ABSTRACT
To investigate immune checkpoint inhibitors (ICIs) induced pancreatic injury (ICIPI), the prognostic effect of COVID-19 vaccine on cancer patients, and whether COVID-19 vaccine increases the incidence of ICIPI. We conducted a retrospective study of 256 stage IV cancer patients treated with ICIs at The First Affiliated Hospital of Anhui Medical University from January 2020 to November 2022. Data collected included pancreatic enzyme levels, treatment outcomes, and vaccination status. Statistical significance was determined using the χ2 test and Kaplan-Meier method (p < .05). Compared to the control group, the vaccinated group (p < .0001) and the group with elevated pancreatic enzyme levels (p = .044) demonstrated higher disease control rates, indicating a direct benefit of vaccination and enzyme monitoring on treatment outcomes. Additionally, vaccinated patients demonstrated longer overall survival versus unvaccinated patients (23.9 months [95% CI, 22.3–25.5] vs 23.6 months [95% CI, 21.1–26.2], HR = 0.45 [95% CI, 0.24–0.86], p = .015) and progression-free survival (17.2 months [95% CI, 14.3–20.1] vs 13.7 months [95% CI, 11.3–16.1], HR = 0.54 [95% CI, 0.36–0.82], p = .004). Importantly, the analysis revealed no significant association between vaccination and pancreatic injury (p = .46). Monitoring pancreatic enzymes can effectively evaluate the therapeutic impact in patients using ICIs. Patients vaccinated against COVID-19 experience better immunotherapy outcomes without an increased risk of ICIPI.
Introduction
The advent of immune checkpoint inhibitors (ICIs) has revolutionized the treatment of advanced cancers, significantly enhancing patient survival rates. By inhibiting pathways that suppress the immune response against tumors, ICIs unleash the immune system’s potential to combat cancer.Citation1,Citation2 However, their therapeutic success is accompanied by the risk of immune-related adverse events (IrAEs), which can affect multiple organ systems, including the pancreas, albeit infrequently.Citation3,Citation4
Amid the global spread of COVID-19, cancer patients, especially those receiving ICIs, face heightened challenges.Citation5 Their compromised immune systems increase their risk of severe outcomes from COVID-19, underscoring the importance of effective vaccination strategies. The interaction between COVID-19 vaccinations, ICIs, therapeutic efficacy, and the incidence of ICI-induced pancreatic injury (ICIPI) remains underexplored, raising critical concerns about the safety and efficacy of administering COVID-19 vaccines to this vulnerable group and the potential impact on cancer prognosis and treatment continuity.
Current research suggests that vaccination of cancer patients, including with influenza vaccines and COVID-19 mRNA vaccines such as BNT162b2 and mRNA-1273, generally does not increase the occurrence of common immune-related adverse events (irAEs) during treatment with immune checkpoint inhibitors (ICIs).These findings, derived from retrospective medical record reviews and cohort studies, demonstrate the short-term safety of such vaccinations, indicating no emergence of new irAEs or worsening of existing ones. This evidence supports the safety of vaccinating cancer patients, as detailed in Table S2.Citation6–8
Existing studies commonly suggest that combining ICIs with vaccines does not heighten the incidence of common immune-related adverse reactions. However, research into immune-related pancreatic adverse reactions remains scant. This article aims to determine whether COVID-19 vaccination increases the risk of immune-related pancreatic adverse events (IrPanAEs) in patients with advanced cancer, particularly those treated with ICIs. Considering the critical role of treatment continuity in patient prognosis, this study will also explore how COVID-19 vaccination impacts the efficacy of ICI therapy and survival outcomes. Ultimately, this research seeks to address existing gaps in the literature and provide a stronger scientific foundation for vaccinating cancer patients against COVID-19.
