ABSTRACT
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infections in young children and associated with most bronchiolitis- and pneumonia-related hospitalizations. A new preventive monoclonal antibody (MAb), nirsevimab, has been launched in the United States, Luxembourg, and France, and was recently approved to be given in a population-based manner throughout Spain. This study aimed to have a first pre-immunization insight into the Spanish parental knowledge about bronchiolitis, RSV, and nirsevimab immunization. Parents in Murcia with children <2 years of age up to the date of September 1, 2023, were selected to complete a questionnaire. The primary endpoint was the parental knowledge about bronchiolitis, RSV, and nirsevimab. A total of 3,217 responses were analyzed. The majority (95.8%) were aware of bronchiolitis. Meanwhile, 46.6% of the respondents knew about RSV, most of them only after the first child’s birth. Information about RSV or bronchiolitis was mainly obtained from family members, with only 4.8% reporting having been informed by Health care Professionals (HCPs). Only 11.2% of respondents were aware of nirsevimab. Nonetheless, these were not entirely satisfied with the information received (score of 3.3 out of 5) and shared that HCPs should be the primary source of information. The present survey then highlights the need for better and more efficient educational strategies directed to all parents/legal guardians. It also sheds some light on the different factors that should be considered to improve awareness of RSV immunization to decrease its burden in Spain and beyond.
Introduction
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infections in young children, with a traditional seasonality between November and March.Citation1,Citation2 RSV is also considered the most common cause of death in young infants with estimations of 2% or 3.6% of RSV-related deaths in children aged between 0 and 60 months or 28 days and 6 months, respectively.Citation3
In 2019, a total of 33 million RSV-associated respiratory infection episodes in children aged 0–60 months were estimated worldwide. Indeed, it is expected that 75% of children at 1 year and about 90% at 2 years of age will have been infected by RSV, including infants without risk factors and born full-term.Citation2–4 Relevantly, RSV is responsible for 60–80% of bronchiolitis and 40% of childhood pneumonia, but epidemiological data also suggest a link between RSV infection in the first 3 years of life, and long-term respiratory morbidity, decreased lung function, and allergic sensitization.Citation2,Citation5–7
In Spain, higher RSV-associated hospitalization rates were observed, especially in the 3–6 months group, when compared with Scotland, England, Finland, and the Netherlands.Citation8,Citation9 Data from 1997 to 2011 showed a total of 326,175 hospital discharges for children up to 5 years old, with an annual incidence of 1,072 patients per 100,000 children.Citation10 Between 2012 and 2018, the number of RSV-related hospitalizations was still high (almost 200,000) in children up to 14 years old.Citation11
Until now, only the monoclonal antibody (MAb) monthly administered palivizumab was available to limited at-risk groups during the RSV season.Citation5,Citation12,Citation13 However, in October 2022, the extended half-life anti-RSV MAb nirsevimab was approved by the European Union for preventing RSV in infants during their first RSV season.Citation14 Nirsevimab is currently administered in the United States (US),Citation15 France,Citation16 Luxembourg,Citation17 and Spain.Citation5 Particularly, the Spanish campaign for the 2023–2024 RSV season was the first initiative to administer nirsevimab in a population-based manner for the prevention of RSV infection in the following pediatric populationCitation5: (1) infants at high risk for severe RSV disease, including preterm (gestational age <35 weeks) infants with less than 12 months old, and patients with congenital heart disease with significant cyanosing or non-cyanosing hemodynamic involvement, bronchopulmonary dysplasia or other underlying pathologies that pose a high risk of severe RSV bronchiolitis up to 24 months old; and to (2) infants <6 months of age born between April 1, 2023, and March 31, 2024.Citation5 Yet, regardless of the availability, in the 2023–2024 season, there were not many countries introducing the nirsevimab immunization.
Parental knowledge about RSV was studied in eight countries, including Spain, in 2020.Citation2 However, no studies have evaluated the attitude of the parents toward nirsevimab. Therefore, the present pilot study reevaluates the knowledge of parents/legal guardians about bronchiolitis, RSV pathology, and especially their awareness about nirsevimab as a preventive strategy for RSV outbreaks.
Materials and methods
Study design
Cross-sectional observational study carried out with the participation of the parents/guardians of 27,668 children <2 years old up to September 1, 2023.
