ABSTRACT
This study aimed to explore the clinical profile and the impact of vaccination status on various health outcomes among COVID-19 patients diagnosed in different phases of the pandemic, during which several variants of concern (VOCs) circulated in South Carolina (SC). The current study included 861,526 adult COVID-19 patients diagnosed between January 2021 and April 2022. We extracted their information about demographic characteristics, vaccination, and clinical outcomes from a statewide electronic health record database. Multiple logistic regression models were used to compare clinical outcomes by vaccination status in different pandemic phases, accounting for key covariates (e.g. historical comorbidities). A reduction in mortality was observed among COVID-19 patients during the whole study period, although there were fluctuations during the Delta and Omicron dominant periods. Compared to non-vaccinated patients, full-vaccinated COVID-19 patients had lower mortality in all dominant variants, including Pre-alpha (adjusted odds ratio [aOR]: 0.33; 95%CI: 0.15–0.72), Alpha (aOR: 0.58; 95%CI: 0.42–0.82), Delta (aOR: 0.28; 95%CI: 0.25–0.31), and Omicron (aOR: 0.29; 95%CI: 0.26–0.33) phases. Regarding hospitalization, full-vaccinated parties showed lower risk of hospitalization than non-vaccinated patients in Delta (aOR: 0.44; 95%CI: 0.41–0.47) and Omicron (aOR: 0.53; 95%CI: 0.50–0.57) dominant periods. The findings demonstrated the protection effect of the COVID-19 vaccines against all VOCs, although some of the full-vaccinated population still have symptoms to varying degrees from COVID-19 disease at different phases of the pandemic.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a virus that caused COVID-19 and rapidly evolved over time. The World Health Organization (WHO) named the new strains/mutations of the virus as variants of concern (VOC). New SARS-CoV-2 VOC emerged in late December 2020 and was first described in the United Kingdom (named B.1.1.7 in Pangolin classification or Alpha in WHO classification), South Africa (B.1.351 or Beta), Brazil (P.1 or Gamma) and India (B.1.167.2 or Delta).Citation1,Citation2 In the beginning of the pandemic (wild-type variant period), most COVID-19 infections remain mild or asymptomatic. The development of significant viral load among asymptomatic individuals facilitated the virus transmission because it’s challenging to identify and isolate asymptomatic cases,Citation3 which indicated the necessity of sustained COVID-19 testing. The real-time reverse transcription polymerase chain reaction (RT-PCR), the gold standard of COVID-19 diagnosis, has low sensitivity to identify asymptomatic virus carriers, but serological screening has been proven to increase RT-PCR sensitivity.Citation4 Literature has shown that compared to the original “wild type” variant, VOCs increased disease severity and the risk of death.Citation5,Citation6
In the United States (US), VOC was first identified between late December 2020 (Alpha variant in Colorado) and late January 2021 (Beta variant in South Carolina, Maryland; Gamma in Minnesota).Citation7 Initially identified in India, the Delta variant spread rapidly and became predominant in the US between May 2021 and November 2021.Citation8,Citation9 Since December 2021, the Omicron variant (B.1.1.529) has become the main variant circulating in the US.Citation10 Different VOCs showed different patterns of symptoms, transmissibility, and immune response. In the early phase of the pandemic, the Alpha variant had been reported to be more transmissible (increased in vivo replication) than pre-Alpha variants and, in some studies, more virulent, causing more severe illness.Citation5,Citation11–13 When it emerged, the Delta strain was found to be more infectious, associated with more severe disease and poorer clinical outcomes, and more capable of vaccine breakthrough infections than wild-type and Alpha variants.Citation14–18
The COVID-19 vaccine rollout has been associated with a reduction in severe illness in those who are fully vaccinated.Citation17,Citation18 In the US, the Moderna and Pfizer-BioNTech COVID-19 vaccine was first rolled out in December 2020 under emergency use authorization (EUA) status, initially targeting vulnerable populations in congregate settings and essential workers.Citation19 In February 2021, FDA issued the third EUA use of the Janssen COVID-19 vaccine.Citation19 Since March 2021, COVID-19 vaccine eligibility has been expanded to all Americans, along with the development of different vaccine products.Citation19 In SC, the vaccination roll-out plan followed national guidelines and policy. Despite some adverse events occurring after the vaccination (e.g., fever and fatigue), the COVID-19 vaccine has been shown to be highly protective against early SARS-Cov-2 strains.Citation20 However, the effectiveness of the vaccine might vary between different VOCs. For example, during the Delta dominance period, Moderna (mRNA-1273) and Pfizer/BioNTech (BNT162b2) vaccines were highly effective against the Delta variant, particularly in preventing severe and critical disease, but their effectiveness is somewhat lower compared with their effectiveness against the previous variants.Citation21–24 In a retrospective study conducted in an Italian University Hospital, 98% of the study population were fully vaccinated from January 2021 to August 2021, but 30% were also laboratory-confirmed COVID-19 positive.Citation25 A large population-based study in Hong Kong found that despite high overall vaccination coverage (>70% with at least two doses), high mortality observed during the Omicron BA.2 wave indicated limited protection effect of vaccination against Omicron BA.2 infections.Citation26 However, the predominant focus of existing literature examining the effectiveness of vaccination has been on individual VOC rather than concurrently exploring the effectiveness in multiple VOCs. There is a need for additional real-world population-based data to explore the impact of the COVID-19 vaccination on the adverse COVID-19 outcomes across phases with different VOCs.
