ABSTRACT
Hepatoid adenocarcinoma of the lung (HAL) is a rare and aggressive subtype of lung cancer. The prognosis for patients with HAL is generally poor and currently, there are only limited treatment options. Here, we present a case of a 47-year-old male diagnosed with locally advanced-stage HAL who achieved a remarkably long disease-free survival after receiving neoadjuvant and adjuvant camrelizumab plus chemotherapy and surgery. This case highlights the potential of immunochemotherapy plus surgery in improving outcomes for patients with HAL.
Introduction
Hepatoid adenocarcinoma of the lung (HAL) is an uncommon variant of lung cancer characterized by morphological and immunohistochemical features resembling hepatocellular carcinoma,Citation1 and it is generally associated with poor survival.Citation2,Citation3 The survival for patients with resectable HAL spans from 3 to 108 months, with less than 50% beyond 24 months.Citation4
Conventional treatment modalities such as surgery, chemotherapy, radiotherapy, and targeted therapy have shown limited efficacy.Citation5 Due to its rarity and aggressive nature, the optimal treatment approach for HAL remains unclear, and novel treatment options are urgently needed to improve outcomes for these patients. Immune checkpoint inhibitors, including camrelizumab, have already demonstrated encouraging results in lung cancer.Citation6 Camrelizumab is a humanized high-affinity IgG4-kappa anti-programmed death-1 (PD-1) monoclonal antibody developed by China Jiangsu Hengrui Pharmaceuticals Co., Ltd. It inhibits the PD-1 and its downstream signaling pathways, releasing the immunosuppressive state and restoring the immune mechanism against tumor cells.Citation7 However, the application of immunotherapy in HAL and its potential for achieving prolonged survival has not been thoroughly investigated yet. Herein, we present a noteworthy case of locally advanced-stage HAL, in which the patient achieved an extended period of disease-free and overall survival after receiving camrelizumab plus surgery.
Patient presentation
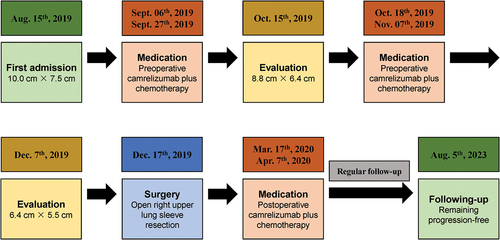
A 47-year-old male presented to our hospital with a history of persistent cough and occasional hemoptysis for over two weeks (). The patient had a history of hypertension for over 5 years and a 30-pack-year history of cigarette smoking. Physical examination revealed decreased breath sounds in the right lung. A chest computed tomography (CT) scan demonstrated a large mass (10.0 cm × 7.5 cm) located in the right upper lobe of the lung with obstruction in the apical and posterior segment bronchi, accompanied by multiple enlarged mediastinal and hilar lymph nodes (). Positron emission tomography-computed tomography (PET-CT) only revealed18F-fluoro-deoxyglucose (FDG)-avid lung lesions. No abnormalities were observed in brain magnetic resonance imaging (MRI) or abdominal ultrasound examinations. Additionally, all tumor biomarkers fell within the normal range, including alpha-fetoprotein (AFP) being 2.8 ng/ml. Hence, the initial staging for this patient was determined as T4N0M0, stage IIIa.
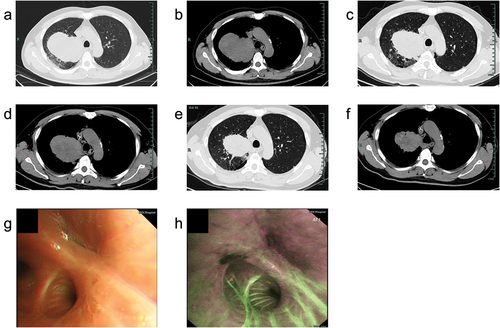
Figure 2. Radiological and bronchoscopic examination of the mass. (a,b) Computed tomography (CT) image of the mass at baseline; (c,d) CT image of the lesion after the 2nd cycle treatment; (e,f) CT image of the lesion after the 4th cycle treatment; (g) White light bronchoscopic image of obstruction by neoplasm in the apical segment of the right upper lobe; (h) Autofluorescence bronchoscopic image of obstruction by neoplasm in the apical segment of the right upper lobe.
