ABSTRACT
In Singapore, population aging and rising life expectancy are increasing herpes zoster (HZ) burden, which may be reduced by vaccination. The present study modeled the public health impact of HZ vaccination in Singapore using ZOster ecoNomic Analysis (ZONA) model adapted with Singapore-specific key model inputs, where available. Base case analysis was conducted in adults ≥ 50 years of age (YOA), exploring three vaccination strategies (no vaccination, recombinant zoster vaccine [RZV], zoster vaccine live [ZVL]) under mass vaccination setting (30% coverage). Scenario and sensitivity analyses were performed. Out of 1.51 million adults in 2021 (base case population), 406,513 (27.0%) cases of HZ, 68,264 (4.5%) cases of post-herpetic neuralgia (PHN), and 54,949 (3.6%) cases of other complications were projected without vaccination. RZV was estimated to avoid 73,129 cases of HZ, 11,094 cases of PHN, and 9,205 cases of other complications over the subjects’ remaining lifetime; ZVL would avoid 17,565 cases of HZ, 2,781 cases of PHN, and 1,834 cases of other complications. The number needed to vaccinate to prevent one case of HZ/PHN was lower for RZV (7/41) than ZVL (26/163). Among all five age-stratified cohorts (50–59/60–64/65–69/70–79/≥80 YOA), RZV (versus no vaccination/ZVL) avoided the largest number of cases in the youngest cohort, 50–59 YOA. Results were robust under scenario and sensitivity analyses. Mass vaccination with RZV is expected to greatly reduce the public health burden of HZ among Singapore individuals ≥ 50 YOA. Findings support value assessment and decision-making regarding public health vaccination strategies for HZ prevention in Singapore.
Plain Language Summary
Risk of shingles (herpes zoster) increases with age, especially from 50 years. Shingles is a major public health concern in Singapore, given its rapidly aging population. Vaccination can prevent shingles and reduce its public health burden. Two shingles vaccines are available in Singapore: recombinant zoster vaccine (RZV) since 2021, zoster vaccine live (ZVL) since 2008. To understand the value of preventing shingles via vaccination, this study assessed the public health impact of shingles vaccination. Three vaccination strategies (no vaccination, vaccination with RZV, vaccination with ZVL) were compared in 1.51 million Singapore adults aged 50 years and above. Without vaccination, public health burden of shingles would be high; an estimated 406,513 (27.0%) would have shingles, 68,264 (4.5%) would have shingles-related long-term nerve pain, 54,949 (3.6%) would have other shingles-related complications, and 17,762 (1.2%) would be hospitalized due to shingles. Shingles vaccination could reduce this public health burden: RZV avoided 73,129 cases of shingles, 11,094 cases of shingles-related long-term nerve pain, 9,205 cases of other shingles-related complications, and 2,827 hospitalizations due to shingles, which was 4–6 times that avoided with ZVL (shingles: 17,565; shingles-related long-term nerve pain: 2,781; other shingles-related complications: 1,834; hospitalizations due to shingles: 484). Shingles vaccination for adults aged 50 years and above, especially early vaccination from 50–59 years, could reduce its public health burden more than vaccination at later ages and contribute toward healthy aging, preventive care, and the Healthier SG initiative. Results support local public health value assessments and decision-making for shingles prevention.
Introduction
Herpes zoster (HZ) is a viral infection characterized by a painful, blistering rash typically distributed along 1–2 adjacent dermatome(s).Citation1–3 HZ occurs when latent varicella-zoster virus (VZV) is reactivated, brought about when VZV-specific cell-mediated immunity falls below a critical threshold due to age-related decline in immunity and/or immunosuppression.Citation1
HZ skin lesions usually subside within 2–4 weeks,Citation4 however, patients are significantly impacted by acute pain from HZ, particularly in terms of quality of life.Citation5 Furthermore, patients may develop complications associated with HZ.Citation6 Post-herpetic neuralgia (PHN), a type of persistent neuropathic pain lasting ≥90 days after rash onset,Citation7 is the most common HZ-related complication and can affect up to 30% of HZ patients globally.Citation2 The impact of PHN on patients’ lives may be seen across their physical, psychological, social, and functional domains of health.Citation6 Other HZ-related complications include ocular and neurological complications, such as stroke, and may result in long-term physical impairment.Citation6–Citation8-10 HZ and its complications may have spill-over effects on the patient’s family, caregivers, and society,Citation11 in the form of caregiver burden and public health burden due to increased healthcare utilization.
Globally, the burden of HZ is substantial, with approximately one in three individuals expected to develop HZ in their lifetime.Citation3 The risk of HZ grows with age, particularly from 50 years of age (YOA).Citation12 For individuals who live to 85 YOA or later, the estimated lifetime risk of HZ increases from one in three to one in two.Citation13 In Singapore, a recent study in the primary care setting reported that the mean age of HZ diagnosis in 2018–2020 was approximately 60 years and HZ was more prevalent among older adults ≥ 50 YOA (410–829 cases per 100,000 population) compared with younger adults 21–49 YOA (147–210 cases); expectedly, older age (≥50 years) was found to be a key risk factor for HZ.Citation14 With the proportion of individuals aged ≥50 years in Singapore increasing from 29.8% in 2011 to 38.3% in 2022, the resident population is rapidly aging.Citation15 Therefore, the burden of HZ and its complications is estimated to be significant and is expected to increase with population aging. Notably, the seroprevalence of VZV antibodies has been reported in approximately 88% of Singapore adults ≥ 25 YOA,Citation16 suggesting that majority of adults are predisposed to developing HZ.
A single-center study of patients ≥ 50 YOA seeking medical attention for HZ or PHN locally (N = 347) found that up to over 80% of patients reported prodromal (e.g., pain, itch, and insomnia) and clinical symptoms (e.g., pain or discomfort, blistering rashes followed by scarring, and insomnia or sleep disturbance) of HZ.Citation17 Pain was reported as the most unbearable HZ-associated symptom, with a large proportion of patients (55.6%) tapping into healthcare resources for analgesics.Citation17 Generally, HZ may be treated with anti-virals and/or managed with analgesicsCitation18; in Singapore, traditional Chinese medicine (i.e., acupuncture) may also be considered for treatment.Citation19 Specifically, anti-virals should be initiated within 72 hours of rash onset (i.e., acute HZ) for optimal efficacyCitation3,Citation20; however, patients may be delayed in seeking medical attention and pharmacologic treatment,Citation21 due in part to nonspecific symptoms at HZ onset,Citation6 or potentially low awareness of HZ burden.Citation22 Studies have shown that anti-virals and/or analgesics may be considered suboptimal in the management of HZ due to limited efficacy against PHN,Citation23–26 and minimal impact on patient quality of life.Citation6,Citation26
Proactive prevention against HZ via vaccination would be an effective way to reduce the burden of HZ and its complications,Citation27–29 and therefore deliver preventive care and support healthy aging which are key objectives of Healthier SG.Citation30 The vaccines presently licensed for HZ prevention in adults ≥ 50 YOA in Singapore include the non-live, adjuvanted recombinant zoster vaccine (RZV),Citation31 and zoster vaccine live (ZVL).Citation32 RZV has demonstrated high and sustained vaccine efficacy estimates against HZ (89.8–97.2%) among adults ≥ 50 YOA in global clinical trials.Citation33,Citation34 When comparing the efficacy of RZV versus ZVL indirectly across separate studies, RZV showed higher and longer-lasting estimates.Citation33–38
RZV and ZVL are available in Singapore through the private market and were registered for use in 2021 and 2008, respectively.Citation39 While the 2023 Society of Infectious Diseases Singapore guideline recommends HZ vaccination for immunocompetent adults ≥ 60 YOA or adults with prior HZ,Citation40 RZV and ZVL are not currently listed in the local National Adult Immunization Schedule.Citation41
Public health impact (PHI) studies are helpful in understanding the value of vaccination in HZ prevention and its unmet public health need; such studies on RZV from other locales or regions such as the United States of America (USA), Canada, Germany, Japan, Hong Kong, and Beijing, China, have demonstrated superior PHI of RZV over no vaccination, ZVL (where evaluated), or both.Citation42–48 Therefore, this study aims to model the PHI of HZ vaccination in Singapore by comparing vaccination with RZV or ZVL versus no vaccination among adults ≥ 50 YOA.