Materials and methods
Study design and population
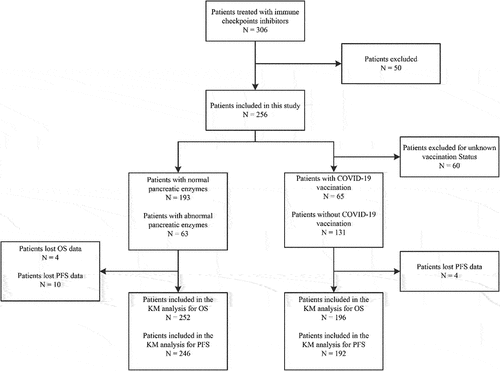
We conducted a retrospective, descriptive study of adult patients who received ICIs therapy at The First Affiliated Hospital of Anhui Medical University from January 2020 through November 2022. The study protocol was reviewed and approved by our hospital’s ethics committee. The inclusion criteria are as follows: (1) aged 18 years or older, (2) received ICIs therapy, (3) TNM stage: stage Ⅳ(The pathological diagnosis of these patients is clear and the imaging has measurable lesions.); Exclusion criteria: (1) There are definite severe heart and lung diseases, autoimmune diseases and immunodeficiency diseases (The indexes of rheumatism immunity and humoral immunity in these patients are obviously abnormal.); (2) Before treatment, there were obvious abnormality of pancreatic enzymes in laboratory and CT showed pancreatic lesions, with a history of pancreatitis ().
Data collection
Basic clinical data
The following data were collected from the medical record database of The First Affiliated Hospital of Anhui Medical University: age, gender, type of cancer, type of immune checkpoint inhibitor, initial treatment upon diagnosis, date of disease progression, pancreatic-related adverse events (lipase, amylase, imaging, and patient’s clinical signs and symptoms), date of death, and cause of death. The status of influenza vaccination and some parts of the overall survival (OS) data were obtained through telephone follow-up.
Efficacy evaluation and pancreatic toxicity grading
Efficacy evaluation was in accordance with irRECIST, and imaging data were confirmed by at least 2 clinical oncologists. Comprehensive efficacy evaluation was divided into complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). The primary endpoint of this study was progression-free survival (PFS), and the secondary endpoints were overall survival (OS), objective response rate (ORR), disease control rate (DCR). PFS was defined as the time from the initiation of immunotherapy to disease progression or death. OS was defined as the time from the initiation of immunotherapy to the death of patients due to any cause. The objective response rate (ORR) was calculated by CR+PR, and the disease control rate (DCR) was calculated by CR+PR+SD. Enzyme levels exceeding the hospital laboratory’s normal reference values are defined as enzyme abnormalities. The grading of pancreatic enzymes is according to Common Terminology Criteria for Adverse Events (CTCAE) v4.03 from April 2011 through April 2018.
Statistical analysis
Data processing was performed using SPSS 22.0 version software. Enumeration data were described by the number of cases and percentages, and the chi-square (χ2) test was used for group comparisons. Survival analysis was conducted using the Kaplan-Meier method and landmark analysis, with the log-rank test employed to assess differences in survival. A p-value of less than .05 was considered to indicate statistical significance. GraphPad Prism was used for graphical representation.
Results
Patient characteristics
From January 2020 to November 2022, 256 Patients with advanced cancer were included in the study. Among the 256 patients, 6 patients went back to the local hospital for further treatment, which could not clearly describe PFS and OS. 4 patients lost their visit. The average age of patients is 59.9 years old,there are 171 males and 85 females. 9 patients used two anti-PD-1 antibodies, 6 patients were given anti-PD-L1 antibody, only one patient used anti-PD-L1/CTLA4 double antibody (Cadonilimab). The remaining 249 patients were treated with PD-1 monoclonal antibody. The cancer species we collected include melanoma, respiratory system, digestive system, urinary system, gynecological system, etc. Most of them are digestive tract tumors (61.3%) and respiratory tract tumors (19.3%). 63 patients developed pancreatic enzyme elevation (24.6%), and 6 patients developed immune-related pancreatitis (2.3%).64 were vaccinated with COVID-19 vaccine, 127 were not vaccinated, and 57 were uncertain whether they were vaccinated with COVID-19 vaccine ().
Table 1. Patient characteristics.
Pancreatic enzymes
Among the 256 patients, 63 (24.6%) patients had increased lipase and/or amylase in different degrees, during which there were 48 cases in G1, 11 cases in G2 and 4 cases in G3–4. We classified similar symptoms of pancreatitis such as abdominal pain, nausea and vomiting, elevated blood sugar and bleeding (excluding other causes) as symptomatic patients, including symptomatic patients and asymptomatic patients. Only 5 cases showed pancreatitis in imaging. The time is calculated from the first day of using ICIs to the first time when pancreatic enzyme abnormality occurs. According to our data (), we think that enzyme elevation generally occurs after the second treatment and before the third treatment, and the median time is about 49 days. Among them, lipase increased after the second treatment, with a median time of about 45.5 days, while amylase increased after the third treatment, with a median time of about 62 days.
