ABSTRACT
While the number of immunocompromised (IC) individuals continues to rise, the existing literature on influenza vaccine effectiveness (VE) in IC populations is limited. Understanding the vaccine effectiveness (VE) of the seasonal influenza vaccines in immunocompromised (IC) populations remains paramount. Using 2017–2018 US Flu VE Network data, we examined the VE of the 2017–2018 seasonal influenza vaccine against symptomatic influenza in outpatient settings among IC adults. We used logistic regression and adjusted for enrollment site, race, self-reported general health status, age, and onset date of symptoms. The VE among non-IC was 31% (95% CI: 22, 39) and among IC participants was −4% (95% CI: −66, 35), though the difference was not statistically significant. This study demonstrates the capacity to study a large IC population using an existing influenza VE network and contributes to the literature to support large, multicenter VE studies for IC populations.
SUMMARY
Influenza vaccine effectiveness studies are lacking among immunocompromised patients. The VE among non-IC and IC outpatient adults ≥18 years were 31% (95% CI: 22, 39) and −4% (95% CI: −66, 35), respectively, for 2017–2018 seasonal influenza vaccines.
Introduction
During the 2017–2018 influenza season, there were an estimated 41 million cases of influenza, including approximately 710,000 hospitalizations and 52,000 deaths in the United States.Citation1 The predominant strain was A(H3N2) with low-level circulation of influenza B virus later in the season. Influenza hospitalizations disproportionately affect adults 65 years and older and individuals with high-risk conditions, with immunocompromised (IC) individuals having a 5–8-fold higher risk of hospitalizations from acute respiratory illnesses compared to non-immunocompromised (non-IC) individuals.Citation2,Citation3 Although annual influenza vaccination continues to be recommended to prevent influenza infections and influenza-related hospitalizations among IC adults, influenza vaccine effectiveness (VE) among IC adults remains under researched.Citation4
While the number of IC adults continues to increase annually due to advances and expanded uses of biologics, medications, and chemotherapy, vaccine research in this population remains stagnant, and the data on prevention of influenza by vaccination in IC adults is limited. Existing IC adult influenza vaccine research is limited to vaccine immunogenicity and efficacy studies leaving major gaps in our understanding of the influenza VE among IC adults,Citation5–13 While vaccine efficacy can provide investigators insight into reduced risk in the population, it does not entirely reflect how the vaccine will work in the “real-world,” among groups of people. Additionally, most influenza vaccine immunogenicity and efficacy studies on IC individuals are small and lack the statistical power needed to confirm the results or be clinically relevant,Citation5–13 VE studies, which exist outside of controlled settings, utilize real-world data to understand the protection afforded by a vaccine and the groups of individuals most at risk. Influenza VE studies focus on VE estimates for the general population, and there is a lack of data for IC individuals.
As IC individuals represent 2.7% of the US adult population, the role of influenza vaccination in the prevention of influenza and subsequent influenza-associated hospitalizations is of increasing importance.Citation14 Understanding the level of protection afforded by influenza vaccination in a real-world setting can inform vaccine recommendations for the immunocompromised population and if there is a need to evaluate new vaccines. This study investigated the role of the seasonal influenza vaccine in preventing influenza among outpatient IC adults.
Methods
Study design and enrollment
The US Flu VE Network study is a multicenter, prospective, test-negative, case-control study to estimate the VE of the annual influenza vaccine among children and adults who seek care for acute respiratory illness with cough in outpatient settings (including emergency departments). Methods for the US Flu VE study have been previously described,Citation15–19 Briefly, adults, and children ≥6 months of age presenting to one of the enrolling outpatient centers in Michigan, Pennsylvania, Texas, Washington, or Wisconsin with an acute respiratory illness with a new/worsening cough seen within 7 days of symptom onset were eligible for the study. Demographics, symptoms related to influenza-like illness (ILI), vaccination status, date of symptom onset, self-reported general health status, smoking status, and functional status questions were obtained through patient interviews and confirmed through electronic medical records (EMR). The number of underlying high-risk conditions and prior year hospitalizations and outpatient visits were extracted from the EMR. High-risk conditions are CDC-defined chronic health conditions that place an individual at higher risk of influenza complications.Citation20 For this study, “high-risk conditions” was defined as having one or more ICD-10-CM codes corresponding to the following conditions: chronic pulmonary or cardiovascular conditions, renal, hepatic, neurologic, hematologic, or metabolic disorders, and immunosuppression caused by medications, HIV infection, and primary immunodeficiency. The onset date of illness reported at enrollment was divided into tertiles and categorized as pre-peak, peak, and post-peak based on the tertile breakpoints. To allow for comparison to other adult influenza VE studies, this analysis focused solely on adult participants aged ≥18 years.
