ABSTRACT
This study aimed to elucidate the seroprevalence of antibodies to tetanus and pertussis among Chinese health care workers. Blood specimens from health care workers were collected during the 2021 annual medical examination at the First People’s Hospital of Wuhu. Commercial ELISA kits were employed to quantify serum IgG antibodies against tetanus toxin (anti-TT IgG) and both IgG and IgA antibodies against pertussis toxin (anti-PT IgG, anti-PT IgA). A concentration of anti-TT IgG exceeding 0.1 IU/ml was deemed seroprotective against tetanus, while concentrations of anti-PT IgG ≥ 50 IU/ml or anti-PT IgA ≥ 15 IU/ml were indicative of a prior pertussis infection. The overall seroprotective rate for anti-TT IgG stood at 10.43% (92/882), with the highest seroprotective rate (13.91%) in the 20–29 age group, followed by the 30–39 age group (10.57%), 40–49 age group (5.80%), and 50–59 age group (5.63%). Eighteen (2.04%) of the studied subjects were positive to anti-PT IgG, and the positive rate in 20–39 age group and 40–59 age group was 1.19% (8/673) and 4.78% (10/209), respectively. Thirty (3.40%) subjects displayed anti-PT IgG levels ≥100 IU/ml and/or anti-PT IgA ≥ 15 IU/ml, suggesting a recent pertussis infection within the preceding year. Over half (503/882, 57.03%) had undetectable anti-PT IgG antibodies. The majority of health care workers in China appear susceptible to tetanus and pertussis, and a significant subset has experienced pertussis infection. The implementation of booster vaccinations against these diseases for Chinese health care workers is recommended.
Introduction
TetanusCitation1 and pertussis (whooping cough)Citation2 are vaccine-preventable infectious diseases caused by the bacteria Clostridium tetani and Bordetella pertussis, respectively. Tetanus toxin (TT)-incorporated vaccines have significantly curtailed tetanus morbidity. Post-2020, neonatal tetanus cases reported annually in China have remained below 50 (32 cases in 2020, 23 cases in 2021, and 18 cases in 2022).Citation3 However, the epidemiological profile of adult tetanus remains ambiguous due to its non-notifiable status in China. The increasing burden of tetanus in older demographics is further exacerbated by misdiagnoses and missed diagnoses.Citation4,Citation5
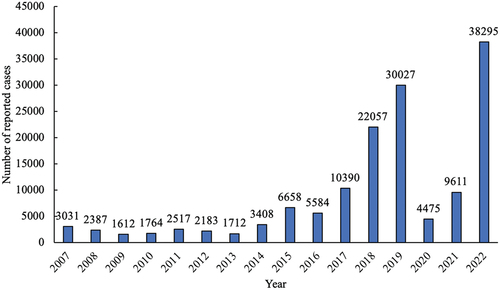
A pertussis resurgence has been observed globally, even in nations with robust vaccination programs.Citation6–8 In China, the number of reported pertussis cases surged from 5584 in 2016 to 10,390 in 2017, with a subsequent escalation. Although strict public health measures in response to the COVID-19 pandemic temporarily reduced pertussis cases in 2020 and 2021, a swift resurgence to 38,295 cases was noted in 2022 upon relaxation of these measures. The number of pertussis cases reported to WHO from 2007 to 2022 in ChinaCitation9 is visualized and shown in . Still, these numbers may underrepresent the actual epidemic due to diagnostic limitations and clinician underreporting.
Figure 1. Number of reported pertussis cases in China, 2007–2022.Citation9.
