire.com/news-release/2023/07/06/2700292/25416/en/Vaxart-Announces-Positive-Preliminary-Topline-Data-from-Dose-Ranging-Phase-2-Study-of-its-Bivalent-Norovirus-Vaccine-Candidate.html.
- Lopez P, Lopez-Medina E, Saez-Llorens X, deAntonio R, Masuda T, Mendelman PM, Sherwood J, Baehner F, Borkowski A. Immunogenicity and tolerability of a bivalent virus-like particle norovirus vaccine candidate in children from 6 months up to 4 years of age: a phase 2 randomized, double-blind trial. Hum Vaccin Immunother. 2023 Dec 31;19(1):2204787. doi:10.1080/21645515.2023.2204787.
- Olson D, Krager S, Lamb MM, Rick AM, Asturias EJ. Knowledge of norovirus and attitudes toward a potential norovirus vaccine in rural Guatemala: a cross-sectional exploratory survey. Am J Trop Med Hyg. 2018 May;98(5):1498–1501. doi:10.4269/ajtmh.17-0771.
- Vesikari T, Saez-Llorens X, Blazevic V, Lopez P, Lopez E, Masuda T, Mendelman PM, Liu M, Sherwood J, Baehner F, et al. Immunogenicity of a bivalent virus-like particle norovirus vaccine in children from 1 to 8 years of age: a phase 2 randomized, double-blind study. Vaccine. 2022 Jun 9;40(26):3588–3596. doi:10.1016/j.vaccine.2022.04.089.
- Biospace. Vaxart doses first subject in the phase 2 clinical trial of its bivalents norovirus candidate; [ accessed 2023 May 11]. https://www.biospace.com/article/releases/vaxart-doses-first-subject-in-the-phase-2-clinical-trial-of-its-bivalent-norovirus-candidate/.
- Schmidt MA, Groom HC, Rawlings AM, Mattison CP, Salas SB, Burke RM, Hallowell BD, Calderwood LE, Donald J, Balachandran N, et al. Incidence, etiology, and healthcare utilization for acute gastroenteritis in the community, United States. Emerg Infect Dis. 2022 Nov;28(11):2234–2242. doi:10.3201/eid2811.220247.
- Leuchter RK, Jackson NJ, Mafi JN, Sarkisian CA. Association between covid-19 vaccination and influenza vaccination rates. N Engl J Med. 2022 Jun 30;386(26):2531–2532. doi:10.1056/NEJMc2204560.
- Soorapanth S, Cheung R, Zhang X, Mokdad AH, Mensah GA. Rural–urban differences in vaccination and hesitancy rates and trust: US COVID-19 trends and impact survey on a social media platform, May 2021–April 2022. Am J Public Health. 2023 Apr 13;113(6):1–9. doi:10.2105/AJPH.2023.307274.
- Services MDoHaH. Prioritization of hepatitis a vaccination for food handlers and healthcare workers in responseto the hepatitis a outbreak in michigan: frequently asked questions; 2023 Oct 27 [accessed 2023 Oct 27]. https://www.michigan.gov/-/media/Project/Websites/mdhhs/Folder3/Folder35/Folder2/Folder135/Folder1/Folder235/FAQ_-_Vaccination_Prioritizing_for_Food_Handlers_and_Healthcare_Workers_11-3-17.pdf?rev=9ab290266cfe41b7824e70d41ab9f1b8.
- Gallant AJ, Nicholls LAB, Rasmussen S, Cogan N, Young D, Williams L, Gesser-Edelsburg A. Changes in attitudes to vaccination as a result of the COVID-19 pandemic: a longitudinal study of older adults in the UK. PLoS One. 2021;16(12):e0261844. doi:10.1371/journal.pone.0261844.
- Lee Mortensen G, Harrod-Lui K. Parental knowledge about respiratory syncytial virus (RSV) and attitudes to infant immunization with monoclonal antibodies. Expert Rev Vaccines. 2022 Oct;21(10):1523–1531. doi:10.1080/14760584.2022.2108799.
- Jones JM, Fleming-Dutra KE, Prill MM, Roper LE, Brooks O, Sánchez PJ, Kotton CN, Mahon BE, Meyer S, Long SS, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendations of the advisory committee on immunization practices – United States, 2023. Morb Mortal Wkly Rep. 2023 Aug 25;72(34):920–5. doi:10.15585/mmwr.mm7234a4.
- Burke RM, Mattison CP, Marsh Z, Shioda K, Donald J, Salas SB, Naleway AL, Biggs C, Schmidt MA, Hall AJ, et al. Norovirus and other viral causes of medically attended acute gastroenteritis across the age spectrum: results from the medically attended acute gastroenteritis study in the United States. Clin Infect Dis. 2021 Aug 16;73(4):e913–e920. doi:10.1093/cid/ciab033.
- Aliabadi N, Tate JE, Haynes AK, Parashar UD. Centers for disease C, prevention. Sustained decrease in laboratory detection of rotavirus after implementation of routine vaccination-United States, 2000-2014. Morb Mortal Wkly Rep. 2015 Apr 10;64(13):337–42.
- Getachew HB, Dahl RM, Lopman BA, Parashar UD. Rotavirus vaccines and health care utilization for diarrhea in US children, 2001 to 2015. Pediatr Infect Dis J. 2018 Sep;37(9):943–948. doi:10.1097/INF.0000000000001988.