Using SC statewide electronic health records (EHR) data, we aimed to compare the clinical profiles, disease severity, hospitalization, and mortality among adult COVID-19 patients with different vaccination statuses (e.g., full vaccination and partial vaccination) during different phases of the pandemic, which were defined by different dominant variants that circulated in SC.
Methods
Data source and study population
This study is an observational cohort study. We derived COVID-19 infection-related data (e.g., lab-confirmed and probable COVID-19 cases, hospitalization/ICU, and case demographics) from the SC statewide Case Report Form (CRF) issued by the SC Department of Health and Environmental Control (DHEC).Citation27 According to SC Law and Regulation, all COVID-19 infections are required to be reported to DHEC.Citation28,Citation29 Information about COVID-19 vaccination (i.e., vaccination dates, locations, and product name) was extracted from the Statewide Immunization Online Network (SIMON) in SC DHEC, which is a new Immunization Information System used to house individual-level allocations of vaccine doses. Documentation sources for SIMON include hospital records, vaccination cards, and state vaccine registries. Based on the standardized surveillance case definition for COVID-19, 861,526 people were diagnosed with COVID-19 from January 2, 2021, to April 13, 2022,Citation27 and they were included in the analysis for the current study. (Supplemental Figure S1) We chose January 2, 2021, as the beginning date of the study period because this is the first day that vaccination information becomes available in the SIMON database. The SC RFA office assigned each participant a unique Revenue and Fiscal Affairs (RFA) ID, which was used to link de-identified HIV, COVID-19 testing, and vaccination datasets. Institutional review boards at the University of South Carolina and relevant SC state agencies approved the protocol of this study (Pro00100854).
Measures
Different phases of the pandemic and case demographics
The first case of COVID-19 in South Carolina (SC) was reported in March 2020, and different VOCs characterized the four major phases before April 2022, the end of the study period. The first phase (phase 1) started in late 2020 and ended in March 2021 with wild-type dominance. The second phase occurred in late March 2021 and lasted until June 2021 with Alpha dominance. The third phase occurred suddenly after June 2021 and lasted until early December 2021, with Delta dominance.Citation10 The first Omicron variant case was detected in late December 2021, starting the fourth phase. As of July 2022, the Omicron variant remains the main variant circulating during this phase.Citation10 Based on the timeline for different dominant variants in SC, we categorized the study periods into four time windows, including Pre-alpha (January 2, 2021-March 17, 2021), Alpha (March 18-June 30, 2021), Delta (June 30, 2021-November 30, 2021), and Omicron (November 30, 2021-April 13, 2022) dominant periods.Citation10 Each patient is categorized based on which window their COVID-19-positive diagnosis date falls into.
Case demographics information included age (18–49, 50–64, and ≥65 years old), race (Black, White, Asian, and Other/Unknown), ethnicity (non-Hispanic/Latino, Hispanic/Latino, and Unknown), sex/gender (male, female, and Other/Unknown), residential status (rural, urban) and comorbidity burden. Based on the Rural-Urban Commuting Area (RUCA) codes, residential status was categorized into rural and urban areas.Citation30 The comorbidity burden of each participant was measured based on the Charlson Comorbidity Index (CCI) score. According to the CCI scoring system, 19 different comorbid disease categories were identified based on the International Classification of Disease 10th revision (ICD-10) diagnostic codes.Citation31 Each patient’s comorbidity diagnostic codes before their COVID-19 diagnosis day were retrieved from their EHR data, and their total CCI score was calculated using the specific weighted score system for CCI. Their CCI score was further categorized into three levels, including ‘CCI = 0,’ ‘CCI = 1,’ and ‘CCI ≥2.’Citation31 Missing information for all variables was kept in the analysis by putting them in the category “Unknown” or “Other/Unknow,” and no missing data imputation was conducted.