On September 1, before the start of the seasonal 2023–2024 immunization campaign against RSV, parents/guardians available in the population database of the Region of Murcia (PERSAN) received a mobile text message with a link to an electronic data collection form (Supplementary Table S1). The questionnaire was accessible, with a clear format, and easy to answer by all parents, regardless of being HCP. All participants were informed about the reason for conducting the study and were notified that the completion of the form was voluntary and anonymous, thereby guaranteeing the privacy of the respondent.
Participants were selected to complete the questionnaire if they were parents/guardians of children <24 months old and agreed to participate. Parents/guardians of children >24 months old by September 1, 2023, and participants who did not accept to participate in the study were excluded.
The study was performed per the “Note for Guidance on Good Clinical Practice” of May 1, 1996, the Royal Decree of February 2004 and the up-to-date Declaration of Helsinki. It was further approved by the Ethics Committee for Research with Medicines of Area 1-Hospital Clínico Universitario Virgen de la Arrixaca.
Study endpoints
The primary endpoint was the parental knowledge about bronchiolitis, RSV, and nirsevimab as a preventive strategy. For that, the following qualitative variables were evaluated: (1) Knowledge about bronchiolitis by parents; (2) Knowledge about RSV by parents/guardians; and (3) Knowledge about nirsevimab for the prevention of RSV disease. The secondary endpoints meant to evaluate the parental attitudes about the upcoming introduction of an MAb for population-based immunization of children <6 months of age, and finally, the main sources of information from which parents/guardians obtain information about vaccinations and immunizations.
The secondary variables analyzed are represented in supplementary Table S2. Likert scale questions from 1 to 5, 7, 8 or 9 points were presented to measure participants’ opinions and perceptions with greater distinction being 1 the lowest value.Citation18
Statistical analysis
Sociodemographic data and other baseline study characteristics of parents/guardians were described using descriptive statistical indices. Continuous variables were expressed using measures of central tendency (mean and median) and measures of dispersion (standard deviation, range, and interquartile range). Categorical variables were described by absolute and relative frequencies. Missing data were excluded upon calculating percentages. To analyze the possible factors that influence the knowledge of bronchiolitis, RSV, and nirsevimab, as well as parents’ proactivity against nirsevimab, a univariate logistic regression model was performed, considering as possible factors the sociodemographic characteristics and the reception of information about the immunization. Then, a multivariate logistic regression model was performed with the univariate significant variables (p < .10) with a backward selection of variables giving rise to the actual significant factors (p < .05). The statistical analysis was carried out with the SAS v9.4 statistical program.
Results
Study population
The questionnaire was sent to parents/guardians of 27,668 children and was available between the 7th and 20th of September 2023. Relevantly, the questionnaire was sent before the launching of the RSV informative immunization campaign, and in a moment, when there were still no news in the press about RSV immunization or nirsevimab (baseline). A total of 3,457 responses were obtained achieving a response rate of 12.5%. Of them, 240 had children >24 months old by the time of September 1st, 2023, and were therefore excluded.
The children from the surveyed parents/guardians (N = 3,217) had a mean age of 11.4 months (range: 0–24), 256 (8.0%) were preterm, 2,658 (82.6%) had been vaccinated with a vaccine non-funded by Public Health and only 80 (2.5%) had a chronic disease, with congenital heart problems being the most common (0.6%). Children’s additional socio-demographic data are presented in .
Table 1. Socio-demographic data from surveyed parents and respective children.
As for the parents/guardians, 2,812 (87.4%) were Spanish, 1,945 (60.5%) had a university degree and 617 (19.2%) were HCP ().
