Dear Editor,
We read R. Awasthia et al.’s paper “Kymriah® (tisagenlecleucel) – An overview of the clinical development journey of the first approved CAR-T therapy”Citation1 with great interest. The authors describe the pathway of clinical evolution of tisagenlecleucel, the first autologous anti-CD19 chimeric antigen receptor (CAR) T-cell immunotherapy approved for the treatment of three relapsed/refractory (r/r) hematologic malignancies: pediatric and young adult B-acute lymphoblastic leukemia (B-ALL), diffuse large B-cell lymphoma (DLBCL), and follicular lymphoma (FL). We congratulate the authors for the qualitative description of this topic.
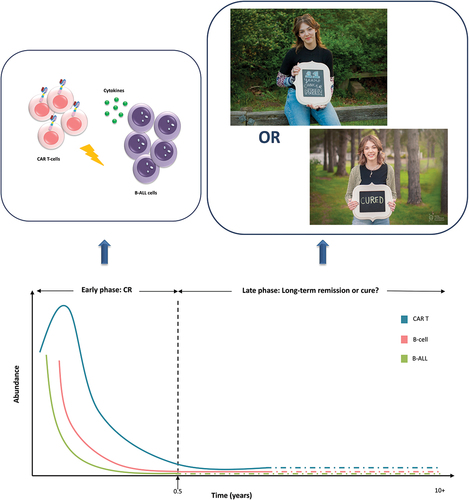
In Figure 1 of R. Awasthia et al.'s paper, the phrase “First pediatric patient, Emily Whitehead, treated with CAR T therapy 10 years ago remains cancer-free” is presented as Kymriah’s achievement in 2022. Additionally, the first paragraph of the Efficacy section mentions “… the first patient treated with tisagenlecleucel in a clinical trial for pediatric ALL recently celebrated 10 years in remission.” In both points, the authors argue that the young girl remains in long-term remission but not declared as cured. As the evidence around CAR T-cell therapies increases, the researchers feel more confident in believing that Emily is actually cured.Citation2 In light of the findings on long-lived functionally active CAR T-cells over a decade in two chronic lymphocytic leukemia (CLL) patientsCitation3 and given the absence of relevant long-term data on Emily’s case, her actual disease status remains unclear; was the B-ALL burden completely eradicated early soon after the CAR T-cell infusion, or some residual B-ALL cells remained over a decade and were being destroyed by potentially remaining anti-CD19 CAR T-cells?
Early complete disease remission (CR) in the child patient was associated with a persistent molecular remission. Analysis of minimal residual disease (MRD) by deep DNA sequencing of the total rearranged IGH gene locus revealed the malignant clone was undetected in the blood or marrow on day 180 post infusion.Citation4 If a residual leukemic clone was suppressed by remaining anti-CD19 CAR T-cells without relapsing for 11 consecutive years, the following questions are reasonably raised:
During this period, have CAR T-cells been detected in the blood or marrow, contributing to the alleged long-term suppression of the remaining leukemic clone and even at undetectable MRD levels?
How long B-cell aplasia lasted and has immunoglobulin replacement been administered?