In China, a whole-cell pertussis vaccine combined with diphtheria and tetanus toxoids (DTwP) was incorporated into the National Immunization Program (NIP) in 1978. The diphtheria-tetanus-acellular pertussis (DTaP) vaccine began to be used in parallel with DTwP in 2007, and completely replaced DTwP in 2013. The immunization strategy for DTwP or DTaP vaccine used in China is three primary doses administered at 3, 4, and 5 months, with a subsequent booster at 18–24 months. A booster dose of diphtheria and tetanus (DT) vaccine will be given when the child is 6 years old, without protection for pertussis. Several studies have shown that pertussis vaccine-induced immunity lasts only a few years,Citation10–12 and the protective effect is not sufficient to establish herd immunity.Citation13
Given that pertussis toxin (PT) is the sole antigen exclusive to Bordetella pertussis, anti-PT antibody levels serve as a reliable serological metric for recent infection or vaccination.Citation14,Citation15 While numerous studies have explored anti-PT IgG antibody levels across various regionsCitation16,Citation17 and age groups,Citation18,Citation19 scant data exists on health care workers – a demographic at heightened risk of infection and transmission. This study seeks to bridge this knowledge gap by assessing the seroprevalence of tetanus and pertussis antibodies among Chinese health care workers.
Materials and methods
Study subjects and serum samples
Residual serum samples were sourced from health care workers of the First People’s Hospital of Wuhu who underwent their 2021 annual health checkup. The First People’s Hospital of Wuhu, located in Wuhu City, Anhui Province, is a large general hospital integrating pediatric and adult services, with nearly 1,200 employees. Individuals with any sign of respiratory diseases or immunocompromised conditions were excluded. Demographic and epidemiological data, including age, gender, and cough history in the past year, were documented. Vaccination histories were unavailable. The exemption from informed consent for study was approved by the ethics committee of the First People’s Hospital of Wuhu.
Finally, a total of 882 serum samples were procured and stored at −80°C until this investigation. The study subjects included not only doctors (266, 30.16%) and nurses (403, 45.69%), but other clinical workers such as technician (62, 7.03%), pharmacist (29, 3.29%), and non-clinical workers (122, 13.83%).
Serological testing for anti-TT IgG
Anti-TT IgG antibodies were quantified using commercial ELISA kits (Euroimmun, Lübeck, Germany), and the results were expressed in international units per milliliter (IU/ml). Based on the manufacturer’s guidelines and previous studies,Citation17,Citation20 results were categorized into four tiers: <0.01 IU/ml (seronegative), 0.01 – <0.1 IU/ml (basic immunity), ≥0.1 IU/ml (seroprotective), >0.5 IU/ml (long-term protection).
Serological testing for anti-PT IgG
Quantitative determination of anti-PT IgG antibodies was performed using ELISA kits (Euroimmun, Lübeck, Germany), calibrated against the “First International Standard for Pertussis Antiserum,” NIBSC code: 06/140, World Health Organization (WHO). Results interpretation adhered to European Centre for Disease Prevention and Control (ECDC) guidelines.Citation21 Concentration of anti-PT IgG antibodies ≥100 IU/ml serves as an indication of a recent infection within a year, 50–<100 IU/ml as an indication of infection in the past few years, and <50 IU/ml considered as negative. The cut off of the test was 5 IU/ml, therefore those who have anti-PT IgG antibodies <5 IU/ml were interpreted as undetectable.
Serological testing for anti-PT IgA
For samples exhibiting detectable anti-PT IgG (≥5.0 IU/ml), anti-PT IgA levels were assumed using available ELISA kits (Institut Virion/Serion GmbH, Germany). Concentration of anti-PT IgA ≥ 15 IU/ml was considered to be indicative of a recent pertussis infection.Citation22,Citation23
Statistical analysis
For statistical analysis, antibody concentrations below the detection line were assigned as half of the detection line (0.005IU/ml for anti-TT IgG, and 2.5 IU/ml for anti-PT IgG). Data were analyzed using Excel (Microsoft Excel Software, Version 16.30, Redmond, WA, USA), SPSS Statistics (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY, USA) and Prism 9.0 software (GraphPad Software, San Diego, CA, USA). Serum antibody concentrations in different age groups and genders were expressed by geometric mean concentration (GMC) calculated by GraphPad and were analyzed by a normality test. Normally distributed continuous variables of two or more groups were compared using a Student’s t-test or one-way ANOVA. Chi-square (and Fisher’s exact) test was used to compare rates between different gender groups or among different age groups. Two-tailed p values < .05 were considered statistically significant.