Table 2. Pancreatic enzymes.
COVID-19 vaccine and pancreatic enzymes
Among the information we collected, 65 patients were vaccinated, including 14 patients with elevated enzyme (21.5%) and 131 patients without vaccination, including 34 patients with abnormal enzyme (26.1%), a total of 196 patients. We compared the pancreatin between patients injected with COVID-19 vaccine and those who did not(p = .46<.05). The results showed that the injection of COVID-19 vaccine had no obvious effect on Enzyme abnormality of cancer patients treated with immune checkpoint inhibitors ().
Table 3. COVID-19 vaccine and pancreatic enzymes.
Efficacy evaluation
In our study, we analyzed data from 63 patients with enzyme abnormalities (enzymes exceeding normal reference values) and 193 patients with normal enzyme levels. Among these, 10 patients with abnormal enzymes were initially included to calculate the average duration of enzyme abnormalities; however, due to lost follow-up, they were excluded from the efficacy evaluation. Additionally, 4 unvaccinated patients were lost to follow-up for various reasons and were not included in the efficacy analysis. The disease control rate (DCR) for patients with enzyme abnormalities was significantly higher (p = .044 < .05) than for those without such abnormalities. Similarly, the DCR for patients who received the COVID-19 vaccine was significantly higher (p < .0001) than for those who were unvaccinated, as indicated in . These results suggest that patients vaccinated against COVID-19 experienced superior therapeutic outcomes compared to the control group.
Table 4. Efficacy evaluation.
Survival analysis
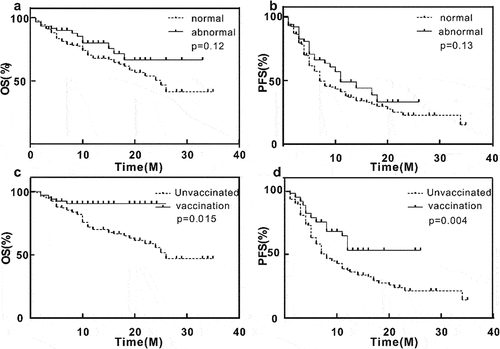
The average follow-up time was about 12.0 months. We defined the increase of enzyme as abnormal enzyme, and the non-increase as normal enzyme. The average OS of patients with abnormal enzymes was 25.4 [95% CI, 21.8–28.9] months, while that of patients with normal enzymes was 22.2 [95% CI, 19.8–24.6] months, with no statistical significance (p = .12>.05), hazard ratio (HR) = 0.66 [95% CI, 0.39–1.11]. The average PFS of patients with enzyme abnormality was 14.2 [95% CI, 11.1–17.2] months, while that of patients with enzyme normality was 13.8 [95% CI, 11.6–15.9] months, with no statistical significance (p = .13>.05), HR = 0.73 [95% CI, 0.49–1.09]. The average OS of patients injected with COVID-19 vaccine was 23.9 [95% CI, 22.3–25.5] months after the start of immunotherapy, and that of patients without vaccine was 23.6 [95% CI, 21.1–26.2] months, which was statistically significant (p = .015<.05), HR = 0.45 [95% CI, 0.24–0.86]. The average PFS of patients injected with COVID-19 vaccine was 17.2 [95% CI, 14.3–20.1] months, and that of patients without vaccine was 13.7 [95% CI, 11.3–16.1] months, which was statistically significant (p = .004<.05), HR = 0.54 [95% CI, 0.36–0.82] ().
Discussion
Immune checkpoint inhibitors generally involve skin, digestive tract, liver, endocrine, lung and other organs, and there are few reports on pancreatic toxicity.Citation3,Citation9,Citation10 Most guidelines do not recommend routine determination of serum amylase and lipase, and the clinical significance of these laboratory abnormalities is still unclear.Citation10 In one study investigating lipase level changes in 909 patients treated with antiePD-1 or antiePD-L1 and 2.3% of them had increased lipase, and the incidence of immune-related pancreatitis was 0.3%.Citation10 Therefore, they suggest that these patients receiving anti-PD-1/PD-L1 therapy should not routinely measure serum amylase and lipase according to the guidelines. The increase rate of pancreatic related enzymes and the incidence of immune-related pancreatitis in our study are higher than those reported above.
In our study, 63 patients had abnormal enzymes, and we found that 20 patients (32%) had elevated Lipase levels, and 6 patients (9.5%) developed immune-related pancreatitis. Notably, those patients with higher enzyme levels responded better to DCR, indicating a potential biomarker of ICI efficacy ().