Methods
Model overview
The ZOster ecoNomic Analysis (ZONA) model is a static multi-cohort Markov model developed in Microsoft Excel to simulate outcomes of HZ vaccination.Citation44,Citation45 This study adapted the existing ZONA model to the Singapore setting through updating key model inputs, as well as undertaking relevant scenario and sensitivity analyses. Supplementary Figure S1 provides an overview of the ZONA model structure used.Citation44
The model has a cycle length of one year, following cohorts from the year of vaccination over their remaining lifetime. Transition probabilities between health states occur using an annual time step and probabilities of moving between health states are age-specific and to the extent possible, based on country-specific data. The model does not apply an age for the end of lifetime. Instead, age-specific all-cause mortality probabilities () are progressively applied across the age cohorts over their remaining lifetime and the model cycles until no individuals are alive. All subjects remain in their initial cohort and all subsequent events are counted in that cohort only.
Table 1. Model inputs for demographic, epidemiology, and vaccination parameters.
The base case target population was the 2021 population of adults ≥ 50 YOA in Singapore. The population was stratified into five age groups (50–59, 60–64, 65–69, 70–79, and ≥ 80 YOA), and the modeled impact of vaccination was analyzed for the entire study cohort ≥ 50 YOA (i.e., all five age groups combined) and for each individual age group.
The model explored three vaccination strategies: (1) no vaccination, (2) vaccination with RZV (two-dose), and (3) vaccination with ZVL (one-dose). Depending on the corresponding vaccination coverage for strategies (2) and (3), and the assumed second-dose compliance rate for strategy (2) only, individuals could be fully or partially compliant with the vaccine dosing schedule, or not vaccinated at all.
Adults ≥65 YOA in Singapore make up the Merdeka and Pioneer Generations eligible for additional government social support, including healthcare subsidies.Citation58,Citation59 Hence, one scenario analysis was performed to assess the impact of vaccination with RZV or ZVL versus no vaccination, within an aggregated cohort of adults ≥ 65 YOA.
To assess the robustness of the base case results to uncertainties surrounding the model inputs, a deterministic one-way sensitivity analysis (DSA) was conducted on the number of HZ cases avoided with vaccination with RZV versus no vaccination in the mass vaccination setting (30% vaccination coverage).
Model inputs
The model inputs were categorized into (1) demographics, (2) epidemiology, and (3) vaccine efficacy, coverage, and second-dose compliance. reports the complete list of model inputs obtained from Singapore-specific data sources where possible. Inputs for which Singapore-specific databases were unavailable were derived from other locales or regions (described below).
Demographics
The model applied population and mortality figures from the Singapore Department of Statistics 2021; the estimated resident population (including citizens and permanent residents) ≥50 YOA in Singapore in 2021 was 1.51 million.Citation15 Age groups were aggregated to match the ZONA age cohorts, with gender data weighted and combined.
Epidemiology
HZ epidemiology in Asia-Pacific has generally been consistent with global data,Citation2,Citation60 with higher incidence reported in Asia-Pacific.Citation61 Therefore, relevant articles from a separate regional systematic literature review (SLR; Burden of HZ in selected locales in Asia-Pacific; unpublished) were identified to inform the epidemiological inputs in this study. The key epidemiological parameters of interest included age-stratified incidence of HZ, PHN, and other non-PHN complications (i.e., ocular, neurological, cutaneous, and other non-pain).
The selected studies and selection process for each epidemiological model input are as described below. Full details are reported in Appendix 1.
The epidemiological inputs for HZ and PHN were derived from Lin et al.Citation49 (2010) and Jih et al.Citation50 (2009), respectively, which reported on the burden of HZ and its epidemiology in Taiwan. Lin et al.Citation49 (2010) and Jih et al.Citation50 (2009) collected and reported data from the National Health Insurance Research Database (NHIRD), which is a population-level database in Taiwan and currently offers the largest possible sample size nationally. PHN epidemiological inputs were derived from Jih et al.Citation50 (2009) as it accounted for differences in the incidence of PHN across age groups. Gender-specific HZ and PHN incidence rates were weighted and combined according to each of the ZONA age groups. Sensitivity was tested at ± 20% of the base value for HZ incidence; Chen et al.Citation17 (2015) and Goh et al.Citation51 (1997) were used to determine the lower and upper bounds of PHN incidence, respectively, in the DSA.
The incidence of recurrent HZ was assumed to be the same as the incidence of initial HZ, as supported by published data.Citation62–64 As with HZ, the incidence of recurrent PHN was also assumed to be the same as that of its initial event.
For all non-PHN complications assessed (i.e., ocular, neurological, cutaneous, and other non-pain), a USA publication, Yawn et al.Citation52 (2007), was used as a single data source to ensure consistency. Chen et al.Citation17 (2015) was used to determine the upper bound for ocular complications in the DSA.
Separately, the probability for all HZ-related complications (including PHN) was conservatively assumed to be the same regardless of vaccination status. Although this assumption does not account for the potential impact of vaccination on the severity of HZ, a shorter duration and lower severity of pain have been shown in vaccinated versus non-vaccinated individuals.Citation29
HZ-related mortality data were sourced from a USA publication, Le et al.Citation54 (2015), as no regional or local publications identified from the literature search had reported these data. The use of a non-local source was based on the assumption that differences in HZ mortality across countries are negligible since HZ has usually been associated with morbidity rather than mortality, and HZ-associated deaths are expected to be low.Citation65,Citation66 It was also assumed that no additional mortality would be attributable to PHN specifically, on top of that attributed to HZ.
The inputs for hospital resource utilization (mean number of hospitalizations per case of HZ, by age cohort) were derived based on the proportion of cases of HZ with HZ-related hospitalizations described by Lu et al.Citation53 (2018), a Taiwan cohort study reporting data from the NHIRD. As described above, the NHIRD may be considered a nationally representative data source.
Vaccine efficacy, coverage, and second-dose compliance
Model inputs for vaccine efficacy and its waning rates for RZV and ZVL have previously been described by Curran et al.Citation44,Citation45 (2017, 2021) and remained unchanged during adaption of the ZONA model to the Singapore setting.