Results
Characteristics of study population
A cohort of 882 health care workers were recruited and classified into four age groups: 20–29 years (266, 30.16%), 30–39 years (407, 46.15%), 40–49 years (138, 15.65%), and 50–59 years (71, 8.05%). Of these, 238 (26.98%) were male, and 644 (73.02%) were female.
Seroprevalence of anti-TT IgG
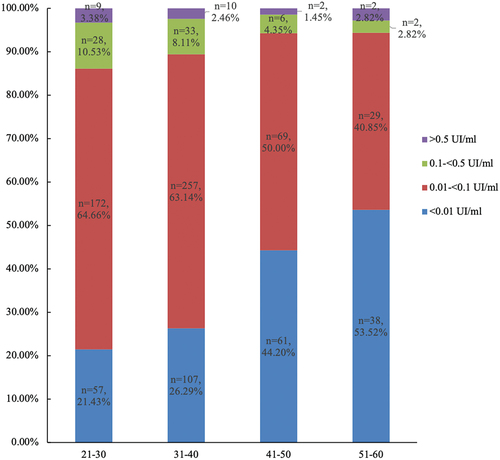
The geometric mean concentration (GMC) of anti-TT IgG antibodies across the 882 samples was 0.02 IU/ml, with no discernible variation across age or gender groups. The aggregate seroprotective rate for anti-TT IgG was 10.43% (92/882), with the highest seroprotective rate (13.91%) in the 20–29 age group, followed by 30–39 age group (10.57%), 40–49 age group (5.80%), and 50–59 (5.63%) age group. The distribution of anti-TT IgG antibody concentrations across age groups is depicted in .
Table 1. Concentration and positivity of anti-TT IgG and anti-PT IgG antibodies across age/gender groups.
Seroprevalence of anti-PT IgG
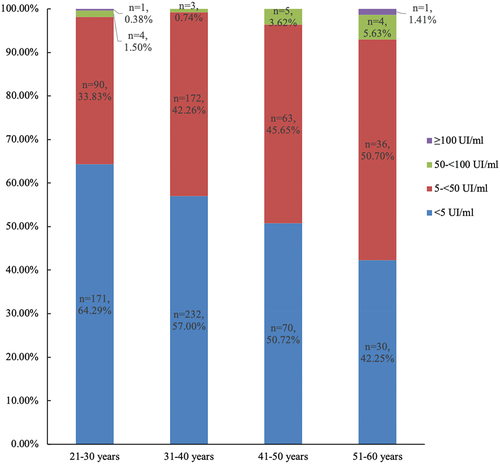
The GMC of anti-PT IgG antibodies for the 882 samples was 5.02 IU/ml. Of these, 361 subjects (40.93%) exhibited anti-PT IgG antibodies between 5 and 50 IU/ml, while 18 (2.04%) surpassed 50 IU/ml. Of these 18 subjects, 16 (1.81%) had a concentration between 50 and 100 IU/ml, and 2 (0.23%) had a concentration higher than 100 IU/ml. The non-detectable rate of anti-PT IgG antibodies was 57.03% (503/882). The distribution of these antibody concentrations across age groups is illustrated in .
The GMC and positivity of anti-PT IgG antibodies among/between different age/gender groups was shown in . The anti-PT IgG antibody levels in the 50–59 age group were higher than those in the other three age groups (p = .0002). To meet the statistical requirements, we combined the 20–29 and 30–39 age groups and the 40–49 and 50–59 age groups, respectively, when comparing the positive rates of anti-PT IgG. The positive rate of anti-PT IgG antibody in 20–39 age group and 40–59 age group was 2.23% (15/673) and 7.18% (15/209), respectively, and the difference was statistically significant (p = .0028). There was no difference in the concentration or positive rate of anti-PT IgG antibodies between males and females.