Primary endpoint
Parental knowledge about bronchiolitis and RSV
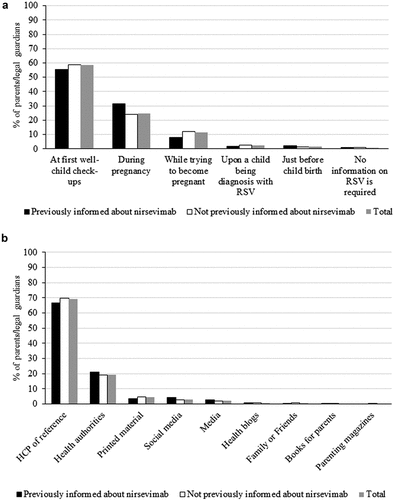
When asked about their knowledge on vaccines/immunizations in general, parents/guardians shared that their main source of information was their HCP of reference (mean score of 5.3 out of 7) and declared to be somewhat satisfied with the information received (mean score 3.6 out of 5; Supplementary Table S3). Regarding bronchiolitis, of the 3,217, 95.8% (95% CI 95.0–96.4) knew about this pathology, of whom 41.6% became aware of it before pregnancy, 7.9% during pregnancy and 39.8% after the birth of the first child (). Of the total respondents, 38.1% declared to have previous experience with bronchiolitis, having required pediatric consultation (30.9%) or hospital emergency care (28.1%) in most cases. Parents declared to have a mean level of satisfaction with the information received of 3.3 out of 5.
Table 2. Parents/guardians’ knowledge on bronchiolitis and RSV.
When asked about RSV, 46.6% of the respondents (95% CI 44.9–48.3) knew about this virus [previously informed (PI) group]. Most of them declared to be aware of this infection only after the birth of the first child (42.1%), 31.2% received information about RSV or bronchiolitis through family and friends and 25.8% from previous experiences with the disease, whereas only 4.8% of the respondents declared having received information from the HCP ().
Addressing the concerns about RSV, no differences were found between the PI group and the remaining parents. The majority showed concerns about RSV severity (63.8%), its frequency in children (48.5%) and contagiousness (47.9%). There were no differences between groups concerning the estimated severity of RSV infection. However, the PI group seemed more enlightened about the estimated risk of RSV-associated illness in children (mean score of 3.4 vs 3; Supplementary Table S4).
Parental knowledge about nirsevimab
Only 359 respondents (11.2%; 95% CI 10.1–12.3%) were aware of MAb nirsevimab (PI group). Among these respondents, nirsevimab was considered to have a mean estimated benefit of 4.3 out of 5, which was significantly superior to the one considered by the remaining parents (3.6. out of 5; p < .0001; ). Regarding the information received about RSV immunization, both the PI group and the remaining respondents considered that the most important thing was to receive adequate information (mean score of 7.5 out of 9) and receive information about efficacy and safety (7.4 out of 9; ). Also, they considered the appropriate time and way to receive proper information to be during the first checkups of the child (58.4%) through the referring HCP (69.3%; ).
Figure 1. Parents’ opinion on the importance of having information about RSV immunization.
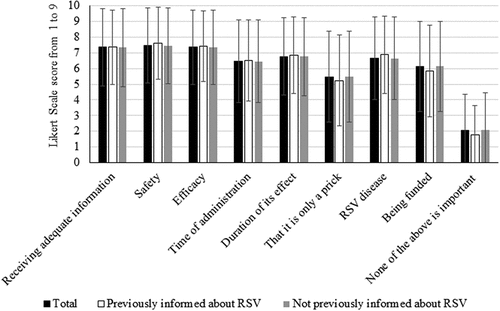
Table 3. Parents’ knowledge of nirsevimab.
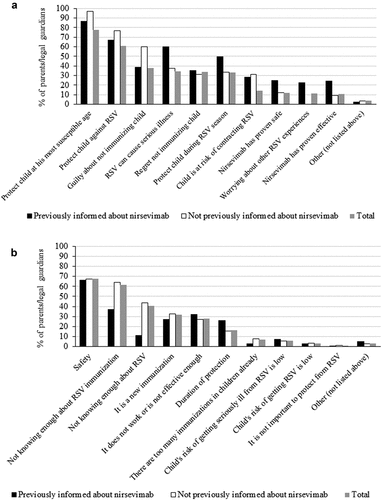
Regarding the main reasons for accepting nirsevimab (), no significant differences were observed between the PI and the non-informed group. The majority (77.7%) described the main reason to be to protect the child from RSV at their most susceptible age. On the other hand, regarding the main doubts about nirsevimab, fewer parents in the PI group (77.2%) declared to have doubts about the immunization with nirsevimab, compared with 95.4% of the remaining respondents (). For both groups, the main reasons for doubting nirsevimab were its safety or adverse effects (67.5%). Within the non-informed respondents, 64.2% also shared concerns about the lack of information on RSV infection ().