RZV efficacy against HZ and PHN was derived from the clinical studies ZOE-50 and ZOE-70,Citation33,Citation34 and their ZOE-049 long-term follow-up (LTFU) extension study.Citation67 As RZV efficacy against PHN can be attributed to the vaccine’s effect against HZ and given the limited number of HZ cases in ZOE-50 and ZOE-70,Citation68 RZV efficacy against PHN was assumed the same as its efficacy against HZ. RZV efficacy was not adjusted for ethnicity since no variation was identified in an Asian subgroup analysis of global ZOE-50 and ZOE-70 trial data.Citation69 Overall, initial vaccine efficacy (i.e., take) was estimated at 98.9% (95% confidence interval [CI], 94.0–100%) and 95.4% (95% CI, 89.7–100%) for adults 50–69 YOA and ≥ 70 YOA, respectively.Citation45
RZV efficacy waning rates were extrapolated by applying linear approximation to annual vaccine efficacy estimates from ZOE-50, ZOE-70, and up to eight years of follow-up data from ZOE-049 LTFU.Citation67 Consequently, annual waning rates for RZV efficacy were estimated to be 1.5% (95% CI, 0.0–3.4%) and 2.3% (95% CI, 0.3–4.4%) for individuals 50–69 YOA and ≥ 70 YOA, respectively.Citation45
For ZVL, initial vaccine efficacy against HZ was derived from clinical data from the Shingles Prevention Study,Citation35 the Short-Term Persistence Substudy,Citation36 and the Long-Term Persistence Substudy.Citation37 Efficacy estimates were 69.8% (95% CI, 54.1–80.6%) for 50–59 YOA, 63.9% (95% CI, 56.0–71.0%) for 60–64 and 65–69 YOA, 40.9% (95% CI, 28.0–52.0%) for 70–79 YOA, and 18.3% (95% CI, 0.0–48.0%) for ≥ 80 YOA. These data were modeled to obtain ZVL efficacy waning rate estimates of 5.4% for the first four years of vaccination and 5.1% thereafter, for all age cohorts.
A 30% vaccination coverage was assumed and applied for vaccination with both RZV and ZVL under the base case government-subsidized mass vaccination setting. As there were no HZ vaccines recommended in the Subsidised Vaccine List or National Immunization Schedule in Singapore, there were no appropriate local vaccine coverage data to reference. The coverage applied was hence estimated based on the 2020 national vaccination target (30%) set and achieved for HZ in the USA.Citation55,Citation56
RZV is a two-dose vaccine and its second dose is recommended for administration 2–6 months following the first dose.Citation31 In this study, the second-dose compliance rate was assumed to be 82.5%, which represented an average of compliance in the real-world setting in the USA (approximately 70%)Citation57 and in pivotal clinical trials (approximately 95%).Citation33,Citation34
Outcomes
The primary health outcomes of interest were the number of cases of HZ, PHN, other HZ-related complications (including ocular, neurological, cutaneous, and other non-pain), and HZ-related deaths. The secondary health outcome of interest was hospital resource utilization, measured by the number of cases of HZ-related hospitalizations. Outcomes of interest were explored under the mass vaccination setting (30% coverage).
The potential PHI of HZ vaccination was analyzed and presented for the base case population, with stratifications made for age (five age cohorts: 50–59, 60–64, 65–69, 70–79, and ≥ 80 YOA). Results were accumulated over the entire time horizon under each strategy of no vaccination, vaccination with RZV, or vaccination with ZVL. The incremental value per outcome was calculated to report the impact of vaccination with RZV or ZVL versus no vaccination.
The number needed to vaccinate (NNV) to avoid one case of HZ and PHN, respectively, was also calculated.
Compliance with ethics guidelines
The analyses performed were based on mathematical modeling with inputs extracted from published data or existing literature. No patients or prospective patient-level data were involved in the analyses.
Results
Disease burden of HZ in Singapore
Out of the total base case population of 1.51 million adults ≥ 50 YOA in Singapore, an estimated 406,513 (27.0%) cases of HZ, 68,264 (4.5%) cases of PHN, and 54,949 (3.6%) cases of other complications were projected and could occur in their remaining lifetime in the absence of HZ vaccination ().
Table 2. Base case results in the mass vaccination setting (30% coverage).
Base case results in the mass vaccination setting (30% coverage)
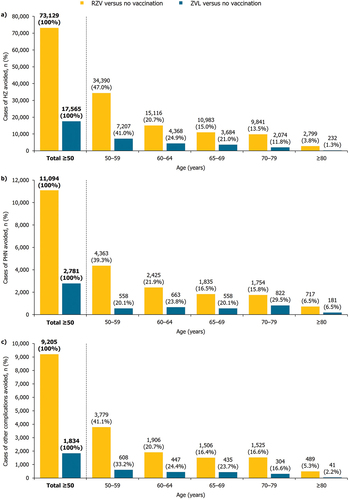
With 30% coverage in the mass vaccination setting, the total vaccinated cohort of adults ≥ 50 YOA was 451,742. Mass vaccination with RZV (versus no vaccination) was estimated to avoid 73,129 cases of HZ, 11,094 cases of PHN, and 9,205 cases of other complications (; ) in the base case population. These results showed that vaccination with RZV avoided 4.2, 4.0, and 5.0 times the number of cases of HZ, PHN, and other complications, respectively, compared with ZVL, which avoided 17,565 cases of HZ, 2,781 cases of PHN, and 1,834 cases of other complications.
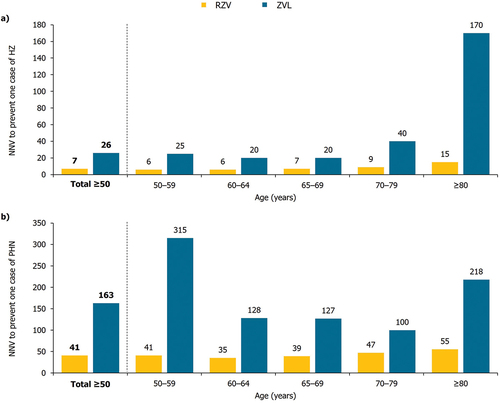
Figure 1. Number of cases of (a) HZ, (b) PHN, (c) other complications avoided in the mass vaccination setting (30% coverage), by age cohort.
Compared with no vaccination, mass vaccination with RZV would also prevent 2,827 hospitalizations and 8 HZ-related deaths, while mass vaccination with ZVL would prevent 484 hospitalizations and 1 HZ-related death ().
In the overall cohort of adults ≥ 50 YOA, the NNV with RZV to prevent one case of HZ and one case of PHN was 7 and 41, respectively; and with ZVL, 26 and 163, respectively (). Comparatively, the NNV with RZV to prevent one case of HZ and one case of PHN, respectively, was 3.7 and 4.0 times lower than with ZVL.
Base case results in the mass vaccination setting (30% coverage), by age cohort
Among the five age cohorts, mass RZV vaccination (versus no vaccination) reduced the largest number of cases in the youngest cohort of 50–59 YOA, which contributed toward 47.0%, 39.3%, and 41.1% of the total number of cases of HZ, PHN, and other complications avoided, respectively, in the gross cohort of adults ≥ 50 YOA (). The largest number of hospitalizations avoided with RZV vaccination was also observed for the 50–59 YOA cohort (). In general, the number of cases avoided gradually decreased with age in the older age cohorts (; ).
Table 3. Base case results for hospitalizations in the mass vaccination setting (30% coverage), by age cohort.
Similarly, mass ZVL vaccination (versus no vaccination) also reduced the largest number of cases of HZ and other complications in the youngest age group of adults 50–59 YOA and the corresponding number of cases avoided gradually decreased with age (). The number of cases of PHN and hospitalizations avoided with ZVL demonstrated no apparent trend with age, with the 70–79 and 65–69 YOA cohorts showing the greatest number of cases of PHN and hospitalizations, respectively (; ).
With RZV vaccination, the NNV to prevent one case of HZ was the lowest for the youngest age cohorts of 50–59 and 60–64 YOA, and increased with age up to the ≥ 80 YOA cohort, from 6 to 15 (). The NNV to prevent one case of PHN initially decreased from 41 in the 50–59 YOA cohort to 35 in the 60–64 YOA cohort, before increasing to 55 in the ≥ 80 YOA cohort ().
With ZVL vaccination, the NNV to prevent one case of HZ was the lowest in the 60–64 and 65–69 YOA cohorts, and increased substantially with age up to the ≥ 80 YOA cohort, from 25 to 170 (). The NNV to prevent one case of PHN was the lowest in the 70–79 YOA cohort (100), with no age-specific trend ().