Bronchoscopy revealed the presence of a lesion within the right upper lobe bronchus (), consistent with findings from CT scans. In combination with the immunohistochemical (IHC) results of brush specimen and biopsy specimen, the final report indicated poorly differentiated non-small cell lung cancer and the specific subtypes of squamous cell carcinoma, adenocarcinoma, or neuroendocrine tumor could not be confirmed based on the profile: P40 (-), CK7 (+), TTF-1 (-), Napsin A (-), CK5/6 (-), ALK-lung (-). PD-L1 expression was not assessed and gene testing for any mutation, like epidermal growth factor receptor (EGFR) was not considered at the first time of diagnosis.
The disease was initially analyzed as potentially resectable, and after discussion within a multidisciplinary team including thoracic surgeons, radiology experts, respiratory physicians, oncologists, pathologists, and anesthesiologists, the patient was started on systemic treatment with camrelizumab, an anti-PD-1 immune checkpoint inhibitor, plus platinum-based dual-drug chemotherapy. Specifically, the patient received intravenous camrelizumab (200 mg), nab-paclitaxel (260 mg/m2), and cisplatin (75 mg/m2) every three weeks. After two cycles of preoperative camrelizumab plus chemotherapy treatment, a repeat CT scan revealed a partial response with a significant reduction in the size of the lung mass (8.8 cm × 6.4 cm) (). The patient’s symptoms improved, and he experienced a notable gain in quality of life. During the course of preoperative medication, the patient’s treatment-related adverse events (trAEs) were carefully monitored. The patient experienced anemia, impaired liver function, and numbness in fingertips. However, according to the Common Terminology Criteria for Adverse Events (CTCAE) V.5.0, these trAEs were all Grade 1–2, only requiring symptomatic management with medications. Following the completion of four cycles of the medication, a repeat CT scan showed further regression of the lung mass (6.4 cm × 5.5 cm) (), and the patient was deemed a candidate for surgical resection.
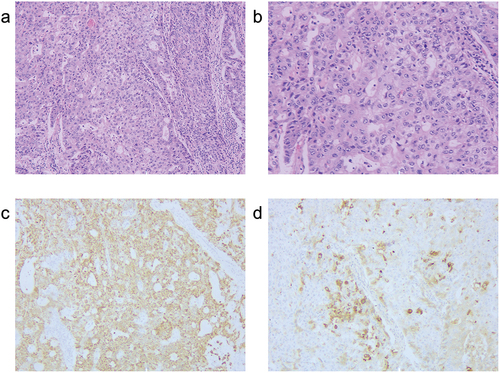
The patient underwent an open right upper lung sleeve resection with complete lymphadenectomy. The operative duration was 151 minutes with an estimated blood loss of 150 milliliters. Postoperative pathology of the patient revealed poorly differentiated adenocarcinoma of the right lung periphery, with negative bronchial margins and no pleural involvement. The pathological response was as follows: 60% viable tumor cells, 20% necrotic area, and 20% stroma, indicating non-major pathological response. There were no metastases observed in 23 lymph nodes from stations 2/4/7/8/10/11. IHC analysis showed positive staining for pan-cytokeratin (pan-CK), cytokeratin 7 (CK7), caudal-related homeobox transcription factor 2 (CDX2), hepatocyte, and α-fetoprotein (AFP), while P40, thyroid transcription factor 1 (TTF-1), Napsin A, chromogranin A (CgA), and synuclein (syn) were negative (). Based on comprehensive clinical examinations and pathological findings, metastases to the liver and gastrointestinal tract were ruled out, leading to the final diagnosis of primary HAL. The postoperative course was uneventful.
Figure 3. Hematoxylin and eosin (HE) staining and immunohistochemical (IHC) staining of the surgical specimen. (a) HE staining (magnification, ×100); (b) HE staining (magnification, ×200); (c) IHC analysis for hepatocytes (magnification, ×100); (d) IHC analysis for AFP (magnification, ×100).