Finally, of the 3,217 respondents, 97.1% declared to accept nirsevimab (proactive group) opposing the remaining 94 parents in the reactive group who did not seem open to nirsevimab immunization ().
Secondary endpoints
Factors influencing the awareness of bronchiolitis, RSV and the knowledge on nirsevimab immunization
Using a univariate logistic regression model, possible factors influencing parents’/guardians’ knowledge of bronchiolitis, RSV, and nirsevimab were identified. Then, a multivariate logistic regression model was performed using the univariate significant variables that allowed the identification of a final set of definite factors with significant impact on the awareness of bronchiolitis, RSV, and the knowledge on nirsevimab immunization (Supplementary tables S5, S6 and S7). It was observed that, for each additional month of age of the child, there is an 8% increase in the probability of knowing bronchiolitis (p = .001). Also, it was seen that the greater the parents’ satisfaction with the information received, the greater the probability of knowing about bronchiolitis (p = .001), RSV (p = .001), and nirsevimab (p = .001). The multivariate analysis also demonstrated that parents/guardians working as HCP are eight times more likely to know bronchiolitis (p = .001), 5 times more likely to know RSV (p = .001) but only three times more likely to know nirsevimab than a non-HCP parents (p = .001). Those with several children were shown to have a 65% more probability of knowing bronchiolitis than those who are first-timers (p = .019). Previous vaccination with a non-financed vaccine (including the rotavirus vaccine that is not funded for children unless preterm birth up to 32 gestation weeks, and the meningococcal B vaccine, not funded for children born before the 1st of January 2023) also showed to increase in 56% of the awareness for bronchiolitis (p = .038) and in 61% for nirsevimab (p = .015). Finally, parents with a university degree were seen to have around 40% increased likelihood of knowing RSV and nirsevimab (p = .001). Among others, the study further highlights that having premature children also interferes significantly (p = .001) with the probability of knowing nirsevimab immunization strategy.
Factors influencing parental proactivity to nirsevimab
Similarly, different variables were observed to significantly influence parents’ proactivity toward nirsevimab (supplementary table S8). The analysis demonstrated that parents who vaccinate their children with an unfunded vaccine are 3.7 times more likely to be proactive toward nirsevimab than those who do not (p = .001). Spanish parents also showed an increased likelihood to be proactive to nirsevimab than a parent/guardian of African or European (non-Spanish) nationality. Lastly, the source of information seemed to influence proactivity as well; receiving information from Primary Care professionals or Public Health campaigns increased the likelihood of parents being proactive to nirsevimab (p = .001 and p = .009, respectively).
Discussion
Parents’ knowledge and attitudes toward immunization are of extreme importance as they are the ones with the responsibility of immunizing their children.
RSV is known to be a major cause of bronchiolitis-related hospitalizations, substantially affecting the health of young children, with consequences beyond acute infection. Hence, the availability of different preventive strategies, both vaccines and MAbs, as well as increasing RSV immunization awareness is urgently needed.Citation19 Despite its severity worldwide, in Spain, while 95.8% of the surveyed parents are aware of bronchiolitis, only 46.6% declared to have knowledge about RSV. According to the multivariate analysis, these findings can be partially justified by the low percentage of respondents with previous experience with bronchiolitis, and the low number of children with chronic diseases or premature births.Citation2 Nonetheless, it is evident the lack of awareness about RSV in this population. On the other hand, it can be relevant to point out that the amount of testing and the efficacy of the RSV vigilance protocols may also interfere with general RSV awareness. Indeed, according to the Acute Respiratory Infection (ARI) Surveillance System in Primary Care (PC) in the Region of Murcia (2022–2023), the vigilance of ARI in PC is based on the information provided by a Sentinel Network, and coverages 5% of the population with a PC team assigned to the Murcia Health Service.Citation20 Since 2023–2024, although it is possible to request a rapid multitest in health centers that allows to rapidly catalog the etiology of the respiratory symptoms, those are only requested under certain circumstances. In this case, it can be questioned whether the low RSV vigilance rate might correlate with a diminished perception of the RSV-associated risk, potentially leading to the observed low levels of RSV knowledge and awareness.