Scenario analysis
Aggregated cohort of adults ≥ 65 YOA, under the mass vaccination setting (30% coverage)
Within the aggregated cohort of 639,008 adults ≥ 65 YOA, 283,835 (44.4%) cases of HZ, 55,078 (8.6%) cases of PHN, and 43,716 (6.8%) cases of other complications were estimated without HZ vaccination ().
Table 4. Scenario analysis results in the aggregated cohort of adults ≥ 65 YOA, under the mass vaccination setting (30% coverage).
With 30% coverage in the mass vaccination setting, the total vaccinated cohort of adults ≥ 65 YOA was 191,702. Mass vaccination with RZV would avoid 55,667 cases of HZ, 10,146 cases of PHN, and 8,295 cases of other complications (), which was 3.9, 2.8, and 4.5 times the number of cases avoided with ZVL, respectively. Mass vaccination with ZVL was estimated to prevent 14,115 cases of HZ, 3,678 cases of PHN, and 1,835 cases of other complications.
Mass vaccination with RZV would also avoid 2,857 hospitalizations and 11 HZ-related deaths, and mass vaccination with ZVL would avoid 589 hospitalizations and 1 HZ-related death (). The number of hospitalizations that would be avoided with mass vaccination (RZV or ZVL) is also presented by age cohort in .
Table 5. Scenario analysis results for hospitalizations in the aggregated cohort of adults ≥ 65 YOA, under the mass vaccination setting (30% coverage), by age cohort.
Deterministic sensitivity analysis (DSA)
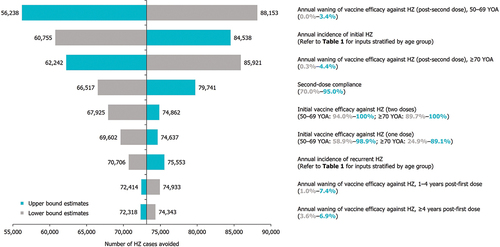
Results were robust under DSA (). The parameters which were shown to have the greatest sensitivity on the number of HZ cases avoided with RZV included (in sequence): (1) annual waning of vaccine efficacy against HZ (post-second dose) for individuals 50–69 YOA, (2) annual incidence of initial HZ, (3) annual waning of vaccine efficacy against HZ (post-second dose) for individuals ≥ 70 YOA, (4) second-dose compliance, and (5) initial vaccine efficacy against HZ (two doses).
Figure 3. DSA on cases of HZ avoided with RZV vaccination versus no vaccination, in the mass vaccination setting (30% coverage).
Discussion
RZV has been registered and available in Singapore since 2021,Citation39,Citation70 and this is the first study evaluating the PHI of the HZ vaccines currently available in Singapore (RZV and ZVL) compared with no vaccination in adults ≥ 50 YOA. The PHI of HZ vaccination in Singapore has previously only been partially explored for ZVL as public health outcomes within a cost-effectiveness analysis.Citation71 The present study used Singapore-specific model inputs, where available, and predicted a high public health burden of HZ in Singapore which can be reduced with HZ vaccination.
As explored in the base case analysis, an approximate 27.0%, 4.5%, and 3.6% of 1.51 million adults ≥ 50 YOA in Singapore in 2021 could develop HZ, PHN, and other complications, respectively, and 1.2% could be hospitalized due to HZ in their remaining lifetime without HZ vaccination. These data are consistent with existing evidence of an estimated 30% lifetime risk of HZ both globally and in the Asia-Pacific region,Citation3,Citation60 and suggest considerable public health burden of HZ in Singapore in the absence of vaccination. Similar findings were further reported in a recent population-based study highlighting the clinical burden and healthcare resource utilization of HZ, as well as the need for HZ vaccination among older adults locally.Citation14
Vaccination can be an effective public health strategy for preventing HZ and its complications, and reducing their public health burden. Compared with no vaccination in the base case analysis, the number of cases of HZ, PHN, other complications, HZ-related deaths, and HZ-related hospitalizations was reduced with mass HZ vaccination in the overall ≥ 50 YOA cohort. Notably, vaccination with RZV and ZVL was projected to avoid 2,827 and 484 HZ-related hospitalizations, respectively.
As in Singapore, the high clinical burden of HZ is similarly documented worldwide.Citation12 For example, the USA set HZ vaccination as one of their public health priorities in 2020,Citation55,Citation56 while other countries have gradually incorporated HZ vaccination into their respective National Immunization Programs (NIPs) targeting older adults (e.g., RZV in Australia,Citation72 Germany,Citation73,Citation74 the United Kingdom,Citation75 and New ZealandCitation76,Citation77). In Germany, the decision to provide HZ vaccination via the NIP considered the increased risk of severe HZ and PHN in older adults, high RZV efficacy, and the anticipated period of protection provided by RZVCitation74; in Australia, listing RZV under the NIP considered its high vaccine efficacy demonstrated in clinical trials, and availability of high quality, long-term data in older adults.Citation72
Vaccination with RZV demonstrated a greater impact on public health outcomes among individuals ≥ 50 YOA versus vaccination with ZVL, where RZV avoided 4–5 times the number of cases of HZ, PHN, or other complications (). In particular, mass vaccination with RZV was estimated to avoid 73,129 HZ cases that would otherwise occur in the absence of vaccination, with an overall NNV of 7 to prevent one case. Even when the upper bound waning rate was explored in the DSA, a reduction of 56,238 cases of HZ was predicted with RZV versus no vaccination; this was more than three times the number of cases avoided with ZVL versus no vaccination. Vaccination with RZV was also more efficient than ZVL in that the NNV to prevent one case of HZ and PHN was 3–4 times lower for RZV (HZ: 7; PHN: 41) than ZVL (HZ: 26; PHN: 163) (). This is due to the higher initial vaccine efficacy and sustained protection conferred by RZV versus ZVL over the projected period. These NNV results are comparable to those from PHI analyses of RZV in Beijing, and of RZV and ZVL in Hong Kong.Citation47,Citation48 NNV was not previously reported in the published cost-effectiveness analysis of ZVL in Singapore.Citation71
The target age group for HZ vaccination may also be important to consider. Early vaccination against HZ (i.e., from 50 YOA) was estimated to have the most substantial PHI. The base case results reported that the greatest percentage of cases avoided was seen in the youngest cohort (50–59 YOA) compared with the older cohorts for both RZV (outcomes of HZ, PHN, and other complications) and ZVL vaccination (outcomes of HZ and other complications only) (). When vaccinated with RZV, the 50–59 YOA cohort accounted for almost half (47.0%) of the total number of cases of HZ avoided, and approximately two-fifths of the total number of cases of PHN (39.3%) and other complications (41.1%) avoided. These results are reasonable considering the 50–59 YOA vaccinated cohort is overall the largest and is expected to have a greater number of life years remaining to benefit from an extended duration of protection through RZV. With ZVL vaccination, the 70–79 YOA cohort reported the largest number of PHN cases avoided (), which may indicate its higher protection against PHN in individuals ≥ 70 YOA than younger individuals. Overall, these findings suggest that early HZ vaccination would result in the most substantial PHI.