Clinical course, symptoms, and vaccination status
Clinical course information included symptom severity during the onset of illness (i.e., symptomatic, asymptomatic, unknown), hospitalization, and mortality (). For symptomatic patients, we described the specific symptoms experienced by the patients during the illness, such as cough, congestion, and headache (). Vaccination status before COVID-19 infection was defined based on individuals’ vaccination history 14 days before their COVID-19 diagnosis day. We defined full vaccination as completion of the recommended dosing regimen of any vaccine (a single dose of Janssen, two doses of Moderna, or two doses of Pfizer-BioNTech);Citation32 partial vaccination as receipt of only one dose of Moderna or Pfizer-BioNTech vaccines; and no vaccination as none of the above vaccines was received.Citation33 Patients were excluded from the analysis if they received COVID-19 vaccines not authorized by the FDA in the United States.
Outcomes
The analysis has three distinct outcomes: mortality, hospitalization, and symptom severity. Based on the most severe symptoms in the most recent COVID-19 diagnosis during each phase, individuals were categorized into three groups, including asymptomatic (no symptom recorded), mild symptoms (any records of mild COVID-19 symptoms or signs, such as fever and headache), and moderate/severe symptoms (any records of having difficulty in breathing or developing pneumonia or acute respiratory distress syndrome). Hospitalization status was categorized into “hospitalized” and “non-hospitalized/unknown” based on the question, “Was the patient hospitalized?” and responses included ‘No,’ ‘Yes,’ and ‘Unknown.’ Patients who didn’t answer this question or chose ‘Unknown’/‘No’ were categorized as ‘0 = no hospital admission/unknow,’ Or else, they were categorized as ‘1 = hospitalized.’ Similarly, mortality status was dichotomized as “death” and “alive/unknown” based on the question, “Did the patient die as a result of this illness?”
Statistical analysis
Demographic characteristics, clinical outcomes, and disease severity of COVID-19 cases were described using count numbers and percentages. The temporal trend of biweekly COVID-19 mortality (death rate) along with different pandemic phases was displayed using a smooth cure with Loess (Local regression, smoothing spam = 0.75) as the smoothing method. Logistic regression models were used to explore the association of vaccination status with mortality and hospitalization. A multinomial logistic regression model was employed to explore the relationship between vaccination status and symptom severity (mild, moderate/severe). All models adjusted potential confounders, such as demographic characteristics and comorbidities. All statistical analyses were performed using SAS software version 9.4 and R software version 3.6.2. A statistically significant level was determined based on a P-value of less than .05.
Results
Demographics and clinical outcomes
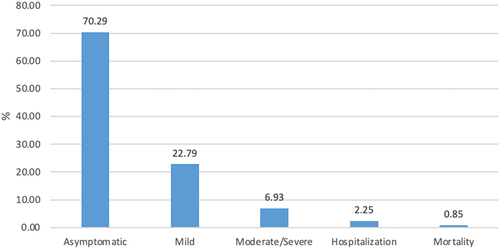
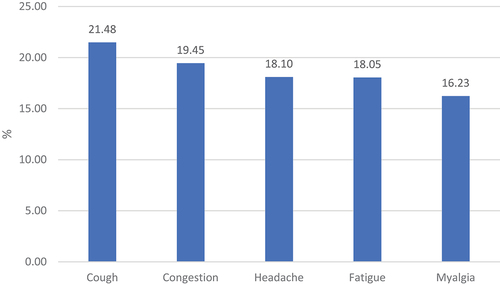
Among 861,526 COVID-19 patients, 55.71% were female, 62.31% were aged 18–49, 60.05% were White, and 72.24% were Not Hispanic or Latino (). In different pandemic phases, the distributions of demographic characteristics were generally comparable (). As presented in , most (70.29%) of the patients were asymptomatic after COVID-19 diagnosis, 22.79% showed mild symptoms, 2.25% were hospitalized, and 0.85% died from COVID-19. The five most frequent symptoms reported were cough (21.48%), congestion (19.45%), headache (18.1%), fatigue (18.05%), and myalgia (16.23%) ().
Table 1. Characteristics distribution among adult COVID-19 patients in different phases of the pandemic in South Carolina, January 2, 2021–April 13, 2022.