An even smaller percentage was knowledgeable about nirsevimab immunization (11.2%). Nirsevimab recent approval against RSV can explain the low data observed. Yet, attention must be given to these worrying numbers, and actions must be taken to provide parents with adequate information, including safety and efficacy data, for them to be able to protect their children at their most vulnerable ages. To counteract this, developing better informative strategies, but also campaigns to increase the etiological diagnosis of RSV-respiratory infections (recognition as a dangerous pathogen, acknowledgment of its severity, or awareness of adequate hygienic practices) should be seen as a priority to increase the level of consciousness of both parents and HCP.Citation21 Indeed, immediately after this survey was conducted, an informative campaign on RSV and nirsevimab immunization was carried out for parents in this region, achieving a 90% of immunization for those born during the RSV season and nearly 87% for those born before the season.Citation22 Based on the high immunization rate observed, it is likely that the informative campaign had a significant impact on raising parents’ awareness of nirsevimab and that this 11.2% no longer reflects the current situation.
The way that information is delivered can also interfere with the level of knowledge of RSV and nirsevimab immunization. As observed, information about bronchiolitis and RSV was mainly delivered to parents/guardians through other family members or friends (31.2%) with only 4.8% of the respondents receiving information from the HCPs. Multivariate analysis demonstrates that having family relatives as the main source of vaccination and immunization information reduces the chance of parents of knowing about RSV or nirsevimab, whereas parents/guardians working as HCPs are more likely to know about bronchiolitis, RSV and nirsevimab. When parents were asked about how this information should be delivered to them, the majority preferred through their HCP. This may underlie the slightly lower level of satisfaction with the information received, emphasizing the need for better education on RSV and nirsevimab immunization routinely delivered in clinics and hospital settings to parents, but especially to pregnant women.
About this topic, an interesting finding in a study from Bell et al., about the parents’/guardians’ views on the acceptability of COVID-19 vaccination, revealed that ethnicity was a determinant of vaccine acceptability demonstrating a decreased level of acceptance in ethnic minorities and lower-income groups.Citation23 Accordingly, this study demonstrates that African, non-Spanish European or South American parents have reduced probabilities of knowing about bronchiolitis compared to Spanish ones. Hence, it emphasizes that not education per se is important,Citation24,Citation25 but a special effort must be made with immigrant parents to address negative views on vaccination and immunization and improve their confidence in them. In line with this, during the campaign that followed the survey, all informative materials were translated to include different nationalities, and may have positively influenced the high percentage of adherence. Yet, strategies such as establishing relations of trust, providing culturally adapted information or facilitating migrant access to vaccination and immunization should also be implemented henceforth to overcome this barrier.Citation26
Interestingly, parents with more than one children were found to have a 65% increased probability of knowing about bronchiolitis than those who are first-timers. Also, the probability of knowing bronchiolitis was observed to increase in 8% each additional month of age of the child. This is in accordance with Lee Mortensen et al. studies demonstrating that RSV and bronchiolitis awareness were higher in experienced parents when compared to new parents (p < .001).Citation2 It can be interpreted that current knowledge on bronchiolitis in this region is time-dependent, likely resulting in a delay in the immunization. Hence, urgent efforts should be made to provide earlier information about RSV-related bronchiolitis infection/consequences, during or even before pregnancy, so that new parents can be aware of RSV immunization in due time.
In fact, the timing is key when addressing RSV immunization. In addition to the earlier described Spanish recommendations,Citation5 in the US, nirsevimab is recommended for all children <8 months old born during or entering their first RSV season and for children aged 8–19 months who are vulnerable to severe RSV disease and entering their second RSV season.Citation15 Even earlier, in France, newborns born from September 15, 2023, onwards, are recommended to receive nirsevimab before leaving the maternity ward.Citation16 Finally, in Luxemburg, nirsevimab is recommended for (1) newborns born during periods of high RSV circulation (also before leaving the maternity ward), for (2) infants <6 months old, who were born outside this high RSV circulation period and for (3) children >12 months old with underlaying conditions.Citation17 Relevantly, 42.1% responded that the best time to receive information about RSV would be “after the birth of the first child” and 36.1% thought it should be given before pregnancy. However, about nirsevimab immunization, most parents responded, “during their child first wellbeing check-up,” which can be considered late when considering the above-described timings of immunization.
