ABSTRACT
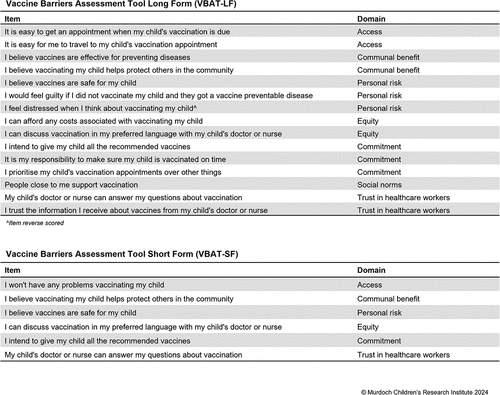
Data on routine childhood vaccination coverage can only tell us who is under-vaccinated; it cannot explain why vaccine coverage is low. Collecting data on the reasons behind under-vaccination is necessary to implement cost-effective strategies that address key barriers and target interventions appropriately. However, no instruments that measure both vaccine acceptance and access factors among parents of children <5 y have been validated in high-income countries. This study aims to develop and validate the Vaccine Barriers Assessment Tool (VBAT) for Australia. We applied three phases of mixed methods data collection and analysis. In Phase 1, we developed a comprehensive list of 80 items reflecting all potential parental barriers to childhood vaccination, derived from published literature and behavioral theory. Through cognitive interviews (n = 28), we refined this list to 45 items. In Phase 2, we conducted a two-wave online survey to test the reliability and validity of these items in an Australian sample of parents (n = 532) with structural equation modeling, further refining the list to 35 items. In Phase 3, we conducted a final parent survey (n = 156), administering these items along with the Parent Attitudes toward Childhood Vaccination (PACV) scale for comparison. We reviewed participants’ immunization register data to assess the predictive validity of the proposed models. The final 6-item short form and 15-item long form Vaccine Barriers Assessment Tool assess access, communal benefit, personal risk, equity, commitment, social norms, and trust in health-care workers. It is being applied for national surveillance in Australia and will be adapted for additional populations and vaccines.
Introduction
As the world begins to recover from the global health emergency of COVID-19, assessing barriers to routine childhood vaccination to improve vaccine uptake and parental vaccine confidence has emerged as an urgent priority.Citation1 Globally, between 2019 and 2021, more than 67 million children under age 5 missed one or more doses of recommended routine vaccines, and measles vaccination coverage declined from 86% to 81%, the largest sustained backslide in immunization in 30 y.Citation1 The largest drops in childhood immunization coverage have been experienced in low- and middle-income countries.Citation1 However, declines in coverage have also been recorded in high-income countries. For example, in the United Kingdom, measles vaccine coverage by age five is at its lowest rate in 10 y (85.2%),Citation2 resulting in significant surges in measles cases in 2023 and 2024.Citation3 New Zealand vaccine coverage among 2-y-olds is 82.5%, a drop of nearly 8% since 2020.Citation4 In Australia, vaccine coverage has dropped below the 95% target for all age groups, with 2-y-old coverage now at 91.2% in the general population and 88.8% among Aboriginal and Torres Strait Islander children.Citation5,Citation6
Unlike many other countries, Australia is able to track changes in childhood vaccination rates through comprehensive registers like the Australian Immunisation Register (AIR), which collects basic demographic data to more accurately define specific low coverage populations. However, vaccination rates from the national register do not provide insights into why coverage gaps exist. They cannot identify parents or carers experiencing access challenges nor those with significant concerns who continue to vaccinate but are at risk of non-vaccination in the future. Understanding the changing social and behavioral drivers of vaccination is key to developing targeted, cost-effective vaccination strategies to improve and sustain uptake.
The two main causes of under- or non-vaccination are acceptance or access barriers or a combination of both.Citation7 Addressing these barriers requires different strategies and policies. Since the start of the COVID-19 pandemic, there is evidence that parental concerns about vaccination have increasedCitation8,Citation9 and the belief that vaccines are important for children has decreased in Australia.Citation1 To address these and other acceptance-related barriers, tailored, dialogue-based communication interventions are among the most effective strategies.Citation10 However, where accessibility issues like getting time off work or traveling to a clinic are the primary barriers, interventions like outreach and extended clinic hours will be more effective than even the most evidence-based communication interventions. Whilst systemic economic and political barriers also impact vaccine uptake, identification of parent-level barriers that can be measured and feasibly addressed is of most value to governments and communities. Importantly, these barriers can change over time, as people are influenced by individual and social factors including exposure to misinformation, disease outbreaks, new vaccine introduction and policy changes.