The onset of ICPIC is still unclear. In the previously reported cases, its onset time varies greatly within 2–16 weeks.Citation11 In our study, the typical onset time of ICPIC is about 17 weeks after treatment, usually after the second cycle, which indicates that it may be valuable for clinicians to monitor enzymes during this period.
Although there is no direct relationship between the increase of enzyme level and the improvement of survival rate, on average, patients with higher pancreatic enzyme level have longer overall survival and progression-free survival. However, due to our small sample size, this discovery is preliminary, which emphasizes the necessity of conducting larger-scale research. Among the patients with elevated enzyme, 16 people stopped ICIs treatment, and the interruption of 5 of them was directly attributed to the increase of enzyme level. Interventions including acid and enzyme inhibitors and methylprednisolone have successfully stabilized pancreatic enzyme levels in these patients (Table S1).
The mechanism behind immune-induced pancreatitis due to ICIs remains largely unknown. It is hypothesized that ICIs may disrupt the balance of autologous tolerance, leading to immune-related adverse effects. These adverse effects might occur due to the removal of negative regulatory factors of cytotoxic T lymphocyte function during ICIs treatment, leading to an inflammatory response in tumor cells and possibly in pancreatic tissue as well. Different ICIs may modulate distinct T cell populations and cytokines, potentially leading to varying immune responses in different tissues, including the pancreas. ICIs can inadvertently enhance immune responses against normal tissues, including the pancreas, by boosting T cell-mediated immune responses against tumors. This heightened T cell activity can trigger an attack on pancreatic cells, resulting in both endocrine and exocrine dysfunction. Treatment with ICIs might also lead to an increase in preexisting autoantibodies, further targeting pancreatic cells. The risk of pancreatic injury may be exacerbated when ICIs are used concurrently with other medications known to cause pancreatitis.Citation12–15
At the time of the epidemic in COVID-19, as a high-risk group of COVID-19 infection, there is no reliable evidence for the safety and effectiveness of tumor patients vaccinated with SARS-CoV-2 vaccine. In the cases we collected, all the vaccinated patients were vaccinated with inactivated vaccine. However, China began to vaccinate on a large scale at the beginning of 2021, and there is no exact guideline in China to tell cancer patients whether they should be vaccinated with COVID-19 vaccine, so relatively few cancer patients who have been vaccinated and used immunosuppressants were collected in this study.
Our results show that the pancreatic enzymes of patients vaccinated with COVID-19 vaccine are not significantly higher than those who were not vaccinated with COVID-19 vaccine. Therefor we consider that COVID-19 vaccine injection has no obvious pancreatic damage to cancer patients who use immune checkpoint inhibitors. However, as far as our current research data are concerned, we only investigated the pancreatic-related toxicity. For other immune-related adverse events, we have no relevant data and dare not make further assertions. However, a study on mRNA COVID-19 vaccine showed that vaccination did not increase immune-related adverse reactions.Citation16 Our data analysis shows that the DCR of patients vaccinated with COVID-19 vaccine are obviously better than those without vaccine, and the OS and PFS are also higher than those without vaccine, which may indicate that vaccination with COVID-19 vaccine plays a positive role in prognosis for patients who use immune checkpoint inhibitors. There are also studies related to our article that have reached similar conclusions.Citation17
In this study, all the vaccinated patients were vaccinated with inactivated virus vaccine, and its main principle of action was to remove infectivity and retain immunogenicity, thus activating the immune system. For patients who use immune checkpoint inhibitors, vaccine-induced immune overactivation may enhance anti-tumor immunity and improve the prognosis of patientsCitation18 In this study, all vaccinated patients received inactivated virus vaccines, the main principles of which include the following: ICIs work by blocking mechanisms such as PD-1/PD-L1 or CTLA-4, which are exploited by tumor cells to suppress the immune system, thereby activating T cells to more effectively target and attack tumor cells. Concurrently, COVID-19 vaccines stimulate the immune system by presenting virus-specific antigens like the spike protein, which enhances the immune memory and response to the SARS-CoV-2 virus. The combination of ICIs and vaccines may lead to a robust activation of immune memory cells, potentially providing a dual defense against tumors and viruses. Both treatments can stimulate the production of various cytokines, such as interferons and tumor necrosis factors, which are pivotal in regulating immune responses, and when combined, may amplify the cytokine response, further boosting the activity and proliferation of immune cells. ICIs therapy, particularly targeting PD-1 and PD-L1, not only aids in fighting cancer by lifting the functional inhibition of T cells but also enhances the immune response to antigens presented by vaccines. Vaccination, in the context of ongoing ICIs therapy, may further activate immune cells that have been “awakened” by ICIs therapy, potentially increasing the vaccine’s effectiveness. However, this enhanced immune activation could also lead to or exacerbate immune-related adverse events (irAEs). Despite these considerations, current research does not indicate a potential for pancreatic-related adverse reactions, although this will be a topic of further investigation within the study’s limitations.Citation17,Citation19,Citation20