Influenza case classification
All enrolled participants provided respiratory specimens (nasal and oropharyngeal swabs) for influenza testing (including influenza type and subtype/lineage) by polymerase-chain reaction (RT-PCR).Citation21 Testing was performed at study site research laboratories using a Centers for Disease Control and Prevention (CDC) RT-PCR protocol with reagents from the International Reagent Resource. Participants positive for influenza were cases, and participants who tested negative were controls.
Influenza vaccination status
Current season influenza vaccination status was confirmed by medical record review, state immunization registry records, occupational health records, health insurance billing claims, or records from patients’ primary care providers. Due to the high potential for bias in self-report, multiple efforts were made to confirm self-reported vaccination with the vaccination administration location information provided by the patient. A participant was considered vaccinated if they received the influenza vaccine ≥14 days before illness onset. Participants vaccinated 0–13 days before illness onset were excluded from the analysis because up to 14 days is required to mount an immune response to vaccination.Citation22
Identification of immunocompromising conditions
In order to correctly identify patients with an immunocompromising condition, all ICD-10-CM codes were collected from EMR data and eight groups of immunocompromising conditions were defined: organ transplantation, stem cell transplantation, underlying immunodeficiency (primary immunodeficiencies), connective tissue disorder, receipt of chemotherapy or radiation therapy, hematologic conditions, chronic steroid use, and HIV (Supplemental Table S1). Patients were categorized as IC if they had ≥1 of the included IC ICD-10 codes, and non-IC if none were identified during visits in the year prior to enrollment. The basis for the groups was a previously described algorithm for identifying patients with active immunosuppression using ICD-10 and CPT codes in a large database of patients with severe sepsis and mirrored the ICD-10 codes utilized in prior influenza vaccine effectiveness studies.Citation3,Citation23,Citation24 However, for this study, the algorithm was modified to only utilize ICD-10 codes as we did not have access to CPT codes.
Table 1. Adult patient characteristics overall and by immunocompromising conditions, US Flu VE network study, 2017–2018 (n = 5671).
Statistical analysis
Demographic and other characteristics of the IC and non-IC groups were compared using the Pearson χ2 test or Fisher exact test for categorical variables and the two-sample t-test for continuous variables.
VE was calculated by comparing vaccination odds among cases and control for the IC and non-IC groups using multivariable logistic regression using influenza positivity as the outcome and vaccination status as the exposure variable, with VE = (1 – adjusted odds ratio) × 100%.Citation25 AIC, BIC, deviance, and pseudo R2 from various models were compared, and prior literature was used to determine which variables to include in the final model.Citation25,Citation26 To determine if VE differed by immunocompromised status, we stratified the sample to IC and non-IC and adjusted for enrollment site, race, self-reported general health status, age, and illness onset date tertile. Differences in VE by IC and non-IC status were assessed by an interaction term between IC status and vaccination status.
Because we did not specifically calculate sample size for this study, we did a post hoc power analysis based on the number of vaccinated (n = 1969) and controls (n = 3687), vaccination rate among controls (52.5%), power of 80%, and a significance level of 0.05. We determined a minimum detectable vaccine effectiveness of 38% in our overall study population during the 2017–2018 influenza season based on these assumptions.