The COVID-19 pandemic highlighted the need for a valid and repeatable measure of access and acceptance barriers to inform policy decisions and target interventions, but currently there is no systematic way to identify and track the drivers of childhood under-vaccination in Australia. Instruments to measure vaccine acceptance or confidence have been developed and validated for adolescents, children, and certain vaccines, but none of these include assessment of access barriers.Citation11–19 Recently, the World Health Organization (WHO) Behavioural and Social Drivers of Vaccination working group developed a flexible set of quantitative and qualitative instruments to help countries measure acceptance as well as access issues, mostly targeted to low- and middle-income country settings.Citation20,Citation21 However, these instruments have not been validated against behavior or vaccine uptake in Australia.
Therefore, we aimed to develop and validate both a short and a long form of the Vaccine Barriers Assessment Tool (VBAT) to comprehensively measure drivers of both parental acceptance and access to vaccination in children under 5 y in Australia. The short form can be applied efficiently or combined with other population assessment tools, while the longer form can be used to provide more detailed insights.
Materials and methods
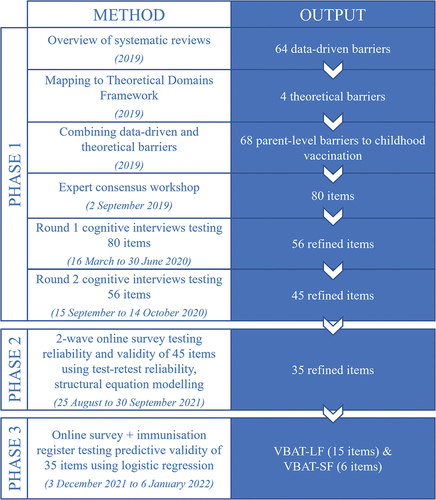
This manuscript presents the three phases of instrument development and validation, which took place between 2019 and 2023. Beginning with a broad item set derived from both behavioral theory and published research evidence, our goal was to refine and reduce the items to reach a final long (VBAT-LF) and short form (VBAT-SF) of the instrument. In all phases, insights generated through relevant qualitative or quantitative methods were considered and contextualized by the core investigator team, comprising eight experts in vaccinology, pediatric medicine, psychology, psychometrics, and social and behavioral science. The project advisory group, which included public health policymakers and researchers, also provided guidance. shows an overview of the methods and outputs of each phase (explained further in this section). Most aspects of the development and validation process took place primarily in Australia, with some data collection in New Zealand. Additional development and validation in the New Zealand context is underway.
The phases of this project received ethical approval from the Royal Children’s Hospital Human Research Ethics Committee (#60277, February 2020, and #75945, July 2021) and the University of Auckland Human Participants Ethics Committee (#024299, May 2020). Participation in all phases of the study was voluntary, and participants could withdraw until the conclusion of the data collection period. Parents or carers (hereafter referred to as ‘parents’) taking part in Phase 1 cognitive interviews were reimbursed for their time. Parents who completed the Phase 2 and 3 surveys were offered the option of entering a prize draw or earning points to be redeemed for gift vouchers.
Phase one: development of the initial item set
We aimed to develop a comprehensive initial item set that reflected all potential parental barriers to childhood vaccination. First, we conducted a global overview of qualitative and quantitative systematic reviews of parent-level barriers (i.e., barriers experienced by or relevant to parents) to vaccination of children aged ≤5 y in both high-income and low- and middle-income settings.Citation7 We extracted data on all described barriers and inductively grouped them into categories. We then deductively mapped the barriers from the systematic reviews against the categories of the Theoretical Domains Framework. This allowed us to identify additional theoretical barriers not described in the literature, such as barriers related to goal setting (e.g., low prioritization of vaccination).Citation22
To draft items for each barrier, we held a 1-d consensus workshop (2 September 2019) with the core investigator team in Australia. Where existing measurement instruments assessed similar barriers (e.g. acceptance tools such as the Parent Attitudes about Childhood Vaccination (PACV),Citation13 the Vaccine Hesitancy Scale,Citation15 Vaccine Confidence Scale),Citation17 we reviewed the wording used in these instruments and aimed to avoid exact duplication. Individual items from a validated scale do not remain ‘validated’ when taken on their own, so there was no advantage to retaining their exact wording. Furthermore, we wanted to ensure that we were guided by the cognitive interviews rather than preconceived bias when selecting or rephrasing items. We framed items positively where possible and followed general criteria for item phrasing, such as avoiding jargon and double-barreled questions. We drafted multiple items for some barriers, particularly those not commonly measured by existing instruments, and included a comprehensive set of access barriers.