Described elsewhere are some of the known reasons that can lead to vaccine hesitancy.Citation27,Citation28 Likewise, many doubts about the new immunization were shared in the survey. Both the PI group and the remaining parents admitted being mainly concerned about its safety and not having enough information about RSV and nirsevimab. Interestingly, although it was demonstrated here that previous vaccinations increase the awareness for bronchiolitis and nirsevimab, having given “too many immunizations” was also a cause of hesitation for 7.2% of the respondents, which is in accordance with previous reports.Citation29
Lastly, the concept of proactive parents was addressed in this survey, and the results identified several factors, such as prior non-funded vaccination, parental nationality, and information source choice, which can be modulated to promote parents’ readiness to immunize.
As for the study's limitations, it can be assumed that parents who responded to the survey perhaps were the ones more concerned about bronchiolitis. On the other hand, it is fair to assume some level of uncertainty associated with the answers given to the question “how information about bronchiolitis/RSV/nirsevimab was received” as some family or friends may have also been HCPs. However, it is still relevant since the goal should be for most individuals to receive well-delivered information from their health system and not from family members or friends (whether they work as health professionals or not).
Also, the percentage of HCPs among the respondents was much higher than the one in the general population,Citation29 and there was an overrepresentation of Spanish parents (60.4% of the survey recipients, and 87.4% of the respondents). Therefore, the level of RSV awareness and nirsevimab acceptance may be overestimated. Finally, although the response rate was low, it was higher than in other previously published studies.Citation30 Likewise, it is not believed that the number of parents who responded to the survey (more than 3,200) could influence the observed results that reflected a low knowledge of RSV and nirsevimab.
Conclusion
The present survey highlights some gaps in parental knowledge, attitudes, and perceptions regarding childhood immunization from a Spanish perspective. A great level of information is still missing about the new immunization strategy with nirsevimab, evidencing the need for better and more efficient educational strategies directed to all parents/guardians, including non-Spanish minorities. In the same way, it sheds some light on the different factors that should be considered to improve awareness of RSV immunization, as well as to increase nirsevimab recommendations and immunization rates worldwide. In fact, accessing the opinions and thoughts of parents in this region has helped us to plan a better way of getting information to parents. We further hope that the results of this study will lead to a better understanding of the attitudes of families toward RSV immunization throughout Spain and abroad.
Ethics approval statement
The study was performed per the “Note for Guidance on Good Clinical Practice” (CPMP/ICH/135/95 of May 1, 1996), Royal Decree 223/2004 of February 2004 and the Declaration of Helsinki in its revised version of Seoul, 2008. It was also approved by the Ethics Committee for Research with Medicines of Area 1-Hospital Clínico Universitario Virgen de la Arrixaca with internal code 2023-9-2-HCUVA.
Participant consent statement
Informed consent was obtained from all participants involved in the study.
REVISION_Supplementary material.docx
Download MS Word (53.4 KB)Acknowledgments
Authors express gratitude to Meisys (Madrid, Spain) for analysis and writing assistance.
Disclosure statement
Jaime Jesús Pérez Martín has collaborated with Sanofi giving talks for continuing medical education. The remaining authors have no other conflicts of interest to disclose.
Data availability statement
The data presented in this study are available on request from the corresponding author.
Supplemental material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2357439
Additional information
Funding
References
- Jain H, Schweitzer JW, Justice NA. Respiratory syncytial virus infection in children. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK459215.
- Lee Mortensen G, Harrod-Lui K. Parental knowledge about respiratory syncytial virus (RSV) and attitudes to infant immunization with monoclonal antibodies. Expert Rev Vaccines. 2022 Oct;21(10):1523–9. doi:10.1080/14760584.2022.2108799.
- Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, Madhi SA, Omer SB, Simões EAF, Campbell H. et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022 May 28;399(10340):2047–64. doi:10.1016/S0140-6736(22)00478-0.
- Gea-Izquierdo E, Gil-Prieto R, Hernandez-Barrera V, Gil-de-Miguel A. Respiratory syncytial virus-associated hospitalization in children aged <2 years in Spain from 2018 to 2021. Hum Vaccin Immunother. 2023 Aug 1;19(2):2231818. doi:10.1080/21645515.2023.2231818.
- Grupo de Trabajo utilización de nirsevimab frente a infección por virus respiratorio sincitial de la Ponencia de Programa y Registro de Vacunaciones. Comisión de Salud Pública del Consejo Interterritorial del Sistema Nacional de Salud. Ministerio de Sanidad, julio; 2023 [accessed 2023 Nov]. https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/comoTrabajamos/docs/Nirsevimab.pdf.