Whether patients who use ICIs can be vaccinated with COVID-19 vaccine is not uniform in different regions. The expert consensus of American Society of Clinical Oncology and National Comprehensive Cancer Network Vaccination Advisory CommitteeCitation21 points out that as long as there are no contraindications to vaccination, cancer patients can be vaccinated with COVID-19 vaccine, including those who are active or receiving cancer treatment. Due to the lack of large-scale clinical research on patients with malignant tumor, it is impossible to determine the safety and effectiveness of novel coronavirus vaccine for patients with malignant tumor. Therefore, at present, China does not recommend that patients with malignant tumors be vaccinated with COVID-19 vaccine, and it is necessary to wait for further clinical data to determine whether they can be vaccinated in the future. As far as the data in this article is concerned, we support patients who use ICIs to inject COVID-19 vaccine.
In general, changes in pancreatic enzymes can be used to evaluate the treatment effects in patients using ICIs. Vaccination with the COVID-19 vaccine does not increase ICPI, but vaccination with the COVID-19 vaccine can improve the prognosis of patients using ICIs. Therefore, we support cancer patients receiving the COVID-19 vaccine.
This study has some limitations. First, the retrospective study design may have led to selection bias, which could affect the generalizability of our results and the interpretation of the relationship between COVID-19 vaccination and ICI treatment outcomes. Second, the diversity of cancer types included in this real-world study suggests that while no differences in vaccination patterns were observed, potential variations in the efficacy of combined CPI and vaccination strategies across different cancer types cannot be definitively ruled out. Third, the limited sample size might have restricted the study’s power to detect certain effects, particularly in the analysis of subgroups. Fourth, the absence of a healthy control group in this study limits our ability to fully understand the comparative immune responses to vaccination in cancer patients versus the general population. Fifth, the median follow-up duration, although adequate for capturing most early-onset IRAEs, may have been too short to identify late-occurring IRAEs, which could impact the long-term safety profile of the treatments under investigation. Sixth, the specific mechanism underlying the improved prognosis in vaccinated patients remains unexplained. Despite these limitations, the study provides valuable insights, and future research should aim to address these constraints with a more robust study design and a larger, diverse cohort that includes a healthy control group for more comprehensive assessments.
Data accessibility statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Revised Supporting Information.docx
Download MS Word (28.2 KB)Acknowledgments
We acknowledge the support received from the Anhui Provincial Department of Education through the Key Project of Natural Science in Anhui Universities, Project Number: 2023AH053283.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2358575
Additional information
Funding
References
- Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, Barron DA, Zehir A, Jordan EJ, Omuro A. et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019 Feb;51(2):202–7. doi:10.1038/s41588-018-0312-8.
- Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–68. doi:10.1038/s41577-020-0306-5.
- Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, Gerber DE, Hamad L, Hansen E, Johnson DB. et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. 2021 June;9(6):e002435. doi:10.1136/jitc-2021-002435.
- Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, Atkins MB, Brassil KJ, Caterino JM, Chau I. et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021 Dec;39(36):4073–126. doi:10.1200/JCO.21.01440.
- Ludi Y, Peiwei C, Jie Y, Xianqun F. Effects of cancer on patients with COVID-19: a systematic review and meta-analysis of 63,019 participants. Cancer Biol Med. 2021;18(1):298–307. doi:10.20892/j.issn.2095-3941.2020.0559.
- Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22(5):581–3. doi:10.1016/S1470-2045(21)00155-8.
- Chen YW, Tucker MD, Beckermann KE, Iams WT, Rini BI, Johnson DB. COVID-19 mRNA vaccines and immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Eur J Cancer. 2021 Sep;155:291–3. doi:10.1016/j.ejca.2021.07.017.