Seroprevalence of anti-PT IgA
Of the 379 samples with detectable anti-PT IgG, 29 exhibited anti-PT IgA concentrations ≥15 IU/ml, translating to a positive rate of 7.65%. The positive rates of anti-PT IgA in 20–29, 30–39, 40–49 and 50–59 age groups were 3.16% (3/95), 6.29% (11/175), 14.71% (10/68), and 12.20% (5/41), respectively.
Cough history
Serological data identified 30 recent pertussis infections, including 1 case with anti-PT IgG ≥ 100UI/ml and anti-PT IgA ≥ 15UI/ml, 1 case with anti-PT IgG ≥ 100UI/ml and anti-PT IgA < 15UI/ml. The remaining 28 cases had anti-PT IgG < 100UI/ml and anti-PT IgA ≥ 15UI/ml. Among the 30 individuals, 13 confirmed a cough history within the past year. Of the 13 subjects with a cough history, 9 had a cough that lasted more than 2 weeks, or more than 4 weeks to be exact. Of these 9 individuals, 5 had some typical pertussis traits including paroxysmal cough, whoop, intense cough with retching or vomiting after cough, or severe cough at night.
Discussion
Since the amount of TT that can cause tetanus disease is not sufficient to produce an immune response, the anti-TT antibodies are often used as indicators for vaccination with TT.Citation24 In this study, 29.82% of the subjects had lower antibody levels than basic immunity, and the higher the age group, the higher the proportion of seronegative. Among them, the proportion of immune-negative subjects in the 50–59 age group was the highest, reaching 53.52%, followed by 40–49 age group with a proportion of 44.20%. This may be due to lack of immunization in these age groups since they had been born before the introduction of tetanus immunization into China’s NIP. This finding concurs with the previous studyCitation17 in that protective tetanus antibody levels diminish with age. But since China is a big country, regional disparities in immunization strategies and coverage may lead to variations in immunity levels in the same age group.
China’s pertussis vaccination protocol has remained consistent since the 1980s, with no booster doses administrated post-age 2. The individual’s immunity to pertussis gradually weakens with age, a phenomenon that has been demonstrated in a number of studies.Citation17,Citation25,Citation26 In the present study, we evaluated the levels of serum anti-PT IgG antibodies among healthy health care workers aged between 20 to 59 years. In the four age groups, the GMCs of anti-PT IgG in the higher age groups were higher than those in the lower age groups. Given that the youngest subject in this study was already 20 years old, more than 18 years since the last vaccination of pertussis, we consider that subjects with anti-PT IgG antibodies ≥50UI/ml have a real Bordetella pertussis infection in recent years. Similarly, the positive rate of pertussis infection in the older age group were higher than in the lower age group, which may be more related to the weakening of pertussis immunity. The correlation between seroprevalence of pertussis and gender was not clear in this study, and this is in line with previous studies.Citation16,Citation19
Compared with the general population, health care workers have a higher chance of coming into contact with pertussis patients and being infected. At present, most studies on pertussis serology in China were carried out in children, adolescents or pregnant women. Only a few reports focused on adults or people of all ages. We tried to compare the positive rates of anti-PT IgG antibodies in different age groups in these reports with the anti-PT IgG positive rates of health care workers in the present study according to age groups. However, there is no uniform criterion for pertussis seropositivity. Many studies have used different cutoffs such as 80 IU/ml,Citation27 40 IU/ml,Citation18,Citation25 30 IU/ml,Citation28 and 20 IU/mlCitation29 to determine pertussis infection. Since ECDC has published their recommendations recently, our present study referenced the ECDC recommendations of 100 IU/ml and 50 IU/ml as indications of recent infection and positivity.