We then tested the initial item set in two rounds of cognitive interviews with parents to refine the wording of the questions and response options and ensure face validity.Citation23 In round 1 (16 March to 30 June 2020), we tested the full item set (N = 80 items) with Australian parents of children under 5 years. Parents were recruited through the Social Research Centre Melbourne by paid social media advertising and expression of interest invitation emailed to the Social Research Centre’s 170 Victorian database members. We used a maximum variation sampling approach to include parents with a first language other than English and with different levels of education, income, and degree of vaccine hesitancy. Vaccine hesitancy was assessed with a single item based on the Vaccine Communication Framework (VCF).Citation24,Citation25 The VCF has five levels which we collapsed into three categories: parents who give their child all recommended vaccines and have no vaccine concerns (no hesitancy; VCF level 1), those who vaccinate with minor vaccine concerns (low hesitancy; VCF level 2), and parents who refuse, delay, or vaccinate but have a lot of concerns (high hesitancy; VCF levels 3–5). All interviews were conducted either in person or via Zoom and parents provided written informed consent. Parents were asked to read items aloud and describe their understanding of the item and how they would approach responding to it. We summarized feedback on each item and revised wording or removed from the item set those items with significant comprehension or interpretation issues.
The revised item set (N = 56 items) was taken to a second round of cognitive interviews (15 September to 14 October 2020), including interviews conducted in New Zealand with Māori and Pacific parents, to ensure cross-country appropriateness in support of future New Zealand instrument development and validation. New Zealand parents were recruited through social media, e-mail lists, word-of-mouth and the New Zealand team’s networks. Additional parents in Australia at high risk of under-vaccination due to socioeconomic status or hesitancy were recruited via the research team’s networks in the North Coast of NSW and in Newcastle, NSW. Following this second round of cognitive interviewing, the items were revised again to form the VBAT draft 1-item set (N = 45 items).
Phase two: reliability and validity of the VBAT items
We assessed the reliability and validity of the draft 1 item set in an online survey (25 August to 13 September 2021) with parents who were members of an online Australia-wide panel. A sample size of n = 450 was determined using the traditional principal component analysis guideline of 10 participants per item,Citation26 which we increased to n = 500 to ensure we were able to recruit sufficient parents across sampling categories. To avoid non-independence, only one parent from a couple was recruited. Parents were recruited by the Social Research Centre via e-mail invitation using a criterion sampling approach. Parents who were >18 y old with a youngest child aged <5 y were eligible.
The primary sampling criterion was vaccination coverage of residential area, determined by matching postcodes with Australian Bureau of Statistics regional fully immunized coverage data.Citation5 We defined three levels based on Australian coverage, which is typically between 90% and 95%,Citation5 with anything below that considered “low” and anything above considered “high.” Specifically, low coverage was defined as <90% in either of the reported age brackets (12-<15 month or 24-<27 months). Medium coverage was defined as 90 to <95% and high coverage >95%. We aimed to recruit 40% of participants from low, 20% from medium and 40% from high coverage areas. Additional sampling criteria included level of vaccine hesitancy, determined by the VCF survey question used in Phase 1, and socioeconomic status of the participant’s residential area. Socioeconomic status was determined using postcode and was based on the tertiles of the Australian Bureau of Statistics Socio-Economic Indexes for Areas (SEIFA) Disadvantage Index.Citation27 Based on previous community research by our group,Citation25 we aimed for our sample to include at least 15% highly vaccine hesitant parents and 40% from both low and high SEIFA tertiles, but these were targets rather than quotas.