- Valachis A, Rosén C, Koliadi A, Digkas E, Gustavsson A, Nearchou A, Ullenhag GJ. Improved survival without increased toxicity with influenza vaccination in cancer patients treated with checkpoint inhibitors. Oncoimmunology. 2021;10(1):1886725. doi:10.1080/2162402X.2021.1886725.
- Calabrese L, Mariette X. The evolving role of the rheumatologist in the management of immune-related adverse events (irAes) caused by cancer immunotherapy. Ann Rheum Dis. 2018 Feb;77(2):162–4. doi:10.1136/annrheumdis-2017-212061.
- Michot J, Ragou P, Carbonnel F, Champiat S, Voisin A-L, Mateus C, Lambotte O, Annereau M. Significance of immune-related lipase increase induced by antiprogrammed death-1 or death ligand-1 antibodies: a brief communication. J Immunother (Hagerstown, Md: 1997). 2018;41(2):84–5. doi:10.1097/CJI.0000000000000202.
- Ikeuchi K, Okuma Y, Tabata T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: A case report. Lung Cancer. 2016 Sep;99:148–50. doi:10.1016/j.lungcan.2016.07.001.
- Zhang Y, Fang Y, Wu J, Huang G, Bin J, Liao Y, Shi M, Liao W, Huang N. Pancreatic adverse events associated with immune checkpoint inhibitors: a large-scale pharmacovigilance analysis. Front Pharmacol. 2022;13:817662. doi:10.3389/fphar.2022.817662.
- Zhang T, Wang Y, Shi C, Liu X, Lv S, Wang X, Li W. Pancreatic injury following immune checkpoint inhibitors: A systematic review and meta-analysis. Front Pharmacol. 2022;13:955701. doi:10.3389/fphar.2022.955701.
- Wu L, Tsang VHM, Sasson SC, Menzies AM, Carlino MS, Brown DA, Clifton-Bligh R, Gunton JE. Unravelling checkpoint inhibitor associated autoimmune diabetes: from bench to bedside. Front Endocrinol (Lausanne). 2021;12:764138. doi:10.3389/fendo.2021.764138.
- Liu Y, Zhang H, Zhou L, Li W, Yang L, Li W, Li K, Liu X. Immunotherapy-associated pancreatic adverse events: current understanding of their mechanism, diagnosis, and management. Front Oncol. 2021;11:627612. doi:10.3389/fonc.2021.627612.
- Nelli F, Giannarelli D, Fabbri A, Virtuoso A, Giron Berrios JR, Marrucci E, Fiore C, Schirripa M, Signorelli C, Chilelli MG. et al. Immune-related adverse events and disease outcomes after the third dose of SARS-CoV-2 mRNA-BNT162b2 vaccine in cancer patients receiving immune checkpoint inhibitors. Cancer Immunol Immunother. 2023 Oct;72(10):3217–28. doi:10.1007/s00262-023-03489-1.
- Brest P, Mograbi B, Hofman P, Milano G. COVID-19 vaccination and cancer immunotherapy: should they stick together? Br J Cancer. 2022 Jan;126(1):1–3. doi:10.1038/s41416-021-01618-0.
- Keam B, Kang C, Jun K, Moon SM, Suh KJ, Lee D-W, Ock C-Y, Kim M, Choi Y, Lim Y. et al. Immunogenicity of influenza vaccination in patients with cancer receiving immune checkpoint inhibitors. Clin Infect Dis. 2020;71(2):422–5. doi:10.1093/cid/ciz1092.
- Lewis A, Annika F, Scott TCS, Rzeniewicz K, Cerrone M, Byrne F, Carlyle E, Edmonds K, Del Rosario L, Shon J. et al. Cytokine release syndrome in a patient with colorectal cancer after vaccination with BNT162b2. Nat Med. 2021;27(8):1362–6. doi:10.1038/s41591-021-01387-6.
- Chang Kyung K, Hang-Rae K, Kyoung-Ho S, Keam B, Choi SJ, Choe PG, Kim ES, Kim NJ, Kim YJ, Park WB. et al. Cell-mediated immunogenicity of influenza vaccination in patients with cancer receiving immune checkpoint inhibitors. J Infect Dis. 2020;222(11):1902–9. doi:10.1093/infdis/jiaa291.
- Trapani D, Curigliano G. COVID-19 vaccines in patients with cancer. Lancet Oncol. 2021 June;22(6):738–9. doi:10.1016/S1470-2045(21)00250-3.