How unique could the cytotoxic potential of the hypothetical remaining few CAR T-cells be, so that for 11 consecutive years they suppress leukemia? Such a clinical course is not in keeping with the hitherto usual results, where prolonged CRs are rarely achieved and most patients relapse after months.Citation5,Citation6
To date, Kymriah has been administered to more than 7,000 r/r B-ALL, r/r DLBCL, and r/r FL patients in the real-world setting and in clinical trials.Citation1 In addition, more than 34,400 patients worldwide have received commercial CAR T-cell immunotherapies.Citation7 Although CAR T-cells are incredibly potent in killing tumor cells, to the best of our knowledge, only three cases have been published where the remission lasted (or lasts) more than 10 years: a) In 2010, anti-CD19 CAR T-cells were administered in 2 advanced, chemotherapy-resistant CLL patients – first patient Bill Ludwig 65 years old, and second patient Doug Olson 64 years oldCitation3,Citation8; b) in 2012, third patient the 6-year-old girl with r/r pre-B-cell ALL, Emily Whitehead treated with the same product.Citation4 In the forthcoming years, as clinical trial and real-world data are expected to become available, more patients may be found as cancer-free after a decade follow-up.Citation3 Remarkably, the first and third patients were administered very high doses and both became gravely ill due to cytokine release syndrome grade 3/4 (). All three patients were leukemia-free for more than 10 years, and in January 2021, Bill Ludwig died due to COVID-19 infection.Citation9
Both academics and biotechnologists are focused on manufacturing more potent and less toxic CAR T-cells.Citation10,Citation11 Optimal in vivo clinical expansion and functional persistence are important determinants to ensure long-term therapeutic efficacy.Citation3,Citation12 Current CAR T-cell products have been designed to combine robust in vivo expansion with long-term persistence; however, it seems that such cell doses rarely offer prolonged disease remission or even cure, but they can increase patients’ survival from months to a few years.Citation5 Because high-dose regimens have been associated with long-term remissions,Citation5 we speculate that they have the required ability to randomly eliminate the malignant clone early in the first months after infusion.Citation13 The over a decade disease-free duration of the first and third patients could be the outcome of the very high anti-CD19 CAR T-cell doses ().
Therefore, the following combination may represent a new therapeutic strategy:
Very high CAR T-cell numbers infused in a split-dosing manner for safety reasons, with longer intervals between infusions.
High CAR T-cell fitness to promote robust anti-leukemic capacity.
Defined composition of CAR T-cell subpopulations in the infused product to help interpret the outcomes.Citation13
Short-term CAR T-cell persistence to minimize or avoid negative relapses, decrease long-term life-threatening infections and prevent potential secondary malignancies.Citation7,Citation14
Effective control of the disease burden before the infusion.
In contrast, in current applied practice, both parameters of in vivo expansion and persistence are pursued to be augmented. However, the proposed strategy could increase the likelihood of early eradication of the malignancy by chance.
How could one convincingly answer the question ”have B-ALL cells been present for more than a decade in Emily’s blood or marrow, or was she cured early after recovery from the IL-6 shock?”Citation15 If Emily still has persisting CAR T-cells and tiny amounts of remaining B-ALL cells, obviously, the leukemic clone has been inactive for 11 years but there is no such evidence in the literature (). In such a scenario, CAR T-cells are expected to disappear in approximately 14–15 years post infusion due to senescence,Citation13,Citation16,Citation17 eliminating also B-cell aplasia. If the B-ALL clone will not reappear, this can confirm the hypothesis of early cure. In the opposite scenario, if CAR T-cells have no longer been detected and B-cell aplasia has been gradually restored without leukemia reappearance, this constitutes an even stronger argument in favor of a potential early cure.
Figure 1. Long-term remission or cure? The leukemic clone was not detected in the blood or marrow by deep molecular sequencing on day 180 after CAR T-cell administration.Citation4 The actual B-ALL cell and CAR T-cell status of Emily remains unclear, on whether the malignant burden was completely eradicated soon after the CAR T-cell infusion, or whether some potentially remaining active CAR T-cells destroyed very few residual B-ALL cells. Emily Whitehead remains 11-year cancer-free celebrating a cure, with early disease eradication constituting a possible hypothesis. Photo credit: reproduction with permission from the Emily Whitehead Foundation.
Author contributions
DB and SB were involved in the conception and design; DB drafted the original paper; SB revised it critically for intellectual content, edited it, and designed the figure; DB and SB approved the final version to be published.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Awasthi R, Maier HJ, Zhang J, Lim S. Kymriah® (tisagenlecleucel) - an overview of the clinical development journey of the first approved CAR-T therapy. Hum Vaccin Immunother. 2023;19(1):2210046. doi:10.1080/21645515.2023.2210046.
- Whitehead E. First pediatric patient to receive CAR T-cell therapy, celebrates cure 10 years later. Children’s Hospital of Philadelphia [accessed 2022 May 11]. https://www.chop.edu/news/emily-whitehead-first-pediatric-patient-receive-car-t-cell-therapy-celebrates-cure-10-years.