Eligible parents completed all 45 draft items and provided demographic details. Consent was implied by survey completion. Parents were invited to complete a re-test of the same items 2 weeks after the first survey (8 to 30 September 2021).
To determine which items should be taken through to phase 3 validation, we calculated the correlation between individual items and the VCF vaccine hesitancy item (not part of the VBAT item set) and between each item’s test and retest response. For each item, we also described the frequency of each response option. The core investigator team discussed and considered the inclusion of any items with potentially poor performance based on the following data: a) construct validity of acceptance-related items, defined as correlation <0.2 with the VCF vaccine hesitancy item; b) test–retest reliability, defined by the intercorrelation coefficient <0.3; c) ceiling effect, which we defined as >70% responding “strongly agree;” and d) >5% responding “don’t know/can’t say.” All these data were considered by the investigator team, along with and qualitative factors like relevance to policymaking and equity, with the final decision about which items to include in the VBAT draft 2 item set based on the expert consensus.
Phase three: predictive validity of VBAT model
We assessed the predictive validity of the VBAT draft 2 items (N = 35) in a second survey (3 December 2021 to 6 January 2022) using a convenience sample of different parents recruited from the same online panel as used in phase two. Based on the number of items progressed for validation testing in this phase, we aimed to recruit n = 350 parents. As we needed to assess behavior or future vaccine uptake in this validation phase, we only included parents with a youngest child who was due to receive a vaccine in the next 6 months according to the Australian National Immunisation Program schedule (i.e. those aged either <18 months or between 3.5 and 4 y).Citation28 To be eligible, parents also needed to provide consent for access to their youngest child’s AIR record. Due to the more limited pool of eligible panel members, no further quotas were placed on sampling. Exclusion criteria were parents aged <18 y, those where any member of their household had participated in phase two. Consent was implied via survey completion.
The phase three survey included a series of demographic questions, the VBAT draft 2 survey items, and the 15 items of the validated PACV vaccine hesitancy scale. The PACV is widely used to assess vaccine hesitancy, although it does not include any access items and has been shown to be predictive of future vaccine uptake in a United States parent population.Citation29 The PACV was scored as described by its authors, resulting in a composite score from 0 to 100 where ≥50 is considered hesitant.Citation29 Parents also provided their postcode and child’s first name to enable access to their child’s AIR record to allow future vaccine uptake to be accurately assessed. This was classified as “vaccinated on time” (received all vaccines within 1 month of the vaccines being due); “delayed” (received vaccines >1 month after the due date); or “not vaccinated” (had not received due vaccines at the time of database review). Each child’s AIR record was reviewed at least 1 month after the last eligible vaccine was due. We calculated the correlation between individual VBAT items and on-time vaccination. Those who received the vaccine late were combined with those who did not receive the vaccine within the study period for the purposes of this calculation.
The investigator team undertook a modified Nominal Group Technique approachCitation30 to reach consensus on a final short-form VBAT (fewer than 10 items) and long form VBAT (more than 10 items). First, we each independently drafted potential models for the short- and long-form VBAT, based on individual item correlation with uptake and reflecting key theoretical and practical concepts or domains.
We then used logistic regression analysis to test the predictive validity of these models, as well as the PACV for comparison. Logistic regression helped determine the performance of these pre-defined models, with reference to the pseudo R2 value of Nagelkerke.Citation31 We considered a pseudo R2 value of ≥0.1 to be a reasonably strong predictive performance. According to Hensher, Rose & Green,Citation32 these values would correspond to classical shared variance (R2) values greater than 0.3, i.e., approximately three times higher than the average effect size in consumer behavior studies.Citation33
To validate the dimensional structure of the VBAT long form, we conducted a confirmatory composite analysis (CCA),Citation34 a kind of structural equation modeling that enables empirical validation of both traditional latent variables, such as vaccine confidence, and formative constructs, like vaccine accessibility.Citation35,Citation36 Formative constructs are defined by their indicators (i.e., the indicators are not assumed to represent a defined latent variable, but rather are designed to constitute the variable). Because these indicators need not be inter-correlated, analytic methods based on inter-item associations, like factor analysis and item response models, may be uninformative regarding the validity and reliability of instruments that capture formative constructs. The justification for aggregating indicator scores for formative constructs into a composite is not that they uniformly represent an underlying construct, but because the aggregate has utility, as determined by correlations with relevant criteria (i.e. PACV hesitancy score and/or timely vaccine uptake, as described above). Like all structural equation modeling techniques, CCA quantifies the degree of model fit based on the discrepancy between the model-implied and the empirical variance-covariance matrix. Values of the standardized root mean square residual (SRMR) below 0.05 combined with values of the comparative fit index (CFI) above 0.90 are regarded as acceptable.Citation37
After discussing the results for each model, members of the core team individually drafted revised models and again brought them together for discussion and analysis until expert consensus was reached on the final items. The team discussed and agreed on qualitative labels for the domains identified through CCA.