- Rosas-Salazar C, Chirkova T, Gebretsadik T, Chappell JD, Peebles RS Jr., Dupont WD, Jadhao SJ, Gergen PJ, Anderson LJ, Hartert TV. et al. Respiratory syncytial virus infection during infancy and asthma during childhood in the USA (INSPIRE): a population-based, prospective birth cohort study. Lancet. 2023 May 20;401(10389):1669–80. doi:10.1016/S0140-6736(23)00811-5.
- Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, Kjellman B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005 Jan 15;171(2):137–41. doi:10.1164/rccm.200406-730OC.
- Del Riccio M, Spreeuwenberg P, Osei-Yeboah R, Johannesen CK, Fernandez LV, Teirlinck AC, Wang X, Heikkinen T, Bangert M, Caini S, et al. RSV in Europe: estimates of RSV-associated hospitalisations in children under 5 years of age. A systematic review and modelling study. medRxiv. 2023. doi:10.1101/2023.02.10.23285756.
- Wildenbeest JG, Billard MN, Zuurbier RP, Korsten K, Langedijk AC, van de Ven PM, Snape MD, Drysdale SB, Pollard AJ, Robinson H. et al. The burden of respiratory syncytial virus in healthy term-born infants in Europe: a prospective birth cohort study. Lancet Respir Med. 2023 Apr;11(4):341–53. doi:10.1016/S2213-2600(22)00414-3.
- Gil-Prieto R, Gonzalez-Escalada A, Marin-Garcia P, Gallardo-Pino C, Gil-de-Miguel A. Respiratory syncytial virus bronchiolitis in children up to 5 years of age in Spain: Epidemiology and comorbidities: an observational study. Medicine (Baltimore). 2015 May;94(21):e831. doi:10.1097/MD.0000000000000831.
- Heppe-Montero M, Walter S, Hernandez-Barrera V, Gil-Prieto R, Gil-de-Miguel Á. Burden of respiratory syncytial virus-associated lower respiratory infections in children in Spain from 2012 to 2018. BMC Infect Dis. 2022 Mar 31;22(1):315. doi:10.1186/s12879-022-07261-1.
- European Medicines Agency (EMA). Synagis, INN-palivizumab summary of product characteristics; 2004. [accessed 2023 Nov]. https://www.ema.europa.eu/en/documents/product-information/synagis-epar-product-information_en.pdf.
- Eiland LS. Respiratory syncytial virus: diagnosis, treatment and prevention. J Pediatr Pharmacol Ther. 2009 Apr;14(2):75–85. doi:10.5863/1551-6776-14.2.75.
- European Medicines Agency (EMA). Product information beyfortus® (nirsevimab); 2022. [accessed 2023 Nov]. https://www.ema.europa.eu/en/documents/product-information/beyfortus-eparproduct-information_es.pdf.
- Jones JM, Fleming-Dutra KE, Prill MM, Roper LE, Brooks O, Sanchez PJ, Kotton CN, Mahon BE, Meyer S, Long SS. et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendations of the advisory committee on immunization practices — United States, 2023. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72(34):920–5. doi:10.15585/mmwr.mm7234a4.
- Haute Autorité de Santé. Nirsévimab (Beyfortus®) dans la prévention des bronchiolites à virus respiratoire syncytial (VRS) chez les nouveau-nés et les nourrissons; 2023. [accessed 2024 Apr]. https://www.has-sante.fr/jcms/p_3461236/fr/nirsevimab-beyfortus-dans-la-prevention-des-bronchiolites-a-virus-respiratoire-syncytial-vrs-chez-les-nouveau-nes-et-les-nourrissons.
- Le Gouvernement Luxembourgeois. Nouvelle immunisation pour se protéger de la bronchiolite pour les nourrissons et jeunes enfants; 2023. [accessed 2024 Apr]. https://gouvernement.lu/fr/actualites/toutes_actualites/communiques/2023/09-septembre/22-immunisation-bronchiolite-nourrissons.html#:~:text=%C3%80%20partir%20de%202024%2C%20tous,de%20haute%20circulation%20du%20RSV.