Early vaccination against HZ is also supported by the NNV results (). With RZV vaccination, the NNV to prevent one case of HZ was the smallest in the 50–59 and 60–64 YOA cohorts, and the NNV to prevent one case of PHN was the smallest in the 60–64 YOA cohort, compared with other age groups. The NNV with RZV to prevent HZ increased with age, from 6 in the 50–59 YOA cohort to 15 in the ≥ 80 YOA cohort, while the NNV to prevent PHN decreased from 41 in the 50–59 YOA cohort to 35 in the 60–64 YOA cohort and then increased to 55 in the ≥ 80 YOA cohort; these trends in the NNV with RZV in Singapore were similar to those reported for Hong Kong.Citation47 For HZ, the increase in NNV with age would mainly be due to the combined effect of lower initial vaccine efficacy, greater waning of vaccine efficacy, and shorter follow-up period in older adults. On the other hand, the drop in NNV for PHN in the 60–64 YOA cohort was due to the greater burden of PHN at 60 YOA compared to 50 YOA, while its subsequent increase with age was due to increased vaccine efficacy against PHN at 60 YOA compared to 70 YOA.
Given the retirement age of 63 and reemployment age of 68 in Singapore,Citation78,Citation79 both set to further increase by 2030,Citation79 most local adults remain active contributors to the workforce in their 50s and 60s.Citation15,Citation80 Considering the consequent aging workforce,Citation80 avoiding HZ and PHN in these individuals would hence beneficially reduce productivity loss to society. Furthermore, the long life expectancy of residents and the resultant aging population in SingaporeCitation81 raise concerns regarding the growing prevalence of age-related diseases or disabilities and its increasing pressure on the healthcare system. To circumvent this, the Healthier SG Initiative was enacted as a national action plan for healthcare transformation to focus on preventive care, encourage healthy aging, and slow the rate of increase of disease burden and healthcare expenditure.Citation30 To address these objectives of Healthier SG, early HZ vaccination could alleviate the disease burden of HZ, accrue its health benefits over a longer period of time, and free up healthcare expenditure and resources for the provision of medical services for other diseases or conditions.
The results of the base case analysis were based on a vaccination coverage rate for RZV of 30% and a second-dose compliance rate of 82.5%. In general, the vaccination coverage and second-dose compliance of HZ vaccination in Singapore is expected to be higher than observed elsewhere (e.g., >30% and > 70%, respectively, in the USA)Citation55–57 given the ease of accessibility to vaccines via healthcare providers and overall adherence to medical advice in Asian communities.Citation82,Citation83 Additionally, published PHI models of RZV, in majority Chinese populations of Hong Kong and Beijing, also used similar second-dose compliance rates (80.0–82.5%).Citation47,Citation48
Due to limited local epidemiological data, there was a need to apply inputs from neighboring locales or countries, which the authors understand is common practice and generally accepted by payers and prescribers locally. As described in the Methods, this study utilized updated demographic data of Singapore and locally relevant epidemiological data, based on a systematic selection of model inputs that included the conduct of a literature search and evaluation of the selected data source for each parameter. Notably, the Taiwan population-level database was mainly used as an alternative to local data sources and may be considered suitable based on shared population ethnicities (i.e., Chinese majority) with Singapore.
For vaccine-related parameters, model inputs of vaccine efficacy were based on data sources,Citation33,Citation34,Citation67 which align with the studies identified in a recent systematic review and meta-analysis assessing HZ vaccine efficacy in immunocompetent individuals.Citation84
Certain comorbidities (e.g., diabetes, chronic kidney disease, and cardiovascular diseases) may increase risk of HZ,Citation85–91 and hence its complications. In Singapore, rates of comorbidities are high among older adults, with more than 50% of the population developing at least one chronic disease by 50 YOA and a second or more comorbidity (i.e., multimorbidity) by 60 YOA.Citation92 Among patients ≥ 50 YOA seeking medical attention for HZ or PHN in Singapore (N = 347), comorbidities were common and observed in up to 51.3% of patients and a large proportion of all patients were 50–60 YOA.Citation17 The base case population in this study was adults ≥ 50 YOA which, when considering the prominent overlap of comorbidities and old age, would expectedly include those with comorbidities in addition to those without; the PHI of HZ vaccination was analyzed and reported in the overall base case population, and not specifically for the comorbidities subgroup. Future studies may hence explore the effects of HZ vaccination in a subset of patients with specific comorbidities. In particular, diabetes could be of interest as it raises the risk of HZ by 24–38%,Citation93,Citation94 and its growing prevalence rate in Singapore is one of the fastest globally.Citation95 Such analyses may supplement the present PHI study regarding HZ vaccination and account for these specific patient groups in Singapore. A comprehensive value assessment of HZ vaccination and its unmet need locally would require understanding the PHI (described in this study) as well as the cost-effectiveness of HZ vaccination for older adults in Singapore. Since there are currently no available local cost-effectiveness data on RZV based on an economic SLR, a cost-effectiveness analysis of RZV may therefore be explored in the future to complement results from the present study and support discussions regarding HZ vaccination strategies for older adults in Singapore.
In conclusion, this analysis demonstrated that mass HZ vaccination with RZV among adults ≥ 50 YOA in Singapore has a superior PHI, compared to no vaccination. These results may be helpful in supporting value assessment and decision-making regarding public health vaccination strategies for HZ prevention in Singapore.
Authors’ contributions
Substantial contributions to study conception and design: HO, CT, CW, NG, and CN; substantial contributions to analysis and interpretation of the data: HO, CT, CW, NG, and CN; drafting the article or revising it critically for important intellectual content: HO, CT, CW, NG, and CN; final approval of the version of the article to be published: HO, CT, CW, NG, and CN.
Data sharing statement
All data generated or analyzed during this study are included in this published article or as supplementary information files.
Prior presentation
This manuscript is based on work that has been previously presented at the 7th World One Health Congress, Singapore (November 7–11, 2022).
Supplementary Material_PHI of HZ Vx SG_Oh et al_15Apr24_clean.docx
Download MS Word (169.1 KB)Acknowledgments
The authors acknowledge Desmond Curran, GSK, and Sumitra Shantakumar, GSK, for contributions to study concept and data interpretation, and Roeland Van Kerckhoven, GSK, for publication management. The authors also thank Costello Medical for editorial assistance and publication coordination, on behalf of GSK, and acknowledge Ren Ping Yong, Costello Medical, Singapore for medical writing and editorial assistance based on authors’ input and direction.
Disclosure statement
HO and CT: Nothing to disclose; CW: Employed by the GSK group of companies; NG and CN: Employed by and hold shares in the GSK group of companies.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2348839
Additional information
Funding
References
- Chlibek R, Pauksens K, Rombo L, van Rijckevorsel G, Richardus JH, Plassmann G, Schwarz TF, Catteau G, Lal H, Heineman TC. Long-term immunogenicity and safety of an investigational herpes zoster subunit vaccine in older adults. Vaccine. 2016;34(6):863–15. doi:10.1016/j.vaccine.2015.09.073.
- Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833. doi:10.1136/bmjopen-2014-004833.
- Harpaz R, O-S IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recom Rep. 2008;57:1–30. quiz CE32–34.
- Patil A, Goldust M, Wollina U. Herpes zoster: a review of clinical manifestations and management. Viruses. 2022;14(2):192. doi:10.3390/v14020192.
- Drolet M, Brisson M, Schmader KE, Levin MJ, Johnson R, Oxman MN, Patrick D, Blanchette C, Mansi JA. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. CMAJ. 2010;182(16):1731–6. doi:10.1503/cmaj.091711.
- Johnson RW, Bouhassira D, Kassianos G, Leplege A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010;8(1):37. doi:10.1186/1741-7015-8-37.
- Hadley GR, Gayle JA, Ripoll J, Jones MR, Argoff CE, Kaye RJ, Kaye AD. Post-herpetic neuralgia: a review. Curr Pain Headache Rep. 2016;20(3):17. doi:10.1007/s11916-016-0548-x.
- Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, Backonja M, Betts RF, Gershon AA, Haanpaa ML, McKendrick MW. et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(Suppl 1):S1–26. doi:10.1086/510206.
- Volpi A. Severe complications of herpes zoster. Herpes. 2007;14:35–9.
- Wu PH, Chuang YS, Lin YT. Does herpes zoster increase the risk of stroke and myocardial infarction? A comprehensive review. J Clin Med. 2019;8(4):547. doi:10.3390/jcm8040547.
- Weinke T, Glogger A, Bertrand I, Lukas K. The societal impact of herpes zoster and postherpetic neuralgia on patients, life partners, and children of patients in Germany. Sci World J. 2014;2014:749698. doi:10.1155/2014/749698.
- van Oorschot D, Vroling H, Bunge E, Diaz-Decaro J, Curran D, Yawn B. A systematic literature review of herpes zoster incidence worldwide. Hum Vaccin Immunother. 2021;17(6):1714–32. doi:10.1080/21645515.2020.1847582.
- Schmader K. Herpes zoster and postherpetic neuralgia in older adults. Clin Geriatr Med. 2007;23(3):615–632, vii–viii. doi:10.1016/j.cger.2007.03.003.
- Chan XV, Tan NC, Ng MCW, Ng DX, Koh YLE, Aau WK, Ng CJ. Prevalence and healthcare utilization in managing herpes zoster in primary care: a retrospective study in an Asian urban population. Front Public Health. 2023;11:1213736. doi:10.3389/fpubh.2023.1213736.
- Department of Statistics Singapore, SingStat. Population and population structure; 2022 [accessed 2023 Apr]. https://tablebuilder.singstat.gov.sg/statistical-tables/downloadMultiple/zX5lZdqFrk-29wjZN4FFXw.
- Fatha N, Ang LW, Goh KT. Changing seroprevalence of varicella zoster virus infection in a tropical city state, Singapore. Int J Infect Dis. 2014;22:73–7. doi:10.1016/j.ijid.2013.10.003.
- Chen Q, Hsu T-Y, Chan R, Kawai K, Acosta CJ, Walia A, Pan JY. Clinical and economic burden of herpes zoster and postherpetic neuralgia in patients from the National Skin Centre, Singapore. Dermatol Sin. 2015;33(4):201–5. doi:10.1016/j.dsi.2015.04.002.
- Werner RN, Nikkels AF, Marinovic B, Schafer M, Czarnecka-Operacz M, Agius AM, Bata-Csorgo Z, Breuer J, Girolomoni G, Gross GE. et al. European consensus-based (S2k) guideline on the management of herpes zoster - guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV), Part 2: treatment. J Eur Acad Dermatol Venereol. 2017;31(1):20–9. doi:10.1111/jdv.13957.
- Qi T, Lan H, Zhong C, Zhang R, Zhang H, Zhu F, Ji B. Systematic review and meta-analysis: the effectiveness and safety of acupuncture in the treatment of herpes zoster. Ann Palliat Med. 2022;11(2):756–65. doi:10.21037/apm-22-109.
- Gan EY, Tian EA, Tey HL. Management of herpes zoster and post-herpetic neuralgia. Am J Clin Dermatol. 2013;14(2):77–85. doi:10.1007/s40257-013-0011-2.
- Johnson R, McElhaney J, Pedalino B, Levin M. Prevention of herpes zoster and its painful and debilitating complications. Int J Infect Dis. 2007;11(Suppl 2):S43–48. doi:10.1016/S1201-9712(07)60021-6.
- Miyachi M, Imafuku S. Relationship between prior knowledge about herpes zoster and the period from onset of the eruption to consultation in patients with herpes zoster. J Dermatol. 2016;43(10):1184–7. doi:10.1111/1346-8138.13334.
- Chen N, Li Q, Yang J, Zhou M, Zhou D, He L. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst Rev. 2014(2):CD006866. doi:10.1002/14651858.CD006866.pub3.
- Katz J, Cooper E, Walther R, Sweeney E, Dworkin R. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis. 2004;39(3):342–8. doi:10.1086/421942.
- Dworkin R, Schmader K. Treatment and prevention of postherpetic neuralgia. Clin Infect Dis. 2003;36(7):877–82. doi:10.1086/368196.
- Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain. 2005;6(6):356–63. doi:10.1016/j.jpain.2005.01.359.
- Schmader KE, Johnson GR, Saddier P, Ciarleglio M, Wang WW, Zhang JH, Chan IS, Yeh SS, Levin MJ, Harbecke RM. et al. Effect of a zoster vaccine on herpes zoster-related interference with functional status and health-related quality-of-life measures in older adults. J Am Geriatr Soc. 2010;58(9):1634–41. doi:10.1111/j.1532-5415.2010.03021.x.
- Curran D, Matthews S, Rowley SD, Young JH, Bastidas A, Anagnostopoulos A, Barista I, Chandrasekar PH, Dickinson M, El Idrissi M. et al. Recombinant zoster vaccine significantly reduces the impact on quality of life caused by herpes zoster in adult autologous hematopoietic stem cell transplant recipients: a randomized placebo-controlled trial (ZOE-HSCT). Biol Blood Marrow Transplant. 2019;25(12):2474–81. doi:10.1016/j.bbmt.2019.07.036.
- Curran D, Oostvogels L, Heineman T, Matthews S, McElhaney J, McNeil S, Diez-Domingo J, Lal H, Andrews C, Athan E. et al. Quality of life impact of an adjuvanted recombinant zoster vaccine in adults aged 50 years and older. J Gerontol A Biol Sci Med Sci. 2019;74(8):1231–8. doi:10.1093/gerona/gly150.
- Ministry of Health, Singapore. White paper on Healthier SG; 2022 [accessed 2023 May]. https://file.go.gov.sg/healthiersg-whitepaper-pdf.pdf.
- Shingrix [package insert]. Singapore: GSK group of companies; 2022.
- Zostavax [package insert]. Singapore: MSD; 2022.
- Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barbera J. et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–96. doi:10.1056/NEJMoa1501184.
- Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Diez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barbera J. et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–32. doi:10.1056/NEJMoa1603800.
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE. et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–84. doi:10.1056/NEJMoa051016.
- Schmader KE, Oxman MN, Levin MJ, Johnson G, Zhang JH, Betts R, Morrison VA, Gelb L, Guatelli JC, Harbecke R, et al. Persistence of the efficacy of zoster vaccine in the Shingles Prevention Study and the Short-Term Persistence Substudy. Clin Infect Dis. 2012;55(10):1320–8. doi:10.1093/cid/cis638.
- Morrison VA, Johnson GR, Schmader KE, Levin MJ, Zhang JH, Looney DJ, Betts R, Gelb L, Guatelli JC, Harbecke R. et al. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis. 2015;60(6):900–9. doi:10.1093/cid/ciu918.
- Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103–8. doi:10.15585/mmwr.mm6703a5.
- Health Sciences Authority, Singapore. PZ4970 Infosearch - therapeutic products; 2021 [accessed 2023 May]. https://eservice.hsa.gov.sg/prism/common/enquirepublic/SearchDRBProduct.do?action=load&_ga=2.183810082.563179921.1554083187-551332391.1551944793.
- Society of Infectious Disease, Singapore. Handbook on adult vaccination in Singapore 2023; 2023 [accessed 2024 May]. https://www.cfps.org.sg/assets/Circulars/2023/SG-SID-001-Handbook-v15-Highres-bleed.pdf.
- Ministry of Health, Singapore. Nationally recommended vaccines; 2021 [accessed 2023 Apr]. https://www.moh.gov.sg/resources-statistics/nationally-recommended-vaccines.