Temporal trend of COVID-19 mortality
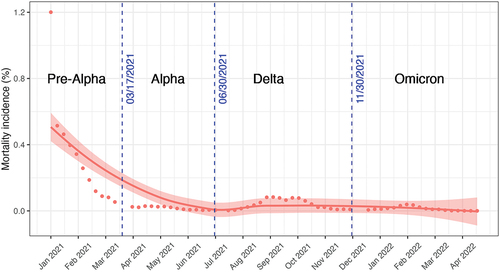
We observed an overall declining trend for the biweekly incidence of COVID-19 mortality in COVID-19 patients after the rollout of vaccination. Relatively substantial reductions in mortality were observed during the Pre-alpha and Alpha dominant period (January 2–June 30, 2021). A fluctuation was observed during Delta dominant phase, with the incidence of mortality gradually increasing in the beginning and then decreasing back to the initial level (June 30, 2021–October 14, 2021). Compared to the Delta dominant phase, a similar but smaller fluctuation was found in the phase when Omicron became dominant (November 30, 2021–April 13, 2022) ().
Clinical outcomes disparities by vaccination status across different COVID-19 pandemic phases
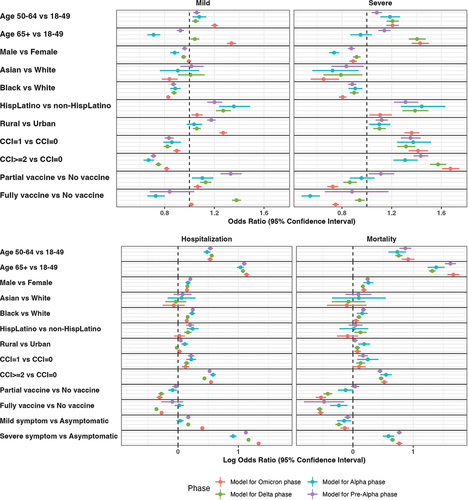
During the Pre-alpha phase, compared to individuals without vaccination, full-vaccinated individuals were less likely to die from COVID-19 (aOR: 0.33; 95%CI: 0.15–0.72), and partial-vaccinated individuals were more likely to exhibit mild (aOR: 1.33; 95%CI: 1.25–1.42) or moderate/severe symptoms (aOR: 1.11; 95%CI: 1.02–1.22). Among all COVID-19 cases diagnosed during the Alpha dominant phase, full-vaccinated patients were less likely to die from COVID-19 (aOR: 0.58; 95%CI: 0.42–0.82) and exhibit mild (aOR: 0.73; 95%CI: 0.66–0.81) or moderate/severe (aOR: 0.54; 95%CI: 0.48–0.62) symptoms than none-vaccinated patients. But partial-vaccinated individuals were more likely to exhibit mild symptoms (aOR: 1.10; 95%CI: 1.02–1,19) and less likely to be hospitalized (aOR: 0.81; 95%CI: 0.69–0.96). In the Delta and Omicron dominant phase, both full-vaccinated and partial-vaccinated patients showed a higher risk of exhibiting mild symptoms but a lower risk of death from COVID-19 and exhibiting moderate/severe symptoms or hospitalization comparing to non-vaccinated patients (p-values were less than .01 for all of them except that no significant difference was observed in mild symptoms for full-vaccinated patients in the Omicron phase) ().
Table 2. Odds ratio of health outcomes by vaccination status in different phases among adult COVID-19 patients in South Carolina, January 2, 2021 - April 13, 2022.
The relationship between some demographic characteristics and COVID-19 clinical outcomes varied when analyzed in separate models with different phases. Across all four phases, compared to patients aged 18–49 years old, those aged 50–64 had a consistently higher risk of mild/severe symptoms, hospitalization, and mortality, while those aged over 65 years old only had a consistently higher risk of hospitalization and mortality. Regarding race, Asian patients were less likely to have mild symptoms only in the Omicron phase and severe symptoms in all phases compared to White patients. Black patients were less likely to have mild or severe symptoms but more likely to be hospitalized or die due to COVID-19 infection across four phases than White patients. Compared to non-Hispanic/Latino individuals, Hispanic/Latino patients were more likely to report mild or severe symptoms across four phases, report hospitalization in three phases (Pre-Alpha, Alpha, and Delta), and report mortality in one phase (Delta). The association of gender and CCI scores with different clinical outcomes was similar across different phases ().