- Melenhorst JJ, Chen GM, Wang M, Porter DL, Chen C, Collins MA, Gao P, Bandyopadhyay S, Sun H, Zhao Z, et al. Decade-long leukemia remissions with the persistence of CD4+ CAR T cells. Nature. 2022;602:503–4. doi:10.1038/s41586-021-04390-6.
- Grupp S, Kalos M, Barrett D, Aplenc R, Porter D, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi:10.1056/NEJMoa1215134.
- Cappell KM, Kochenderfer JN. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. 2023;20(6):359–371. doi:10.1038/s41571-023-00754-1.
- Ledford H. Last-resort cancer therapy holds back disease for more than a decade. Nature. 2022;602(7896):196. doi:10.1038/d41586-022-00241-0.
- Levine BL, Pasquini MC, Connolly JE, Porter DL, Gustafson MP, Boelens JJ, Horwitz EM, Grupp SA, Maus MV, Locke FL, et al. Unanswered questions following reports of secondary malignancies after CAR-T cell therapy. Nat Med. 2024. doi:10.1038/s41591-023-02767-w.
- Kalos M, Levine B, Porter D, Katz S, Grupp S, Bagg A, June C. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi:10.1126/scitranslmed.3002842.
- Ludwig B. Patient who helped pioneer cancer immunotherapy at Penn, dies at 75 of COVID-19. The Philadelphia Inquirer, LLC; 2023 [accessed 2021 Feb 17]. https://www.inquirer.com/obituaries/covid-19-claims-bill-ludwig-cancer-immunotherapy-pioneer-penn-20210217.html.
- Maus M. A decade of CAR T cell evolution. Nat Cancer. 2022;3(3):270–271. doi:10.1038/s43018-022-00347-4.
- Flemming A. Cytotoxic CD4+ CAR T cells implicated in long-term leukaemia remission. Nat Rev Immunol. 2022;22(3):146. doi:10.1038/s41577-022-00689-1.
- Testa U, Sica S, Pelosi E, Castelli G, Leone G. CAR-T cell therapy in B-cell acute lymphoblastic leukemia. Mediterr J Hematol Infect Dis. 2024;16(1):e2024010. doi:10.4084/MJHID.2024.010.
- Bouzianas D, Bouziana S. A decade of CD4+ chimeric antigen receptor T-cell evolution in two chronic lymphocytic leukemia patients: were chronic lymphocytic leukemia cells present? Explor Target Antitumor Ther. 2023;4(5):1128–1135. doi:10.37349/etat.2023.00186.
- Spiegel JY, Jain MD, Nastoupil LJ, Tamaresis J, Ghobadi A, Lin Y, Lekakis LJ, Reagan PM, Oluwole OO, McGuirk JP, et al. Five year outcomes of patients with large B-Cell lymphoma treated with standard-of-care Axicabtagene Ciloleucel: results from the US lymphoma CAR-T cell consortium. Blood. 2023;142(Supplement 1):1032. doi:10.1182/blood-2023-179868.
- Rosenbaum L. Tragedy, perseverance, and chance - the story of CAR-T therapy. N Engl J Med. 2017;377(14):1313–1315. doi:10.1056/NEJMp1711886.
- Biasco L, Scala S, Basso Ricci L, Dionisio F, Baricordi C, Calabria A, Giannelli S, Cieri N, Barzaghi F, Pajno R, et al. In vivo tracking of T cells in humans unveils decade-long survival and activity of genetically modified T memory stem cells. Sci Transl Med. 2015;7(273):273ra13. doi:10.1126/scitranslmed.3010314.
- Oliveira G, Ruggiero E, Stanghellini MT, Cieri N, D’Agostino M, Fronza R, Lulay C, Dionisio F, Mastaglio S, Greco R, et al. Tracking genetically engineered lymphocytes long-term reveals the dynamics of T cell immunological memory. Sci Transl Med. 2015;7(317):317ra198. doi:10.1126/scitranslmed.aac8265.