We have listed in all the available literature information on anti-PT IgG antibody levels in people of various ages. Among all these studies, only the study of Liu D et al.Citation17 clearly marked the calculation method of GMC, which is consistent with the method used in our present study. It was found that the level of anti-PT IgG antibody in health care workers over 40 years old was higher than that in the general population. The comparability of the results is limited by the fact that the other studies did not elaborate on how GMC was calculated, as well as regional differences and different kits used. However, our study found that more than half of health care workers had non-detectable anti-PT IgG antibodies, indicating that they were vulnerable to pertussis. Health care workers are exposed to a large number of people every day, and once infected, it is easy to cause the spread of pertussis. So, it is important to strengthen the surveillance and vigilance of health care workers with pertussis infection.
Table 2. Anti-PT IgG antibody levels in different age groups reported in different studies.
Since the methods for laboratory confirmation on Bordetella pertussis are not routinely used in China, the diagnosis of pertussis is usually based on clinical presentation, which is typically characterized by a paroxysmal cough and whooping. However, pertussis is no longer just a childhood disease. In adults, the typical symptoms of pertussis are not often present, and atypical symptoms such as persistent cough are not common.Citation31 In our present study, only 13 of the 30 subjects who were serologically diagnosed with a recent pertussis infection had cough in the past year. Among these 13 subjects with cough, only 9 had persistent cough, and 5 of these 9 had typical pertussis cough. Therefore, the diagnosis of pertussis only based on clinical manifestations is bound to cause missed diagnosis of this disease. It is not difficult to understand why a number of studies from different regions of China have reported that the actual incidence of pertussis in China has been underestimated.Citation14–16,Citation19
There are some limitations in this study. First, the sample is drawn from a single district, which may not represent the whole country. Multi-center studies should be implemented in the future. Second, there was no definitive cutoff values for antibody level of pertussis infection, various cutoffs were used in different studies. We referenced the recommendations from ECDC, which are the first recommendations published. Third, the vaccination status of participants was unavailable. However, adults included in this study were older than 20 years, and they should have not received any booster vaccination because no booster dose is given after two years of age in China. Therefore, the anti-PT antibodies detected were most likely due to infection rather than vaccination. The distribution of tetanus antibody level by age groups also indicated the real proportion of subjects with vaccination could be probably much lower than the estimate of this study.
In summary, our research suggests that a significant portion of health care workers aged 20–59 in China are susceptible to pertussis, with over 2% showing positive anti-PT IgG antibodies. The seroprotective rate of anti-TT IgG was approximately 10%, declining with age. Booster vaccinations against pertussis and tetanus for health care workers in China warrant consideration.Citation1
Authors’ contributions
Wei Shi,Bingsong Wang, and Kaihu Yao designed the study and handled the manuscript; Wei Shi, Qinghong Meng, Xianlai Zhang, Zhen Li, Fei Ying, and Linyan Cong collected the sera and performed the ELISA experiments; Wei Shi, Bingsong Wang, and Fang He worked with the statistical analysis.
Acknowledgments
The authors sincerely thank the Euroimmun company for providing ELISA kits, and thank the staff of the First People’s Hospital of Wuhu for providing their leftover serum samples.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Centers for Disease Control and Prevention. Tetanus. [accessed 2023 Sep 5]. https://www.cdc.gov/tetanus/.
- Centers for Disease Control and Prevention. Pertussis (whooping cough). [accessed 2023 Sep 5]. https://www.cdc.gov/pertussis/.
- World Health Organization (WHO). Immunization data: comparison of immunization coverage for DTP vaccination coverage and reported cases for tetanus. [accessed 2024 Jan 5]. https://immunizationdata.who.int/compare.html?COMPARISON=type1__WIISE/MT_AD_COV_LONG+type2__WIISE/MT_AD_INC_LONG+option1__DTP_coverage+option2__TTETANUS_cases&CODE=CHN&YEAR=.
- Yao K. Immunization to prevent non-neonatal tetanus. Zhonghua Er Ke Za Zhi. 2020;58(3):259–7. doi:10.3760/cma.j.issn.0578-1310/2020.03.024. (In Chinese).
- Wang C, Liu S, Shao Z, Yin Z, Chen Q, Ma X, Ma C, Wang Q, Wang L, Deng J. et al. Guidelines for the use of post-traumatic tetanus vaccines and passive immune preparation. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):167–72. doi:10.3760/cma.j.issn.0254-6450.2020.02.006. (In Chinese).