- Patterson BJ, Buck PO, Curran D, Van Oorschot D, Carrico J, Herring WL, Zhang Y, Stoddard JJ. Estimated public health impact of the recombinant zoster vaccine. Mayo Clin Proc Innov Qual Outcomes. 2021;5(3):596–604. doi:10.1016/j.mayocpiqo.2021.03.006.
- McGirr A, Van Oorschot D, Widenmaier R, Stokes M, Ganz ML, Jung H, Varghese L, Curran D. Public health impact and cost-effectiveness of non-live adjuvanted recombinant zoster vaccine in Canadian adults. Appl Health Econ Health Policy. 2019;17(5):723–32. doi:10.1007/s40258-019-00491-6.
- Curran D, Van Oorschot D, Varghese L, Oostvogels L, Mrkvan T, Colindres R, von Krempelhuber A, Anastassopoulou A. Assessment of the potential public health impact of herpes zoster vaccination in Germany. Hum Vaccin Immunother. 2017;13(10):2213–21. doi:10.1080/21645515.2017.1345399.
- Curran D, Van Oorschot D, Matthews S, Hain J, Salem AE, Schwarz M. Long-term efficacy data for the recombinant zoster vaccine: impact on public health and cost effectiveness in Germany. Hum Vaccin Immunother. 2021;17(12):5296–303. doi:10.1080/21645515.2021.2002085.
- Watanabe D, Mizukami A, Holl K, Curran D, Van Oorschot D, Varghese L, Shiragami M. The potential public health impact of herpes zoster vaccination of people aged ≥ 50 years in Japan: results of a Markov model analysis. Dermatol Ther (Heidelb). 2018;8(2):269–84. doi:10.1007/s13555-018-0236-3.
- Chan PKS, Wong MCS, Chan M, Ching K, Giannelos N, Ng C. Public health impact of herpes zoster vaccination on older adults in Hong Kong. Hum Vaccin Immunother. 2023;19(1):2176065. doi:10.1080/21645515.2023.2176065.
- Lee C, Jiang N, Tang H, Ye C, Yuan Y, Curran D. Potential public health impact of the adjuvanted recombinant zoster vaccine among people aged 50 years and older in Beijing. Hum Vaccin Immunother. 2021;17(10):3735–46. doi:10.1080/21645515.2021.1932216.
- Lin YH, Huang LM, Chang IS, Tsai FY, Lu CY, Shao PL, Chang LY. Varicella-Zoster Working Group, Advisory Committee on Immunization Practices Taiwan. Disease burden and epidemiology of herpes zoster in pre-vaccine Taiwan. Vaccine. 2010;28(5):1217–20. doi:10.1016/j.vaccine.2009.11.029.
- Jih JS, Chen YJ, Lin MW, Chen YC, Chen TJ, Huang YL, Chen CC, Lee DD, Chang YT, Wang WJ. et al. Epidemiological features and costs of herpes zoster in Taiwan: a national study 2000 to 2006. Acta Derm Venereol. 2009;89(6):612–6. doi:10.2340/00015555-0729.
- Goh CL, Khoo L. A retrospective study of the clinical presentation and outcome of herpes zoster in a tertiary dermatology outpatient referral clinic. Int J Dermatol. 1997;36(9):667–72. doi:10.1046/j.1365-4362.1997.00241.x.
- Yawn B, Saddier P, Wollan PC, St. Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clinic Proc. 2007;82(11):1341–9. doi:10.4065/82.11.1341.
- Lu WH, Lin CW, Wang CY, Chen LK, Hsiao FY. Epidemiology and long-term disease burden of herpes zoster and postherpetic neuralgia in Taiwan: a population-based, propensity score-matched cohort study. BMC Public Health. 2018;18(1):369. doi:10.1186/s12889-018-5247-6.
- Le P, Rothberg MB. Cost-effectiveness of herpes zoster vaccine for persons aged 50 years. Ann Intern Med. 2015;163(7):489–97. doi:10.7326/M15-0093.
- HealthyPeople.gov, U.S. Department of Health and Human Services. Immunization and infectious diseases data details; 2020 [accessed 2023 Apr]. https://wayback.archive-it.org/5774/20210211114047/https://www.healthypeople.gov/node/3527/data-details.
- Wick JY. Pharmacy Times. HP2020 herpes zoster immunization goal has been met; 2020 [accessed 2023 Apr]. https://www.pharmacytimes.com/view/hp2020-herpes-zoster-immunization-goal-has-been-met.
- LaMori J, Feng X, Pericone CD, Mesa-Frias M, Sogbetun O, Kulczycki A. Real-world evidence on adherence and completion of the two-dose recombinant zoster vaccine and associated factors in U.S. adults, 2017–2021. Vaccine. 2022;40(15):2266–73. doi:10.1016/j.vaccine.2022.03.006.
- Ministry of Health, Singapore. Merdeka generation package; 2023 [accessed 2023 Sep]. https://www.moh.gov.sg/healthcare-schemes-subsidies/merdeka-generation-package.
- Ministry of Health, Singapore. Pioneer generation package; 2023 [accessed 2023 Sep]. https://www.moh.gov.sg/healthcare-schemes-subsidies/pioneer-generation-package.
- Chen LK, Arai H, Chen LY, Chou MY, Djauzi S, Dong B, Kojima T, Kwon KT, Leong HN, Leung EM. et al. Looking back to move forward: a twenty-year audit of herpes zoster in Asia-Pacific. BMC Infect Dis. 2017;17(1):213. doi:10.1186/s12879-017-2198-y.
- Curran D, Callegaro A, Fahrbach K, Neupane B, Vroling H, van Oorschot D, Yawn BP. Meta-regression of herpes zoster incidence worldwide. Infect Dis Ther. 2022;11(1):389–403. doi:10.1007/s40121-021-00567-8.
- Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86(2):88–93. doi:10.4065/mcp.2010.0618.
- Kim YJ, Lee CN, Lee MS, Lee JH, Lee JY, Han K, Park YM. Recurrence rate of herpes zoster and its risk factors: a population-based cohort study. J Korean Med Sci. 2019;34(2):e1. doi:10.3346/jkms.2019.34.e1.
- You JHS, Ming WK, Tsang OT, Chan PK. Optimal gender-specific age for cost-effective vaccination with adjuvanted herpes zoster subunit vaccine in Chinese adults. PLOS ONE. 2019;14(1):e0210005. doi:10.1371/journal.pone.0210005.
- Mahamud A, Marin M, Nickell SP, Shoemaker T, Zhang JX, Bialek SR. Herpes zoster-related deaths in the United States: validity of death certificates and mortality rates, 1979–2007. Clin Infect Dis. 2012;55(7):960–6. doi:10.1093/cid/cis575.
- Pan CX, Lee MS, Nambudiri VE. Global herpes zoster incidence, burden of disease, and vaccine availability: a narrative review. Ther Adv Vaccines Immunother. 2022;10:25151355221084535. doi:10.1177/25151355221084535.
- Boutry C, Hastie A, Diez-Domingo J, Tinoco JC, Yu CJ, Andrews C, Beytout J, Caso C, Cheng HS, Cheong HJ. et al. The adjuvanted recombinant zoster vaccine confers long-term protection against herpes zoster: interim results of an extension study of the pivotal phase 3 clinical trials ZOE-50 and ZOE-70. Clin Infect Dis. 2022;74(8):1459–67. doi:10.1093/cid/ciab629.
- European Medicines Agency. Shingrix: EPAR – Product information; 2022 [accessed 2023 Sep]. https://www.ema.europa.eu/en/documents/product-information/shingrix-epar-product-information_en.pdf.