- Clark LA, Watson D. Constructing validity: New developments in creating objective measuring instruments. Psychol Assess. 2019 Dec;31(12):1412–27. doi:10.1037/pas0000626.
- Ruckwardt TJ. The road to approved vaccines for respiratory syncytial virus. NPJ Vaccines. 2023 Sep 25;8(1):138. doi:10.1038/s41541-023-00734-7.
- MurciaSalud [Internet]. Sistema de Vigilancia de Infección Respiratoria Aguda en Atención Primaria. Región de Murcia: Temporada 2022–2023; 2024. [accessed 2024 Apr]. https://www.murciasalud.es/documents/128166/6091445/Vigilancia+de+infecci%C3%B3n+respiratoria+leve+en+Atenci%C3%B3n+Primaria+IRA-AP.+Informaci%C3%B3n+metodol%C3%B3gica.pdf/240218a4-82e1-d54f-7cd6-5cae63555121?t=1700048914558.
- Martinon-Torres F, Miras-Carballal S, Duran-Parrondo C. Early lessons from the implementation of universal respiratory syncytial virus prophylaxis in infants with long-acting monoclonal antibodies, Galicia, Spain, September and October 2023. Euro Surveill. 2023 Dec;28(49):2300606. doi:10.2807/1560-7917.ES.2023.28.49.2300606.
- MurciaSalud [Internet]. Virus respiratorio sincitial - VRS; 2022. [accessed 2024 Apr]. https://www.murciasalud.es/documents/5435832/5545872/Coberturas+provisionales+de+inmunizaci%C3%B3n+con+Nirsevimab.pdf/30328cba-aa1e-6e35-7770-b22e98f8c35f?t=1711099482261.
- Bell S, Clarke R, Mounier-Jack S, Walker JL, Paterson P. Parents’ and guardians’ views on the acceptability of a future COVID-19 vaccine: A multi-methods study in England. Vaccine. 2020 Nov 17;38(49):7789–98. doi:10.1016/j.vaccine.2020.10.027.
- Takagi MA, Hess S, Smith Z, Gawronski K, Kumar A, Horsley J, Haddad N, Noveloso B, Zyzanski S, Ragina N. et al. The impact of educational interventions on COVID-19 and vaccination attitudes among patients in Michigan: a prospective study. Front Public Health. 2023;11:111144659. doi:10.3389/fpubh.2023.1144659.
- Tang S, Liu X, Jia Y, Chen H, Zheng P, Fu H, Xiao Q. Education level modifies parental hesitancy about COVID-19 vaccinations for their children. Vaccine. 2023 Jan 9;41(2):496–503. doi:10.1016/j.vaccine.2022.11.060.
- Daniels D, Imdad A, Buscemi-Kimmins T, Vitale D, Rani U, Darabaner E, Shaw A, Shaw J. Vaccine hesitancy in the refugee, immigrant, and migrant population in the United States: a systematic review and meta-analysis. Hum Vaccin Immunother. 2022 Nov 30;18(6):2131168. doi:10.1080/21645515.2022.2131168.
- Dube E, Gagnon D, Nickels E, Jeram S, Schuster M. Mapping vaccine hesitancy–country-specific characteristics of a global phenomenon. Vaccine. 2014 Nov 20;32(49):6649–54. doi:10.1016/j.vaccine.2014.09.039.
- Kennedy A, Lavail K, Nowak G, Basket M, Landry S. Confidence about vaccines in the United States: understanding parents’ perceptions. Health Aff (Millwood). 2011 June;30(6):1151–9. doi:10.1377/hlthaff.2011.0396.
- Ministerio de Sanidad. Número de profesionales de la medicina que trabajan en el Sistema Nacional de Salud (SNS) en Atención Primaria, Atención Hospitalaria, Servicios de urgencias y emergencias (112/061) y Especialistas en formación según comunidad autónoma; 2022. [accessed 2024 Apr]. https://www.sanidad.gob.es/estadEstudios/sanidadDatos/tablas/tabla13.htm.
- Ricco M, Corrado S, Cerviere MP, Ranzieri S, Marchesi F. Respiratory syncytial virus prevention through monoclonal antibodies: a cross-sectional study on knowledge, attitudes, and practices of Italian pediatricians. Pediatr Rep. 2023 Feb 20;15(1):154–74. doi:10.3390/pediatric15010013.