The patient received an additional 2 cycles of adjuvant camrelizumab plus chemotherapy postoperatively, following the same regimen as preoperatively. Subsequently, regular postoperative follow-up was conducted. Up to the most recent follow-up, the patient experienced an exceptional outcome, remaining disease-free, and the overall survival exceeded almost 5 years from the initial diagnosis.
Discussion
This case report sheds light on the potential benefits of neoadjuvant and adjuvant camrelizumab plus platinum-based dual-drug chemotherapy and surgery in improving survival outcomes for locally advanced HAL patients and underscores the need for further research to elucidate its role in managing HAL.
The most frequently affected individuals are middle-aged or older men who have a history of smoking, and approximately 80% patients have elevated AFP levels.Citation4,Citation8 In this case, the patient exhibited similar clinical characteristics but normal levels of AFP and normal morphology of liver. This discrepancy may be related to the partial positivity of AFP in the IHC examination of the lung lesion in this patient. According to the HAL diagnostic criteria redefined by Haninger,Citation9 any aberration in patients expressing hepatic differentiation markers, regardless of AFP levels, should be diagnosed as HAL. Nevertheless, there is no definite correlation between AFP levels and prognosis in HAL patients.Citation10
The decision to offer neoadjuvant and adjuvant immunochemotherapy instead of chemoradiation for locally advanced non-small cell lung cancer in initially deemed unresectable patients involves several considerations. While clinical trials for neoadjuvant NSCLC have primarily focused on resectable patients, the choice of treatment approach for unresectable cases is influenced by evolving treatment paradigms and individual patient characteristics. Evolving evidenceCitation11 supported that preoperative immunochemotherapy may be considered when there is a possibility of downstaging the tumor, making it amenable to surgical resection.
In this case, camrelizumab plus chemotherapy demonstrated a significant antitumor effect, leading to a partial response and making the patient eligible for curative-intent surgery. The combination of immunochemotherapy and surgical resection may have contributed to the extended survival seen in this patient. Previous studies have reported cases of sintilimab or durvalumab application in advanced-stage HAL.Citation4,Citation12,Citation13 However, the survival benefit was limited due to the development of interstitial pneumonia or infectious complications following immunotherapy. In cases of resectable hepatoid adenocarcinoma of the lung, perioperative immunotherapy has the potential to target micro metastatic disease, effectively downstaging the tumor, reducing the likelihood of disease recurrence and improving long-term survival outcomes.
It is important to acknowledge that this is a single-case report, and definitive conclusions cannot be drawn from a solitary observation. Larger-scale clinical studies, including prospective trials, are warranted to validate the efficacy and safety of this therapeutic approach. Furthermore, understanding the molecular basis of HAL is crucial for identifying potential biomarkers of response to immunotherapy. Investigating the tumor’s immune microenvironment, mutational profile, and expression of immune checkpoint proteins such as PD-L1 may provide insights into the patient’s favorable response to camrelizumab and guide patient selection for similar treatment strategies in the future.
In conclusion, this case report highlights the potential benefits of camrelizumab plus chemotherapy and surgery in patients with locally advanced HAL. Immunotherapy, especially with anti-PD-1 agents, should be considered as a viable treatment option for advanced-stage HAL patients, potentially leading to prolonged disease-free and overall survival. Further studies and clinical trials are needed to validate these findings and elucidate the optimal management for this rare and aggressive lung cancer subtype.
Author contributions statement
Xuhua Huang: Conceptualization; Investigation; Methodology; Writing – original draft; Writing – review & editing. Linhai Zhu: Conceptualization; Investigation; Methodology; Writing – original draft. Weifeng Pan: Investigation; Validation. Jian Hu: Conceptualization; Funding acquisition; Investigation; Resources; Supervision; Writing – review & editing. All authors agree to be accountable for all aspects of the work.