Analyses were completed using SAS, version 9.4. Statistical significance was defined as p < .05 or a 95% confidence interval (CI) excluding the null value. The enrollment protocols were approved by each institution’s Institutional Review Board.
Results
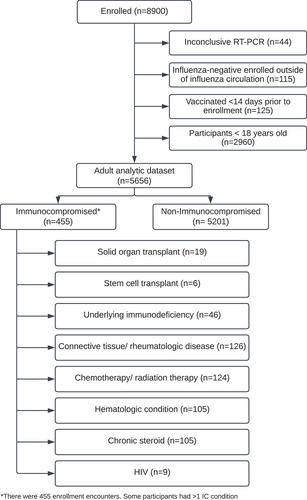
From November 2017 through February 2018, 8900 outpatients were enrolled in the US Flu VE Network. After excluding pediatric participants, 5656 outpatient participants were included in the adult analytic dataset, and among those, there were 455 IC individuals (). There were 1969 (34.8%) confirmed influenza infections (1821 (35.0%) for non-IC and 148 (32.5%) for IC). Influenza A(H3N2) was the predominant influenza strain contributing to 1121 (57.0%) of the total influenza infections detected among the US Flu VE adults. Of the 2967 (52.5%) participants who had documentation of receiving an influenza vaccine, 2642 (50.8%) were non-IC and 325 (71.4%) were IC. The majority (79%) of all participants received the quadrivalent egg-based inactivated influenza vaccine (IIV4) with no difference in vaccine type by IC status. Compared to the non-IC group, the IC group was more likely to be female (71.9% IC vs 63.9% non-IC), older (56.5 years IC vs 47.4 years non-IC), white (81.8% IC vs 77.1% non-IC), vaccinated (71.4% IC vs 50.8% non-IC), have other high-risk conditions in the prior year (98.9% IC vs 55.8% non-IC), have >3 outpatient visits with a high-risk code in the last year (78.7% IC vs 24.9% non-IC), and have >3 hospitalizations with a high-risk code in the last year (3.1% IC vs 0.2% non-IC). IC participants were more likely than non-IC participants to report their health within 30 days prior to enrollment as fair/poor (61.8% IC vs 35.9% non-IC). There were no significant differences in BMI, date of symptom onset, or influenza positivity between IC and non-IC participants ().
Figure 1. US Flu VE Network study population, 2017–2018. Immunocompromised groups were mutually exclusive and followed the order listed here.
After adjusting for enrollment site, race, self-reported general health status, age, and onset date of symptoms, the overall VE against symptomatic influenza among outpatients was 30% (95% CI: 21, 37), 31% (95% CI: 22, 39) among non-IC participants, and −4% (95% CI: −66, 35) among IC participants (). The p-value for interaction by IC-status was 0.100.
Table 2. Adult influenza vaccine effectiveness, US Flu VE Network influenza, 2017–2018.
Discussion
In this large, multicenter, test-negative case-control study, conducted during the 2017–2018 influenza season in the United States, we observed a lower VE with no statistically significant protection against outpatient symptomatic influenza among IC individuals. The overall VE was low among adults during this H3N2 predominant season, resulting in limited power to detect a 30% difference in point estimates. There were significant demographic differences between IC and non-IC participants with IC participants more likely to be female, older, identify as White, receive an influenza vaccine, and to describe their health as good/fair/poor. Among all participants, egg-based inactivated quadrivalent vaccine was the primary influenza vaccine type received. The influenza strains included in the influenza vaccines had good antigenic similarity to the circulating strains of influenza A(H1N1)pdm09, A (H3N2), and B/Yamagata; however, there was considerable antigenic drift from the circulating B/Victoria.Citation27
The overall adjusted VE was 30%; non-IC and IC participants had an adjusted VE of 31% and −4%, respectively. Negative VE point estimates have been discussed in prior papers and do not necessarily indicate an increased risk of infection among vaccinated individuals if the upper bound of the 95% CI is >0.Citation28,Citation29 Possible reasons for a lack of precision of the estimate include increased contact with the healthcare system among vaccinated individuals and increased test-seeking behaviors among IC individuals.Citation29,Citation30 While the test-negative case-control design attempts to reduce bias from healthcare-seeking behaviors, we cannot rule out residual confounding. The wide 95% confidence interval for the VE for the IC participants reiterates that we have little knowledge about the VE for this population and stems from the small sample size, reinforcing the need for a larger sample size in future studies. However, it is unlikely that the lower VE for IC participants is an artifact because immunogenicity studies have demonstrated reduced humoral responses to influenza vaccines in the IC population. In addition, in the 2017–2018 season, influenza VE for preventing influenza hospitalizations was also substantially lower in IC adults vs non-IC adults.Citation24,Citation31