Analysis for phases two and three was performed using SPSS 28Citation38 and MPLUS 7.3.Citation39
Results
A total of 865 parents participated in the study (). Phase 2b parents were a sub-set of those participating in Phase 2a.
Table 1. Participant characteristics at each phase.
Phase one: development of the item set
The global overview of systematic reviews of parent-level barriers to childhood vaccination included 30 reviews describing 64 unique barriers.Citation7 When mapped to the Theoretical Domains Framework, these barriers aligned with 10 of the 14 domains. Four additional theoretical barriers were added to address Optimism, Intentions, Goals, and Behavioural Regulation.Citation22 The 1-d workshop resulted in an initial set of 80 items (see supplemental files). Likert scales proposed as potential response options included scales in both directions (i.e. strongly disagree to strongly agree and vice versa), scales with or without a separate “Not sure/prefer not to say” option, 5-point scales with or 4-point scales without a neutral in-line option (e.g. “Neither agree nor disagree”), and display of options as a list rather than a horizontal scale.
In round 1 of cognitive testing, the whole initial item set was tested with Australian parents (n = 14) (). Six parents (43%) were highly vaccine hesitant (VCF levels 3–5), three (21%) spoke a language other than English, and nine (64%) had a bachelor’s degree or higher. Based on these interviews, we rephrased some items. We reduced the initial item set from 80 to 56 items, removing items that were poorly understood and those where a clearly preferred alternative phrasing was already included. The preferred response option was a vertical 4-point list, with a separate “not sure/prefer not to answer” option.
A second round of cognitive testing was undertaken with 14 parents from both Australia (n = 4) and New Zealand (n = 10). Four parents from New Zealand were highly vaccine hesitant (29%) and four had a bachelor’s degree or higher (29%). All the New Zealand parents self-identified as Māori and Pacific ethnicity. Following round 2, the items were again refined, and the item set was further reduced to 45 items (see supplemental files). The New Zealand parents often wanted an option to select “slightly agree” or “slightly disagree,” so the 4-point scale was revised to strongly agree, slightly agree, slightly disagree, and strongly disagree, with a “don’t know/can’t say” option.
Phase two: reliability and validity of the VBAT items
A total of 532 parents completed the online survey of the 45 VBAT draft 1 items, with a subset of 263 (49%) completing the re-test 2 weeks later (). Characteristics of those who completed the re-test were similar to those who did not.
Twenty-four items demonstrated potential issues in either correlation with the VCF item (n = 11), test–retest reliability (n = 3), ceiling effect (n = 4), high numbers of “Don’t know/can’t say” responses (n = 11) or a combination of these. All these items were discussed with the investigator team, and the expert consensus was to remove 10 items, prioritizing removing those with high rates of “Don’t know/can’t say” responses (). The final number of items posted in this phase was 35.
Table 2. Characteristics of the VBAT draft 1 items reviewed for inclusion.
Phase three: predictive validity of VBAT items and model composition
A total of 305 parents completed the online survey consisting of the 35 VBAT draft 2 items with the intention of validating responses against AIR data to assess their ability to predict behavior. However, data from n = 149 parents could not be used for assessing the predictive validity because the child’s AIR record was unable to be accessed (n = 61); the child’s record was a duplicate (n = 2); the child was ineligible as AIR data indicated that vaccines had been given prior to the survey (n = 79); or the date of the next vaccination was beyond the timeline of the study (n = 7). This left 156 parents with survey data able to be linked to the child’s AIR data. Compared to the parents who provided AIR data, those for whom we could not access AIR data were less likely to live in areas with the highest rates of vaccination and had fewer children under 5 months old. They were similar in all other characteristics (). Of the 156 parents included in Phase 3, 70.5% (n = 110) children were vaccinated on time; 20.5% delayed (n = 32) or 8.9% not vaccinated (n = 14).