- PERISCOPE Consortium. PERISCOPE: road towards effective control of pertussis. Lancet Infect Dis. 2019;19(5):179–86. doi:10.1016/S1473-3099(18)30646-7.
- Winter K, Glaser C, Watt J, Harriman K. Pertussis epidemic–California, 2014. MMWR Morb Mortal Wkly Rep. 2014;63:1129–32.
- Marshall KS, Quinn HE, Pillsbury AJ, Maguire JE, Lucas RM, Dey A, Beard FH, Macartney KK, Mcintyre PB. Australian vaccine preventable disease epidemiological review series: pertussis, 2013–2018. Commun Dis Intell (2018). 2022;46. doi:10.33321/cdi.2022.46.3.
- World Health Organization (WHO). Immunization data: comparison of immunization coverage for DTP vaccination coverage and reported cases for pertussis. [accessed 2023 Sep 5]. https://immunizationdata.who.int/compare.html?COMPARISON=type1__WIISE/MT_AD_COV_LONG+type2__WIISE/MT_AD_INC_LONG+option1__DTP_coverage+option2__PERTUSSIS_cases&CODE=CHN&YEAR=.
- Sheridan SL, Frith K, Snelling TL, Grimwood K, Mcintyre PB, Lambert SB. Waning vaccine immunity in teenagers primed with whole cell and acellular pertussis vaccine: recent epidemiology. Expert Rev Vaccines. 2014;13(9):1081–106. doi:10.1586/14760584.2014.944167.
- Mcgirr A, Fisman DN. Duration of pertussis immunity after DTaP immunization: a meta-analysis. Pediatrics. 2015;135(2):331–43. doi:10.1542/peds.2014-1729.
- Tartof SY, Lewis M, Kenyon C, White K, Osborn A, Liko J, Zell E, Martin S, Messonnier NE, Clark TA. et al. Waning immunity to pertussis following 5 doses of DTaP. Pediatrics. 2013;131(4):e1047–52. doi:10.1542/peds.2012-1928.
- Plans-Rubió P. Vaccination coverage for routine vaccines and herd immunity levels against measles and pertussis in the World in 2019. Vaccines (Basel). 2021;9(3):256. doi:10.3390/vaccines9030256.
- De Melker HE, Versteegh FG, Conyn-Van Spaendonck MA, Elvers LH, Berbers GA, Van Der Zee A, Schellekens JF. Specificity and sensitivity of high levels of immunoglobulin G antibodies against pertussis toxin in a single serum sample for diagnosis of infection with bordetella pertussis. J Clin Microbiol. 2000;38(2):800–6. doi:10.1128/JCM.38.2.800-806.2000.
- Zhang J, Deng J, Yang Y. Pertussis vaccination in Chinese children with increasing reported pertussis cases. Lancet Infect Dis. 2022;22(1):21–2. doi:10.1016/S1473-3099(21)00752-0.
- Zhang C, Hu W, Wang R, Wang Y, Li Y, Lv Y, Li W, Si Y, Zhang S. Seroepidemiology of pertussis and diphtheria among healthy adults in Shaanxi Province, Northwest China: a large - scale cross-sectional study. Hum Vaccin Immunother. 2022;18(6):2133913. doi:10.1080/21645515.2022.2133913.
- Liu D, Cheng X, Wei S, Yuan L, Chen C, Yao K. Decline of serologic immunity to diphtheria, tetanus and pertussis with age suggested a full life vaccination in mainland China. Hum Vaccin Immunother. 2021;17(6):1757–62. doi:10.1080/21645515.2020.1840253.
- Chen Z, Pang J, Zhang N, Chen N, Ding Y, He Q. Seroprevalence study of pertussis in adults at childbearing age and young infants reveals the necessity of booster immunizations in adults in China. Vaccines (Basel). 2022;10(1):84. doi:10.3390/vaccines10010084.