Discussion
This statewide cohort study spans four phases after the implementation of vaccine rollout, including Pre-alpha, Alpha, Delta, and Omicron phases. It provided one of the largest real-world evidence to compare the influence of vaccination status on a series of clinical outcomes among COVID-19 patients in SC. Compared to non-vaccinated COVID-19 patients, reduced mortality was found in all four phases among fully vaccinated patients but only in the Delta and Omicron phases among partially vaccinated patients. Additionally, reduced hospitalization and moderate/severe symptoms were observed among fully vaccinated and partially vaccinated patients in the Delta and Omicron dominance phases. Our findings demonstrated varied protective effects of the COVID-19 vaccines across phases with different dominant variants. The protection effect is not 100%, and some vaccinated individuals still present symptoms of varying degrees of COVID-19.
After the rollout of the COVID-19 vaccine, substantial reductions in mortality were observed in the Pre-alpha and Alpha-dominant phases, followed by slight fluctuations in the Delta and Omicron phases. Substantial reduction of COVID-19 mortality at the beginning of the post-vaccination era but not in the later phases partially raised the hypothesis that the substantial reduction of COVID-19 mortality observed was due to some population-level protection induced by greater disease spread at the beginning of the pandemic.Citation34 Another potential explanation for the decreased mortality is the effects of previous COVID-19 infection on subsequent infection. Lower risk of reinfection and severe infection were found among adults with prior COVID-19 infection.Citation35,Citation36 When Delta and Omicron became the dominant variant, the vaccine’s protection effect was compromised. The delta and Omicron phase fluctuations could also be caused by waning immunity.Citation37 According to Pablo et al., the vaccine effectiveness (VE) of BNT162b2 and mRNA-1273 decreased as time from the vaccine administration elapsed.Citation38 For example, the VE for preventing COVID-19 infection of the complete two-dose Moderna vaccine was 95.2% when 12 to 120 days had elapsed since its administration and 88.3% when more than 120 days had passed.Citation38 In a multicentric European cohort of healthcare workers, the anti-SARS-CoV-2 IgG tier was reduced six months after two doses of vaccination.Citation37 In adjusted models exploring the influence of individual-level vaccination status on health outcomes, fully vaccinated individuals were significantly less likely to die from COVID-19 across all four phases in the post-vaccination era, which proves the protection effect of the COVID-19 vaccine.
The adjusted models found increased moderate/severe symptoms during the Pre-alpha phase but not in later phases among partially vaccinated patients, and there was decreased mortality among fully vaccinated patients. The combined findings could reflect limited immune response after the initial vaccination dose in the pandemic’s early phase. One literature review and meta-analysis of the real-world effectiveness of COVID-19 vaccines suggested that VE for preventing admission to ICU and severe disease was 66.4% and 97.4% for partial and full vaccination, respectively.Citation39 Some fully vaccinated patients were still admitted to hospital or showing moderate/severe symptoms. This could be due to numerous factors, such as ineffective immune response mounted against COVID-19 vaccines among individuals with comorbidities (e.g., use of immunosuppressive agents and older age) and decreased vaccine effectiveness with the emergence of new VOCs.Citation40 To protect individuals from VOCs, booster doses of the COVID-19 vaccine have been recommended in many countries, and one potential strategy to promote COVID-19 booster is vaccine coadministration.Citation41 It has been shown that vaccine coadministration is effective and more cost-effective than programs aiming at promoting one vaccine alone. One systematic review of the coadministration of seasonal influenza and COVID-19 vaccines showed that the coadministration of these two vaccines is safe and does not have immunological interference.Citation42
Our study has limitations. First, RFA randomly shifted COVID-19 diagnosis dates, which disabled us from illustrating an accurate temporal trend of mortality. However, the dates shifted range only from 1 to 14 days, which is relatively short compared to our study period (around 16 months). Thus, we believe the impact was minimal and wouldn’t change our conclusions. Additionally, the biweekly interval used to analyze the mortality trend could also likely minimize the influence of shifting dates. Second, EHR data used in the current study could not capture clinical information for patients who did at-home self-testing. Self-testing kits have been available since September 2020, and the unavailability of their information may underestimate the percentage of asymptomatic or mildly symptomatic patients since they are unlikely to seek healthcare. In addition, the analysis did not include information regarding access to care and health-seeking behavior due to data unavailability. Across different pandemic phases, access to care and health-seeking behavior might vary and influence disease severity and mortality. Third, ‘Unknown’ and ‘No’ responses to the hospitalization question were combined and considered as “not hospitalized” regarding hospitalization status in the analyses. This might skew the results since some patients who responded ‘Unknown’ might experience hospitalization due to COVID-19 infection. However, we have a relatively low proportion of individuals with “unknown” responses (2.68%). It is also supported that the hospitalization rate in our study was comparable with the findings in other studies at the date of this data collection.Citation43 Therefore, we believe that the bias caused by this categorization is minimal. Despite these limitations, this study is one of the largest real-world studies in SC exploring the impact of different vaccination statuses on clinical profiles among COVID-19 patients in different phases of the pandemic.