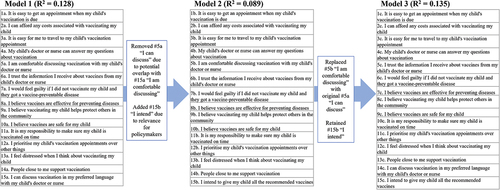
Logistic regression analysis of the predictive performance of the team’s individual models for a VBAT long and short form informed refined models of each (see for example of the process). For benchmarking purposes, we calculated the PACV instrument’s pseudo R2 of Nagelkerke using data from our sample. The PACV generated a pseudo R2 value of 0.088.
The team reached consensus on a final long- and short-form VBAT (). The final short-form VBAT pseudo R2 value was 0.113, and the long form pseudo R2 was 0.135. The short VBAT includes six items representing six domains, while the long VBAT includes 15 items representing seven domains.
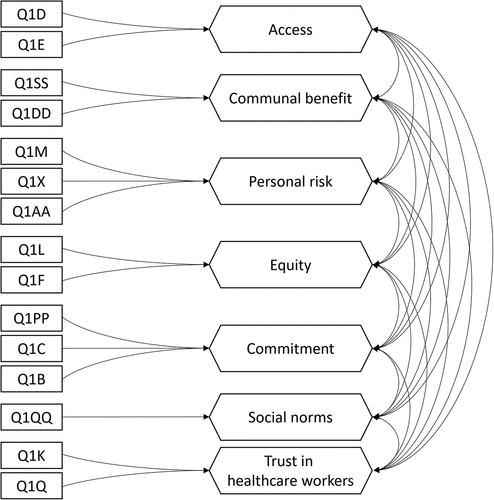
We validated the dimensional structure of the VBAT-LF () with data from all but one (n = 304) phase three participant (one participant had created too many missing values). Model estimation with maximum likelihood terminated normally and without any Heywood cases. While the test of exact fit was significant (χ2 = 188.878; df = 66; p < .001), a standardized root mean square residual of 0.045 and a comparative fit index of 0.928 indicated an acceptable model fit.
Discussion
Understanding the reasons for under-vaccination is key to designing and targeting effective and cost-effective interventions. While vaccine hesitancy is often blamed for suboptimal coverage, the true barriers to uptake are often a complex mix of both access and acceptance issues. However, available measurement instruments focus almost exclusively on the psychosocial factors affecting uptake and do not include practical barriers to vaccination. We aimed to develop a measurement instrument to assess both access and acceptance barriers in the high-income country context of Australia, capitalizing on Australia’s unique national vaccine registry data for validation. Our robust and novel multi-phase process involved comprehensive review of theoretical and practical barriers to vaccination, cognitive item testing, and data-driven psychometric validation to produce a long 15-item form and a short 6-item form of the Vaccine Barriers Assessment Tool for Australia.
In our study population, both forms of the VBAT exhibited an acceptable level of predictive validity, both in absolute and in relative terms. In particular, both forms of the VBAT predicted vaccine uptake to a greater degree than the gold standard PACV tool, which measures vaccine hesitancy. The PACV is one of the most rigorously validated and widely used instruments in this field, with dozens of published studies reporting validation data of translated and adapted versions around the world.Citation40–44 As with most vaccine hesitancy or confidence instruments, the PACV was developed through expert consultation and psychometric testing applying classical test theory methods. However, these methods can only be applied to psychological constructs like attitudes and beliefs. To incorporate access and equity barriers into our instrument, we had to consider different validation approaches. CCA is a novel form of structural equation modeling that has been designed for these kinds of problems. CCA is completely analogous to the well-known workhorse of psychometrics, confirmatory factor analysis, with the difference that CCA models composites instead of common factors. In the current setting, CCA helped to validate the VBAT and provide support for the VBAT’s dimensional structure. In addition to the novel application of CCA, we based our initial item set on both theoretical behavior change literatureCitation22 as well as published evidence on barriers to vaccination around the world.Citation7 The aim of this approach was to capture the broadest possible set of barriers as an initial starting point, which we narrowed through several rounds of assessment with diverse parents, stratified by both socioeconomic and hesitancy factors.