- Kim JH, Diaz-Decaro J, Jiang N, Hwang SJ, Choo EJ, Co M, Hastie A, Hui DSC, Irimajiri J, Lee J. et al. The adjuvanted recombinant zoster vaccine is efficacious and safe in Asian adults ≥ 50 years of age: a sub-cohort analysis of the ZOE-50 and ZOE-70 randomized trials. Hum Vaccin Immunother. 2021;17(7):2050–7. doi:10.1080/21645515.2020.1859321.
- SingHealth. Shingles (herpes zoster) vaccine; 2021 [accessed 2023 May]. https://www.singhealth.com.sg/patient-care/medicine/shingles-herpes-zoster-vaccine/.
- Pan J, Hsu T-Y, Johnson KD, Xu R, Acosta CJ, Kawai K. Cost-effectiveness analysis of herpes zoster vaccine in adults above 50 in Singapore. Dermatol Sin. 2017;35(4):177–81. doi:10.1016/j.dsi.2017.04.011.
- Pharmaceuticals Benefits Advisory Committee. Public summary document – March 2023 PBAC meeting; 2023 [accessed 2023 Jul]. https://www.pbs.gov.au/industry/listing/elements/pbac-meetings/psd/2023-03/files/varicella-zoster-vaccine-psd-03-2023.pdf.
- The Standing Committee on Vaccination, Robert Koch Institute. Immunisation schedule; 2023 [accessed 2023 May]. https://www.rki.de/DE/Content/Infekt/Impfen/Materialien/Downloads-Impfkalender/Impfkalender_Englisch.pdf?__blob=publicationFile.
- Siedler A, Koch J, Garbe E, Hengel H, von Kries R, Ledig T, Mertens T, Zepp F, Uberla K. Background paper to the decision to recommend the vaccination with the inactivated herpes zoster subunit vaccine: statement of the German Standing Committee on vaccination (STIKO) at the Robert Koch Institute. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2019;62(3):352–76. doi:10.1007/s00103-019-02882-5.
- GOV.UK, UK Health Security Agency. Introduction of Shingrix® vaccine for the whole programme and expansion of eligible cohorts letter; 2023 [accessed 2023 Jul]. https://www.gov.uk/government/publications/shingles-vaccination-programme-changes-from-september-2023-letter/introduction-of-shingrix-vaccine-for-the-whole-programme-and-expansion-of-eligible-cohorts-letter.
- Ministry of Health – Manatū Hauora, New Zealand. New Zealand immunisation schedule; 2023 [accessed 2023 May]. https://www.health.govt.nz/our-work/preventative-health-wellness/immunisation/new-zealand-immunisation-schedule.
- Pharmac Te Pātaka Whaioranga, New Zealand. Proposal to widen access to the meningococcal B vaccine and secure supply of the shingles vaccine; 2022 [accessed 2023 May]. https://pharmac.govt.nz/news-and-resources/consultations-and-decisions/2022-10-25-proposal-to-widen-access-to-the-meningococcal-b-vaccine-and-secure-supply-of-the-shingles-vaccine/?keyword=shingrix&type=all&page=1.
- Ministry of Manpower, Singapore. Retirement; 2022 [accessed 2023 Jul]. https://www.mom.gov.sg/employment-practices/retirement.
- Ministry of Manpower, Singapore. Strengthening support for senior workers; 2021 [accessed 2023 Jul]. https://www.mom.gov.sg/-/media/mom/documents/press-releases/2021/1101-rraa—twg-ow-infographic.pdf.
- Ministry of Manpower, Singapore. Resident labour force by age and sex; 2023 [accessed 2023 May]. https://stats.mom.gov.sg/iMAS_Tables1/Time-Series-Table/mrsd_20_Res_labour_force_by_age_sex.xlsx.
- National Population and Talent Division, Strategy Group, Prime Minister’s Office, Singapore. Population in brief 2022; 2022 [accessed 2023 Sep]. https://www.strategygroup.gov.sg/files/media-centre/publications/population-in-brief-2022.pdf.
- HealthHub, Health Promotion Board, Singapore. Stay one step ahead with vaccinations; 2023 [accessed 2023 May]. https://www.healthhub.sg/programmes/163/vaccinate.
- Wang JH, Adams IF, Pasick RJ, Gomez SL, Allen L, Ma GX, Lee MX, Huang E. Perceptions, expectations, and attitudes about communication with physicians among Chinese American and non-Hispanic white women with early stage breast cancer. Support Care Cancer. 2013;21(12):3315–25. doi:10.1007/s00520-013-1902-8.
- Xia Y, Zhang X, Zhang L, Fu C. Efficacy, effectiveness, and safety of herpes zoster vaccine in the immunocompetent and immunocompromised subjects: a systematic review and network meta-analysis. Front Immunol. 2022;13:978203. doi:10.3389/fimmu.2022.978203.
- Sansone RA, Sansone LA. Herpes zoster and postherpetic neuralgia: an examination of psychological antecedents. Innov Clin Neurosci. 2014;11:31–4.
- Chen HH, Lin IC, Chen HJ, Yeh SY, Kao CH. Association of herpes zoster and type 1 diabetes mellitus. PLOS ONE. 2016;11(5):e0155175. doi:10.1371/journal.pone.0155175.
- Tung YC, Tu HP, Wu MK, Kuo KL, Su YF, Lu YY, Lin CL, Wu CH. Higher risk of herpes zoster in stroke patients. PLOS ONE. 2020;15(2):e0228409. doi:10.1371/journal.pone.0228409.
- Ke CC, Lai HC, Lin CH, Hung CJ, Chen DY, Sheu WH, Lui PW. Increased risk of herpes zoster in diabetic patients comorbid with coronary artery disease and microvascular disorders: a population-based study in Taiwan. PLOS ONE. 2016;11(1):e0146750. doi:10.1371/journal.pone.0146750.
- Orhan VA, Avarisli A. Advanced age and multiple comorbidities as important factors in predicting poor prognosis in herpes zoster ophthalmicus. Cureus. 2021;13(9):e18412. doi:10.7759/cureus.18412.
- Li Z, Wang Q, Ma J, Li Z, Huang D, Huang Y, Zhou H. Risk factors for herpes zoster in patients with chronic kidney disease: a case-control study. Vaccines. 2021;9(9):963. doi:10.3390/vaccines9090963.
- Imafuku S, Dormal G, Goto Y, Jegou C, Rosillon D, Matsuki T. Risk of herpes zoster in the Japanese population with immunocompromising and chronic disease conditions: results from a claims database cohort study, from 2005 to 2014. J Dermatol. 2020;47(3):236–44. doi:10.1111/1346-8138.15214.
- Low LL, Kwan YH, Ko MSM, Yeam CT, Lee VSY, Tan WB, Thumboo J. Epidemiologic characteristics of multimorbidity and sociodemographic factors associated with multimorbidity in a rapidly aging Asian country. JAMA Netw Open. 2019;2(11):e1915245. doi:10.1001/jamanetworkopen.2019.15245.
- Marra F, Parhar K, Huang B, Vadlamudi N. Risk factors for herpes zoster infection: a meta-analysis. Open Forum Infect Dis. 2020;7(1):ofaa005. doi:10.1093/ofid/ofaa005.
- Huang CT, Lee CY, Sung HY, Liu SJ, Liang PC, Tsai MC. Association between diabetes mellitus and the risk of herpes zoster: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2022;107(2):586–97. doi:10.1210/clinem/dgab675.
- Bee YM, Tai ES, Wong TY. Singapore’s “war on diabetes”. Lancet Diabetes Endocrinol. 2022;10(6):391–2. doi:10.1016/S2213-8587(22)00133-4.