Conclusions
Our findings provide some incremental evidence regarding the effectiveness of the COVID-19 vaccine and the impact of various dominant variants. Vaccination had a protective effect against severe COVID-19 infection (infection that leads to hospitalization or death) during different phases of the pandemic, which were defined by different dominant variants circulated in SC. However, further studies are needed to identify and mitigate factors related to inadequate vaccine response for those with full or partial vaccination.
Author contribution
XY and FS conceptualized and wrote the first draft and critical revision of the manuscript. JZ set up the statistical test design. HG and SC conducted the data analysis, which was reviewed and verified by JZ. XY and FS prepared tables and figures with input from HG. SW provided clinical input. FS, JZ, AD, LB, SW, BO, and XL reviewed and edited the manuscript. Authorship was determined using ICMJE recommendations.
Supplemental Material
Download PNG Image (22.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data cannot be shared publicly because the data may compromise the privacy of study participants. The restrictions from the ethical application with the University of South Carolina IRB prohibit the authors from making the minimal data set publicly available. Data are available upon reasonable request to the USC IRB (Lisa M. Johnson at [email protected]).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2353491
Additional information
Funding
References
- Faria NR, Claro, IM, Candido D, Franco, LM, Andrade, PS, Coletti, TM, Silva, CA, Sales, FC, Manuli, ER, Aguiar, R, et al. Genomic characterisation of an emergent SARS-CoV-2 lineage in Manaus: preliminary findings. Virological. 2021;372:815–9.
- European Centre for Disease Prevention and Control. SARS-CoV-2 variants of concern as of 26 August 2021. 2021 [accessed 2022 Jul 5]. https://www.ecdc.europa.eu/en/covid-19/variants-concern.
- Bhattacharya S, Basu P, Poddar S. Changing epidemiology of SARS-CoV in the context of COVID-19 pandemic. J Prev Med Hyg. 2020;61(2):E130. doi:10.2139/ssrn.3722801.
- Vimercati L, Stefanizzi P, De Maria L, Caputi A, Cavone D, Quarato M, Gesualdo L, Lopalco PL, Migliore G, Sponselli S. et al. Large-scale IgM and IgG SARS-CoV-2 serological screening among healthcare workers with a low infection prevalence based on nasopharyngeal swab tests in an Italian university hospital: Perspectives for public health. Environ Res. 2021;195:110793. doi:10.1016/j.envres.2021.110793.
- Patone M, Thomas K, Hatch R, Tan PS, Coupland C, Liao W, Mouncey P, Harrison D, Rowan K, Horby P. et al. Mortality and critical care unit admission associated with the SARS-CoV-2 lineage B.1.1.7 in England: an observational cohort study. Lancet Infect Dis. 2021;21(11):1518–28. doi:10.1016/S1473-3099(21)00318-2.
- Lin L, Liu Y, Tang X, He D. The disease severity and clinical outcomes of the SARS-CoV-2 variants of concern. Front Public Health. 2021;9:775224. doi:10.3389/fpubh.2021.775224.
- Walensky RP, Walke HT, Fauci AS. SARS-CoV-2 variants of concern in the United States-challenges and opportunities. JAMA. 2021;325(11):1037–8. doi:10.1001/jama.2021.2294.
- Griffin JB, Haddix M, Danza P, Fisher R, Koo TH, Traub E, Gounder P, Jarashow C, Balter S. SARS-CoV-2 infections and hospitalizations among persons aged ≥16 years, by vaccination status — Los Angeles County, California, May 1–July 25, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1170–6. doi:10.15585/mmwr.mm7034e5.
- Herlihy R, Bamberg W, Burakoff A, Alden N, Severson R, Bush E, Kawasaki B, Berger B, Austin E, Shea M. et al. Rapid Increase in Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant – Mesa County, Colorado, April–June 2021. MMWR Morb Mortal Wkly Rep. 2021;70(32):1084–7. doi:10.15585/mmwr.mm7032e2.
- South Carolina Department of Health and Environmental Control. COVID-19 Variant Dashboard. 2022 [accessed 2022 June 6]. https://scdhec.gov/covid19/covid-19-variants.
- Davies NG, Jarvis CI, Edmunds WJ, Jewell NP, Diaz-Ordaz K, Keogh RH. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021;593(7858):270–4. doi:10.1038/s41586-021-03426-1.
- Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ. 2021;372:n579. doi:10.1136/bmj.n579.
- Frampton D, Rampling T, Cross A, Bailey H, Heaney J, Byott M, Scott R, Sconza R, Price J, Margaritis M. et al. Genomic characteristics and clinical effect of the emergent SARS-CoV-2 B.1.1.7 lineage in London, UK: a whole-genome sequencing and hospital-based cohort study. Lancet Infect Dis. 2021;21(9):1246–56. doi:10.1016/S1473-3099(21)00170-5.
- Liu Y, Rocklov J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J Travel Med. 2021;28(7). doi:10.1093/jtm/taab124.
- Augusto G, Mohsen MO, Zinkhan S, Liu X, Vogel M, Bachmann MF. In vitro data suggest that Indian delta variant B.1.617 of SARS-CoV-2 escapes neutralization by both receptor affinity and immune evasion. Allergy. 2022;77(1):111–17. doi:10.1111/all.15065.
- Teyssou E, Delagrèverie H, Visseaux B, Lambert-Niclot S, Brichler S, Ferre V, Marot S, Jary A, Todesco E, Schnuriger A. et al. The Delta SARS-CoV-2 variant has a higher viral load than the Beta and the historical variants in nasopharyngeal samples from newly diagnosed COVID-19 patients. J Infect. 2021;83(4):1–3. doi:10.1016/j.jinf.2021.08.027.
- Twohig KA, Nyberg T, Zaidi A, Thelwall S, Sinnathamby MA, Aliabadi S, Seaman SR, Harris RJ, Hope R, Lopez-Bernal J. et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2022;22(1):35–42. doi:10.1016/S1473-3099(21)00475-8.
- Ong SWX, Chiew CJ, Ang LW, Mak T-M, Cui L, Toh MPH, Lim YD, Lee PH, Lee TH, Chia PY. et al. Clinical and virological features of SARS-CoV-2 variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta). Clin Infect Dis. 2021; doi:10.2139/ssrn.3861566.
- HHS.gov. COVID-19 vaccines. 2022 [accessed 2022 Aug 4]. https://www.hhs.gov/coronavirus/covid-19-vaccines/index.html.
- Moscara L, Venerito V, Martinelli A, Di Lorenzo A, Toro F, Violante F, Tafuri S, Stefanizzi P. Safety profile and SARS-CoV-2 breakthrough infections among HCWs receiving anti-SARS-CoV-2 and influenza vaccines simultaneously: an Italian observational study. Vaccine. 2023;41(38):5655–61. doi:10.1016/j.vaccine.2023.07.043.
- Nanduri S, Pilishvili T, Derado G, Soe MM, Dollard P, Wu H, Li Q, Bagchi S, Dubendris H, Link-Gelles R. et al. Effectiveness of Pfizer-BioNTech and moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) variant - national healthcare safety network, March 1-August 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1163–6. doi:10.15585/mmwr.mm7034e3.
- Bernal JL, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, et al. Effectiveness of COVID-19 vaccines against the B. 1.617. 2 (Delta) variant. N Engl J Med. 2021;386:585–594.
- Tang P, Hasan MR, Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, AlMukdad S, Coyle P, Ayoub HH, Al Kanaani Z. et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021;27(12):2136–43. doi:10.1038/s41591-021-01583-4.
- Puranik A, Lenehan, PJ, Silvert E, Niesen, MJ, Corchado-Garcia J, O’Horo, JC, Virk A, Swift, MD, Halamka J, Badley, AD, et al. Comparison of two highly-effective mRNA vaccines for COVID-19 during periods of Alpha and Delta variant prevalence. Med. 2022;3:28–41.e8.
- De Maria L, Sponselli S, Caputi A, Pipoli A, Giannelli G, Delvecchio G, Zagaria S, Cavone D, Stefanizzi P, Bianchi FP. et al. Comparison of three different waves in healthcare workers during the COVID-19 pandemic: a retrospective observational study in an Italian University Hospital. J Clin Med. 2022;11(11):3074. doi:10.3390/jcm11113074.
- Yang B, Lin Y, Xiong W, Liu C, Gao H, Ho F, Zhou J, Zhang R, Wong JY, Cheung JK. et al. Comparison of control and transmission of COVID-19 across epidemic waves in Hong Kong: an observational study. Lancet Reg Health–West Pac. 2024;43:43. doi:10.1016/j.lanwpc.2023.100969.
- Council of State and Territorial Epidemiologists. Standardized surveillance case definition and national notification for 2019 Novel Coronavirus Disease (COVID-19). 2020. https://cdn.ymaws.com/www.cste.org/resource/resmgr/2020ps/Interim-20-ID-01_COVID-19.pdf.