Among the range of available measurement instruments, only the WHO BeSD quantitative data collection instrument also captures access barriers or practical issues. The VBAT domains align generally with the overarching BeSD categories, with some differences between the specific constructs and items. For example, the VBAT domains of “communal benefit” and “personal risk” are similar to BeSD constructs within the category of “what people think and feel.” Both instruments assess vaccine affordability, but this falls into the “equity” domain in VBAT and the “practical issues” category in the BeSD instrument. Developed by an expert global working group and cognitively tested in a range of countries, the BeSD tool is designed for flexible use primarily in low- and middle-income countries.Citation20,Citation21 The VBAT is not intended to replace the BeSD tool but is instead tailored specifically for the high-income country setting of Australia. The BeSD tool has been used in multiple studies since its launch in 2022 and formal validation is underway but has not yet been published. A unique feature of validation in Australia is the ability to use actual future vaccine uptake from the Australian Immunisation Register as the behavioral variable, as opposed to intention or self-reported vaccine uptake. Few countries have such a comprehensive register to support robust validation.
We created a short and a long form of the VBAT to maximize the ways in which it can be applied, following the examples of the BeSD and PACV instruments. The WHO BeSD working group note that countries may choose to use some items and not others as their setting requires but suggest including a minimum of five key indicators, while the PACV has 5-item and 15-item versions. The 16-item VBAT-LF includes a broader range of items, allowing for more specific identification of barriers. This specificity can improve cost-effective targeting of interventions. It can also identify areas, topics or populations where additional in-depth qualitative research may be needed. The VBAT-LF may be useful for researchers conducting primary research and intervention evaluations, where asking parents to spend 5–10 min on a survey is feasible. The breadth of factors assessed in the VBAT-LF makes it a potentially suitable evaluation tool for multicomponent interventions that involve both vaccine communication and service delivery elements, which may have different mechanisms of effect.Citation45 We considered three factors when determining the length of the VBAT-SF: brevity/feasibility for use in population surveys; explanation of a high degree of variance (i.e., performance); and breadth of barriers assessed. Through discussion with the core study team and project advisory group, we determined that six items were the maximum length that would be feasible for most government surveys. We selected the six items that performed best and included both access- and acceptance-related items, which is the key point of differentiation from other scales. Since the domain of access contains more varied barriers than the other domains, we determined that a general accessibility item was more appropriate than selecting one type of access to assess in the VBAT-SF. Adding a social norms item lengthened the scale but did not increase its explanatory power. Where social norms are considered particularly relevant, we advise users to apply the VBAT-LF. Both forms include an intention item which can be used as a proxy for vaccine uptake if register data are not available.
Validated measurement instruments are increasingly relevant in a global era of data for decision-making and learning health systems. The COVID-19 pandemic highlighted policymakers’ and program managers’ need for surveillance of vaccine acceptance and accessibility issues to inform tailored and effective interventions. The VBAT is ideal for regular application to identify trends and changes in the vaccine uptake landscape, both at a national and regional level. The Australian Commonwealth has commissioned a pilot of this approach commencing in 2024, with an accompanying qualitative study to explore barriers in an identified priority population, e.g., certain cultural groups, and the VBAT-SF was applied in the 2023 New South Wales population health survey. Additional validation with a larger sample of Australian parents is also underway to inform the development of a scoring system for the VBAT. In addition to applying the current VBAT, several projects are underway to expand and adapt it for other populations, where the existing tool may not adequately capture the most relevant data. Validation in New Zealand is ongoing, with a specific focus on the Māori and Pacific Islander populations. In Australia, we are working with Aboriginal and Torres Strait Islander researchers and communities to create a culturally tailored version appropriate for this population, which may include alternative modes of administration (e.g. through individual or group discussions). We also aim to test the validity of the existing VBAT for childhood influenza vaccines and HPV vaccines, revising as necessary to capture unique barriers such as concerns about fertility or challenges related to the school-based consent process.