This study had two major limitations. First, the number of IC participants relative to the overall sample size was small resulting in an underpowered study. Some participants with IC may have been missed; work on a previous study demonstrated that compared to ICD-10 codes, interview questions better capture chemotherapy and radiation therapy.Citation24 In our study, these participants were not asked about their chemotherapy and radiation therapy during the interview.Citation24 Second, because of the small sample size, we were unable to examine if VE differed by vaccine type (recombinant vs. high dose vs. standard dose) or by different types of IC, and the analysis was underpowered. It is still unknown if enhanced vaccine options such as high dose and recombinant vaccines could improve the VE in the IC population. Recent studies examining recombinant and standard-dose influenza vaccines found the recombinant influenza vaccine provided superior protection against influenza, but immunocompromised patients were excluded or not identified.Citation32,Citation33 Future outpatient influenza VE studies can address limitations highlighted in this study by recruiting from specialized clinics that care for the IC population, ascertaining chemotherapy and radiation therapy use directly from the participants, and expanding the number of enrollment sites to increase sample size. Increasing the sample size will allow investigators to determine if VE varies by vaccine type or IC type.
Influenza vaccination for IC individuals is recommended by the CDC’s Advisory Committee on Immunization Practices.Citation4,Citation34 Although lower influenza VE was observed in IC individuals, influenza vaccination should still be encouraged, and vaccination rates need to improve. The benefit of influenza vaccination in preventing influenza infection for an individual patient cannot be predicted and, even in cases where vaccination did not prevent influenza infection, it reduced severity of illness.Citation35,Citation36 Continuing to expand the literature on this topic has the potential to improve patient outcomes and decrease healthcare costs.
This study was an important step in establishing literature to support large, comprehensive VE studies in IC populations and supports development of more immunogenic and effective vaccines for immunocompromised individuals. By demonstrating the ability to enroll a substantial IC population in a study where the primary focus was not IC individuals, we have shown the feasibility of enrolling IC populations in future studies. These future IC-specific VE studies may lead to greater knowledge about specific protections afforded by the influenza vaccine such as decreased influenza-related hospitalizations and severity of disease. The study also demonstrated a difference in influenza VE in IC individuals compared to non-IC individuals and the need to consider additional confounding variables when analyzing the IC population. Finally, future studies should examine whether VE among IC individuals varies by vaccine type, which should be considered when selecting which influenza vaccine product to administer to IC individuals. To analyze VE by vaccine type, future studies will need to increase the sample size to provide sufficient power which can be accomplished through multi-center trials and electronic health record extraction studies.
Author contributions
Involved in the conception and design: Hughes Kramer, Zimmerman, Haggerty, Silveria; Analysis and interpretation of the data: Hughes Kramer, Zimmerman, Balasubramani, Silviera; Drafting of the paper, revising it critically for intellectual content: Hughes Kramer, Zimmerman, Haggerty, Balasubramani, Norwalk, Martin, Gaglani, Phillips, Belongia, Chung, Silveira; Final approval of the version to be published: Hughes Kramer, Zimmerman, Haggerty, Balasubramani, Norwalk, Martin, Gaglani, Phillips, Belongia, Chung, Silveira. All authors agree to be accountable for all aspects of the work.
CDC disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Ethical approval
The findings of this paper were presented in poster format at IDWeek 2023.