- SCDHEC. South Carolina List of Reportable Conditions. 2021. https://scdhec.gov/health-professionals/south-carolina-list-reportable-conditions.
- SC Department of Health and Environmental Control. COVID-19 Compendium of Reporting. 2021 [accessed 2022 Nov 2]. https://scdhec.gov/sites/default/files/Library/CR-012859.pdf.
- Policy OORH. List of rural counties and designated eligible census tracts in metropolitan counties. 2010. https://www.hrsa.gov/sites/default/files/hrsa/ruralhealth/resources/forhpeligibleareas.pdf.
- Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel J-M, Sundararajan V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–82. doi:10.1093/aje/kwq433.
- Tenforde MW, Self WH, Adams K, Gaglani M, Ginde AA, McNeal T, Ghamande S, Douin DJ, Talbot HK, Casey JD. et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA. 2021;326(20):2043–54. doi:10.1001/jama.2021.19499.
- Sun J, Zheng Q, Madhira V, Olex AL, Anzalone AJ, Vinson A, Singh JA, French E, Abraham AG, Mathew J. et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA internal medicine. JAMA Internal Medicine. 2022;182(2):153–62. doi:10.1001/jamainternmed.2021.7024.
- Vinceti M, Filippini T, Rothman KJ, Di Federico S, Orsini N. The association between first and second wave COVID-19 mortality in Italy. BMC Public Health. 2021;21(1):1–9. doi:10.1186/s12889-021-12126-4.
- Hall V, Foulkes S, Insalata F, Kirwan P, Saei A, Atti A, Wellington E, Khawam J, Munro K, Cole M. et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386(13):1207–20. doi:10.1056/NEJMoa2118691.
- Tenforde MW, Link-Gelles R, Patel MM. Long-term protection associated with COVID-19 vaccination and prior infection. JAMA. 2022;328(14):1402–4. doi:10.1001/jama.2022.14660.
- Collatuzzo G, Visci G, Violante FS, Porru S, Spiteri G, Monaco MGL, Larese Fillon F, Negro C, Janke C, Castelletti N. et al. Determinants of anti-S immune response at 6 months after COVID-19 vaccination in a multicentric European cohort of healthcare workers–ORCHESTRA project. Front Immunol. 2022;13:986085. doi:10.3389/fimmu.2022.986085.
- Chico-Sánchez P, Gras-Valentí P, Algado-Sellés N, Jiménez-Sepúlveda N, Vanaclocha H, Peiró S, Burgos JS, Berenguer A, Navarro D, Sánchez-Payá J. et al. The effectiveness of mRNA vaccines to prevent SARS-CoV-2 infection and hospitalisation for COVID-19 according to the time elapsed since their administration in health professionals in the Valencian Autonomous Community (Spain). Prev Med. 2022;163:107237. doi:10.1016/j.ypmed.2022.107237.
- Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022;114:252–60. doi:10.1016/j.ijid.2021.11.009.
- Juthani PV, Gupta A, Borges KA, Price CC, Lee AI, Won CH, Chun HJ. Hospitalisation among vaccine breakthrough COVID-19 infections. Lancet Infect Dis. 2021;21(11):1485–6. doi:10.1016/S1473-3099(21)00558-2.
- Fitz-Patrick D, Young M, Yacisin K, McElwee K, Belanger T, Belanger K, Peng Y, Lee D-Y, Gruber WC, Scott DA. et al. Randomized trial to evaluate the safety, tolerability, and immunogenicity of a booster (third dose) of BNT162b2 COVID-19 vaccine coadministered with 20-valent pneumococcal conjugate vaccine in adults≥ 65 years old. Vaccine. 2023;41(28):4190–8. doi:10.1016/j.vaccine.2023.05.002.
- Janssen C, Mosnier A, Gavazzi G, Combadière B, Crépey P, Gaillat J, Launay O, Botelho-Nevers E. Coadministration of seasonal influenza and COVID-19 vaccines: A systematic review of clinical studies. Hum Vaccines Immunother. 2022;18(6):2131166. doi:10.1080/21645515.2022.2131166.
- Anzalone AJ, Horswell R, Hendricks BM, Chu S, Hillegass WB, Beasley WH, Harper JR, Kimble W, Rosen CJ, Miele L. et al. Higher hospitalization and mortality rates among SARS‐CoV‐2‐infected persons in rural America. J Rural Health. 2023;39(1):39–54. doi:10.1111/jrh.12689.