Ethics statement
The Clinical Research Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine (FAHZU) approved the study (Grant No. IIT20230461A), and written informed consent was obtained before all the treatments from the patient.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bonis A, Dell’amore A, Verzeletti V, Melan L, Zambello G, Nardocci C, Comacchio GM, Pezzuto F, Calabrese F, Rea F, et al. Hepatoid adenocarcinoma of the lung: a review of the most updated literature and a presentation of three cases. J Clin Med. 2023;12(4):12. doi:10.3390/jcm12041411.
- Chen Z, Ding C, Zhang T, He Y, Jiang G. Primary hepatoid adenocarcinoma of the lung: a systematic literature review. Onco Targets Ther. 2022;15:609–4. doi:10.2147/OTT.S364465.
- Hiroshima K, Iyoda A, Toyozaki T, Haga Y, Baba M, Fujisawa T, Ishikura H, Ohwada H. Alpha-fetoprotein-producing lung carcinoma: report of three cases. Pathol Int. 2002;52:46–53. doi:10.1046/j.1440-1827.2002.01311.x.
- Chen L, Han X, Gao Y, Zhao Q, Wang Y, Jiang Y, Liu S, Wu X, Miao L. Anti-PD-1 therapy achieved disease control after multiline chemotherapy in unresectable KRAS-Positive hepatoid lung adenocarcinoma: a case report and literature review. Onco Targets Ther. 2020;13:4359–4364. doi:10.2147/OTT.S248226.
- Chen HF, Wang WX, Li XL, Xu CW, Du KQ, Zhu YC, Fang M-Y. Hepatoid adenocarcinoma of the lung with EGFR mutation and the response to Tyrosine Kinase inhibitors. J Thorac Oncol. 2019;14:217–219. doi:10.1016/j.jtho.2019.04.032.
- Hou X, Shi X, Luo J. Efficacy and safety of camrelizumab (a PD-1 inhibitor) combined with chemotherapy as a neoadjuvant regimen in patients with locally advanced non-small cell lung cancer. Oncol Lett. 2022;24:215. doi:10.3892/ol.2022.13336.
- Markham A, Keam SJ. Camrelizumab: first global approval. Drugs. 2019;79:1355–1361. doi:10.1007/s40265-019-01167-0.
- Yao Y, Guan X, Bao G, Liang J, Li T, Zhong X. Whole-exome sequencing and bioinformatics analysis of a case of non-alpha-fetoprotein-elevated lung hepatoid adenocarcinoma. Front Pharmacol. 2022;13:945038. doi:10.3389/fphar.2022.945038.
- Haninger DM, Kloecker GH, Bousamra Ii M, Nowacki MR, Slone SP. Hepatoid adenocarcinoma of the lung: report of five cases and review of the literature. Mod Pathol. 2014;27(4):535–542. doi:10.1038/modpathol.2013.170.
- Hou Z, Xie J, Zhang L, Dai G, Chen Y, He L. Hepatoid Adenocarcinoma of the lung: a systematic review of the literature from 1981 to 2020. Front Oncol. 2021;11:702216. doi:10.3389/fonc.2021.702216.
- Lei J, Zhao J, Gong L, Ni Y, Zhou Y, Tian F, Liu H, Gu Z, Huang L, Lu Q, et al. Neoadjuvant Camrelizumab plus platinum-based chemotherapy vs chemotherapy alone for Chinese patients with resectable stage IIIA or IIIB (T3N2) non–small cell lung cancer. JAMA Oncol. 2023;9:1348–1355. doi:10.1001/jamaoncol.2023.2751.
- Basse V, Schick U, Guéguen P, Le Maréchal C, Quintin-Roué I, Descourt R, Simon H, Uguen A, Quéré G. A mismatch repair–deficient hepatoid adenocarcinoma of the lung responding to anti–PD-L1 durvalumab therapy despite no PD-L1 expression. J Thorac Oncol. 2018;13:120–122. doi:10.1016/j.jtho.2018.03.004.
- Li Y, Liu L, Wang D, Tan X, Sui Y, Yang H. Hepatoid adenocarcinoma of the lung with PD-L1 expression and the response to anti-PD-1 therapy: a case report. Front Oncol. 2023;13:1257931. doi:10.3389/fonc.2023.1257931.