Supplemental Material
Download PDF (61.2 KB)Disclosure statement
Richard K. Zimmerman: has received grants from Sanofi Pasteur. Mary Patricia Nowalk: as received grants from Merck. E. T. M. has received personal fees from Pfizer and grants from Merck. Manjusha Gaglani: has received grants from Centers for Disease Control and Prevention-Abt Associates.
Data availability statement
Study data was provided by the Centers for Disease Control and Prevention and is available upon request.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2354013
Additional information
Funding
References
- CDC. Burden of Influenza. Centers for Disease Control and Prevention; 2011 June 11 [Accessed 2021 July 19]. https://www.cdc.gov/flu/about/burden/index.html.
- Appiah GD, Chung JR, Flannery B, Havers FP, Zimmerman RK, Nowalk MP, Monto AS, Martin ET, Gaglani M, Murthy K. et al. Hospitalization following outpatient medical care for influenza: US influenza vaccine effectiveness network, 2011‐12—2015‐16. Influenza Other Respir Viruses. 2019;13(2):133–7. doi:10.1111/irv.12616.
- Patel M, Chen J, Kim S, Garg S, Flannery B, Haddadin Z, Rankin D, Halasa N, Talbot HK, Reed C. et al. Analysis of MarketScan data for immunosuppressive conditions and hospitalizations for acute respiratory illness, United States. Emerg Infect Dis. 2020;26(8):1720–30. doi:10.3201/eid2608.191493.
- Grohskopf LA, Blanton LH, Ferdinands JM, Chung JR, Broder KR, Talbot HK, Morgan RL, Fry AM. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices — United States, 2022–23 influenza season. MMWR Recomm Rep. 2022;71(1):1–28. doi:10.15585/mmwr.rr7101a1.
- Cordero E, Manuel O. Influenza vaccination in solid-organ transplant recipients. Curr Opin Organ Transplant. 2012;17(6):601–8. doi:10.1097/MOT.0b013e3283592622.
- Piñana JL, Pérez A, Montoro J, Giménez E, Gómez MD, Lorenzo I, Madrid S, González EM, Vinuesa V, Hernández-Boluda JC. et al. Clinical effectiveness of influenza vaccination after allogeneic hematopoietic stem cell transplantation: a cross-sectional, prospective, observational study. Clin Infect Dis. 2019;68(11):1894–903. doi:10.1093/cid/ciy792.
- Hanitsch LG, Löbel M, Mieves JF, Bauer S, Babel N, Schweiger B, Wittke K, Grabowski P, Volk H-D, Scheibenbogen C. et al. Cellular and humoral influenza-specific immune response upon vaccination in patients with common variable immunodeficiency and unclassified antibody deficiency. Vaccine. 2016;34(21):2417–23. doi:10.1016/j.vaccine.2016.03.091.
- Vollaard A, Schreuder I, Slok-Raijmakers L, Opstelten W, Rimmelzwaan G, Gelderblom H. Influenza vaccination in adult patients with solid tumours treated with chemotherapy. Eur J Cancer. 2017;76:134–43. doi:10.1016/j.ejca.2017.02.012.
- Garg S, Thongcharoen P, Praphasiri P, Chitwarakorn A, Sathirapanya P, Fernandez S, Rungrojcharoenkit K, Chonwattana W, Mock PA, Sukwicha W. et al. Randomized controlled trial to compare immunogenicity of standard-dose intramuscular versus intradermal trivalent inactivated influenza vaccine in HIV-Infected men who have sex with men in Bangkok, Thailand | clinical infectious diseases | oxford academic. Clin Infect Dis. 2016;62(3):383–91. doi:10.1093/cid/civ884.
- Mazza JJ, Yale SH, Arrowood JR, Reynolds CE, Glurich I, Chyou P-H, Linneman JG, Reed KD. Efficacy of the influenza vaccine in patients with malignant lymphoma. Clin Med Res. 2005;3(4):214–20. doi:10.3121/cmr.3.4.214.