- Zhang Z, Pan J, Chen M, Zhang T, Li J, Lu L. Seroepidemiology of pertussis in China: a population-based, cross-sectional study. Vaccine. 2021;39(12):1687–92. doi:10.1016/j.vaccine.2021.02.032.
- Meng Q, Oian Q, Li L, Liu D, Gao W, Yuan L, Yao K. The maternal antibody against diphtheria, tetanus and pertussis showed distinct regional difference in China. BMC Pediatr. 2019;19(1):480. doi:10.1186/s12887-019-1860-5.
- European Centre for Disease Prevention and Control (ECDC). Laboratory diagnosis and molecular surveillance of Bordetella pertussis. Stockholm: ECDC; 2022.
- Subissi L, Rodeghiero C, Martini H, Litzroth A, Huygen K, Leroux-Roels G, Piérard D, Desombere I. Assessment of IgA anti-PT and IgG anti-ACT reflex testing to improve Bordetella pertussis serodiagnosis in recently vaccinated subjects. Clin Microbiol Infect. 2020;26(5):645. doi:10.1016/j.cmi.2019.10.001.
- Chen Z, Liu X, Zhang Y, Peng X, Zhang N, Chen N, Li Y, He Q. Evaluation of serum anti-pertussis toxin IgA antibodies for the diagnosis of bordetella pertussis infection in young children. J Infect Public Health. 2023;16(8):1167–1173. doi:10.1016/j.jiph.2023.05.028.
- Warrener L, Bwogi J, Andrews N, Samuel D, Kabaliisa T, Bukenya H, Brown K, Roper MH, Featherstone DA, Brown D. Serum anti-tetanus and measles antibody titres in Ugandan children aged 4 months to 6 years: implications for vaccine programme. Epidemiol Infect. 2018;146(9):1151–6. doi:10.1017/S0950268818000948.
- Zhang Y, Chen Z, Zhao J, Zhang N, Chen N, Zhang J, Li S, He Q. Increased susceptibility to pertussis in adults at childbearing age as determined by comparative seroprevalence study, China 2010–2016. J Infect. 2019;79(1):1–6. doi:10.1016/j.jinf.2019.04.011.
- Chen Q, Wang W, Shi X, Xu Y, Zhu Y, Wu Y, Wang Z, Sun H, Sun X. Seroepidemiology of pertussis in the East of China: estimates of incidence of infection in adolescents and adults pre- and post-COVID-19. Front Public Health. 2022;10:1054617. doi:10.3389/fpubh.2022.1054617.
- He H, Zhu Y, Jin M, Zhou Y, Tang X, Yan R, Deng X, Chen K. The decline in immunity and circulation of pertussis among Chinese population during the COVID-19 pandemic: a cross-sectional sero-epidemiological study. Vaccine. 2022;40(48):6956–62. doi:10.1016/j.vaccine.2022.10.020.
- Zhang Q, Han F, Nie Q, Ren H, Zhang B, Liu Q, He Q, Shao Z. Seroprevalence of antibodies to pertussis and diphtheria among healthy adults in China. J Infect. 2011;63(6):441–6. doi:10.1016/j.jinf.2011.07.018.
- Wang CQ, Zhu QR. Seroprevalence of bordetella pertussis antibody in children and adolescents in China. Pediatr Infect Dis J. 2011;30(7):593–6. doi:10.1097/INF.0b013e31820eaf88.
- Chen Z, Pang J, Zhang Y, Ding Y, Chen N, Zhang N, He Q. Seroprevalence of pertussis in adults at childbearing age pre- and post- COVID-19 in Beijing, China. Vaccines (Basel). 2022;10(6):872. doi:10.3390/vaccines10060872.
- Macina D, Evans KE. Bordetella pertussis in school-age children, adolescents, and adults: a systematic review of epidemiology, burden, and mortality in Asia. Infect Dis Ther. 2021;10(3):1115–40. doi:10.1007/s40121-021-00439-1.