- de Roux A, Marx A, Burkhardt O, Schweiger B, Borkowski A, Banzhoff A, Pletz M, Lode H. Impact of corticosteroids on the immune response to a MF59-adjuvanted influenza vaccine in elderly COPD-patients. Vaccine. 2006;24(10):1537–42. doi:10.1016/j.vaccine.2005.10.007.
- Miossi R, Fuller R, Moraes JCB, Ribeiro ACM, Saad CGS, Aikawa NE, Miraglia JL, Ishida MA, Bonfá E, Caleiro MTC. et al. Immunogenicity of influenza H1N1 vaccination in mixed connective tissue disease: effect of disease and therapy. Clinics (Sao Paulo). 2013;68(2):129–34. doi:10.6061/clinics/2013(02)oa02.
- van Assen S, Holvast A, Telgt D, Benne CA, de Haan A, Westra J, Kallenberg CGM, Bijl M. Patients with humoral primary immunodeficiency do not develop protective anti-influenza antibody titers after vaccination with trivalent subunit influenza vaccine | elsevier enhanced reader. Clin Immunol. 2010;136(2):228–35. doi:10.1016/j.clim.2010.03.430.
- Harpaz R, Dahl R, Dooling K. The prevalence of immunocompromised adults: United States, 2013. Open Forum Infect Dis. 2016;3(suppl_1). doi:10.1093/ofid/ofw172.1141.
- Jackson ML, Chung JR, Jackson LA, Phillips CH, Benoit J, Monto AS, Martin ET, Belongia EA, McLean HQ, Gaglani M. et al. Influenza vaccine effectiveness in the United States during the 2015–2016 season. N Engl J Med. 2017;377(6):534–43. doi:10.1056/NEJMoa1700153.
- Ohmit SE, Thompson MG, Petrie JG, Thaker SN, Jackson ML, Belongia EA, Zimmerman RK, Gaglani M, Lamerato L, Spencer SM. et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2014;58(3):319–27. doi:10.1093/cid/cit736.
- McLean HQ, Thompson MG, Sundaram ME, Kieke BA, Gaglani M, Murthy K, Piedra PA, Zimmerman RK, Nowalk MP, Raviotta JM. et al. Influenza vaccine effectiveness in the United States during 2012-2013: variable protection by age and virus type. J Infect Dis. 2015;211(10):1529–40. doi:10.1093/infdis/jiu647.
- Gaglani M, Pruszynski J, Murthy K, Clipper L, Robertson A, Reis M, Chung JR, Piedra PA, Avadhanula V, Nowalk MP. et al. Influenza vaccine effectiveness against 2009 pandemic influenza A(H1N1) virus differed by vaccine type during 2013–2014 in the United States. J Infect Dis. 2016;213(10):1546–56. doi:10.1093/infdis/jiv577.
- Zimmerman RK, Nowalk MP, Chung J, Jackson ML, Jackson LA, Petrie JG, Monto AS, McLean HQ, Belongia EA, Gaglani M. et al. 2014–2015 influenza vaccine effectiveness in the United States by vaccine type. Clin Infect Dis. 2016;63(12):1564–73. doi:10.1093/cid/ciw635.
- CDC. People at High Risk of Flu. Centers for Disease Control and Prevention; 2022 September 6 [Accessed 2023 Mar 20]. https://www.cdc.gov/flu/highrisk/index.htm.
- Harper SA, Bradley JS, Englund JA, File TM, Gravenstein S, Hayden FG, McGeer AJ, Neuzil KM, Pavia AT, Tapper ML. et al. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the infectious diseases society of America. Clin Infect Dis. 2009;48(8):1003–32. doi:10.1086/598513.
- Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, Bousvaros A, Dhanireddy S, Sung L, Keyserling H. et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):e44–100. doi:10.1093/cid/cit684.
- Greenberg JA, Hohmann SF, Hall JB, Kress JP, David MZ. Validation of a method to identify immunocompromised patients with severe sepsis in administrative databases. Ann Am Thorac Soc. 2016;13(2):253–8. doi:10.1513/AnnalsATS.201507-415BC.
- Hughes K, Middleton DB, Nowalk MP, Balasubramani GK, Martin ET, Gaglani M, Talbot HK, Patel MM, Ferdinands JM, Zimmerman RK. et al. Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in immunocompromised adults. Clin Infect Dis. 2021;73(ciaa1927):4353–60. doi:10.1093/cid/ciaa1927.
- Foppa IM, Haber M, Ferdinands JM, Shay DK. The case test-negative design for studies of the effectiveness of influenza vaccine. Vaccine. 2013;31(30):3104–9. doi:10.1016/j.vaccine.2013.04.026.
- Sullivan SG, Tchetgen Tchetgen EJ, Cowling BJ. Theoretical basis of the test-negative study design for assessment of influenza vaccine effectiveness. Am J Epidemiol. 2016;184(5):345–53. doi:10.1093/aje/kww064.
- Garten R, Blanton L, Elal AIA, Alabi N, Barnes J, Biggerstaff M, Brammer L, Budd AP, Burns E, Cummings CN. et al. Update: influenza activity in the United States during the 2017–18 Season and composition of the 2018–19 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67(22):634–42. doi:10.15585/mmwr.mm6722a4.
- Bodner K, Knight J, Hamilton MA, Mishra S. Testing if higher contact among vaccinated can be a mechanism for observed negative vaccine effectiveness. Am J Epidemiol. March 9, 2023:kwad055. doi:10.1093/aje/kwad055.
- Glasziou P, McCaffery K, Cvejic E, Batcup C, Ayre J, Pickles K, Bonner C. Testing behaviour may bias observational studies of vaccine effectiveness. J Assoc Med Microbiol Infect Dis Can. 2022;7(3):242–6. doi:10.3138/jammi-2022-0002.
- Ferdinands JM, Rao S, Dixon BE, Mitchell PK, DeSilva MB, Irving SA, Lewis N, Natarajan K, Stenehjem E, Grannis SJ. et al. Waning of vaccine effectiveness against moderate and severe COVID-19 among adults in the US from the VISION network: test negative, case-control study. BMJ. 2022 October 3;e072141. doi:10.1136/bmj-2022-072141.
- Beck CR, McKenzie BC, Hashim AB, Harris RC, Nguyen-Van-Tam JS, University of Nottingham Influenza and the ImmunoCompromised (UNIIC) Study Group a, Nguyen-Van-Tam JS. Influenza vaccination for immunocompromised patients: systematic review and meta-analysis by etiology. J Infect Dis. 2012;206(8):1250–9. doi:10.1093/infdis/jis487.
- Zimmerman RK, Patricia Nowalk M, Dauer K, Clarke L, Raviotta JM, Balasubramani GK. Vaccine effectiveness of recombinant and standard dose influenza vaccines against influenza related hospitalization using a retrospective test-negative design. Vaccine. 2023;41(35):5134–40. doi:10.1016/j.vaccine.2023.06.056.
- Hsiao A, Yee A, Fireman B, Hansen J, Lewis N, Klein NP. Recombinant or standard-dose influenza vaccine in adults under 65 years of age. N Engl J Med. 2023;389(24):2245–55. doi:10.1056/NEJMoa2302099.
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Bresee JS, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices—United States, 2017–18 influenza season. Am J Transplant. 2017;17(11):2970–82. doi:10.1111/ajt.14511.
- Godoy P, Romero A, Soldevila N, Torner N, Jané M, Martínez A, Caylà JA, Rius C, Domínguez A. Influenza vaccine effectiveness in reducing severe outcomes over six influenza seasons, a case-case analysis, Spain, 2010/11 to 2015/16. Eurosurveillance. 2018;23(43):1700732. doi:10.2807/1560-7917.ES.2018.23.43.1700732.
- Pelton SI, Mould-Quevedo JF, Nguyen VH. The impact of adjuvanted influenza vaccine on disease severity in the US: a stochastic model. Nato Adv Sci Inst Se. 2023;11(10):1525. doi:10.3390/vaccines11101525.