Strengths and limitations
This study applied robust methods to develop and validate the VBAT, underpinned by expert opinion and consultation. However, there were some limitations. Many phases of the study were conducted during the COVID-19 pandemic. This may have had an impact on recruitment and parents’ willingness to consent to providing their child’s AIR data. It may also have affected some people’s vaccine perceptions or their vaccine uptake, though the vaccine uptake data were collected in 2022 when public health restrictions were no longer in place. We used an online panel to recruit parents, which allowed us to stipulate quotas for key sociodemographic characteristics. However, we were unable to recruit a large sample of parents of children with vaccines due in the next 6 months for Phase 3, due to the narrowness of this requirement. Predictive performance of individual items and models could change with larger or more diverse samples, and this is being explored with a larger study in 2024. We did not repeat the Phase 2 assessment with the whole sample from Phase 2a, but the characteristics between the two samples remained similar. In Phase 3, we defined people who did not receive a vaccine in the study period as ‘non-vaccinators,’ but it is possible that they may have vaccinated later and may therefore be ‘delayers’ instead. There could be relevant differences in how non-vaccinators and delayers answer the VBAT items, which this study could not capture or consider. We addressed the unique challenges of validating a tool that combines both psychological constructs and practical issues by utilizing novel techniques drawn from other fields of research, but we were unable to apply classical test theory, so our validation process had to be slightly adapted so that it was also applicable to formative constructs. Finally, we have only validated the survey in Australia and in English. Additional validation should be undertaken with any translations or application in other countries.
Conclusion
The VBAT is the first instrument to measure both access and acceptance barriers to childhood vaccination that has been developed and validated in a high-income country setting against vaccine behavior using immunization registry data. It will be a valuable tool for serial surveillance at a national and regional level to inform vaccine policy and programs and as a measure for evaluating interventions. Future adaptations for other populations, such as Indigenous parents, and additional validation in Australia and New Zealand are underway.
Author contributions
JK, CB, DD, DC, LT and MD were involved in study conception and design. All authors were involved in analysis and interpretation of data, drafting and revising the paper, and final approval of the submitted manuscript. All authors agree to be accountable for all aspects of the work.
Supplemental documents.docx
Download MS Word (25.3 KB)Acknowledgments
We would like to thank Dr Susan Thomas, Dr Katarzyna Bolsewicz and Dr Esther Willing for assisting with cognitive interview data collection and analysis. We also acknowledge the Social Research Centre team for their assistance with recruitment, administration of the surveys and initial descriptive analysis of survey data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, JK, upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2359623
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- United Nations Children’s Fund. The state of the world’s children 2023: for every child, vaccination. Florence: UNICEF Innocenti – Global Office of Research and Foresight; 2023.
- Harker R. Childhood immunisation statistics. commons library research briefing, 1 march 2024. House of Commons Library; 2024.
- Wong C. Measles surge causes alarm: what scientists think. Nature News Explainer. 2024 Jan 31.
- Te Whatu Ora Health New Zealand. Immunisation coverage 2023. https://www.tewhatuora.govt.nz/for-the-health-sector/vaccine-information/immunisation-coverage/.
- Australian Government Department of Health and Aged Care. Immunisation coverage data, surveys and reports 2022. https://www.health.gov.au/topics/immunisation/immunisation-data/childhood-immunisation-coverage/immunisation-coverage-data-surveys-and-reports.
- National Centre for Immunisation Research and Surveillance. Annual immunisation coverage report 2021. New South (UK); 2022.
- Kaufman J, Tuckerman J, Bonner C, Durrheim DN, Costa D, Trevena L, Thomas S, Danchin M. Parent-level barriers to uptake of childhood vaccination: a global overview of systematic reviews. BMJ Glob Health. 2021;6(9):e006860. doi:10.1136/bmjgh-2021-006860.
- Kaufman J, Hoq M, Rhodes A, Measey M, Danchin M. Post-pandemic increase in parent misperceptions about routine childhood vaccines in Australia. Med J Aust. 2024. in press. https://doi.org/10.5694/mja2.52304.
- Wolstenholme A, Smith C. Community attitude research on childhood immunisation 2022. Crows Newst (NSW): Snapcracker; 2022.
- Jarrett C, Wilson R, O’Leary M, Eckersberger E, Larson HJ, Hesitancy SWGo V. Strategies for addressing vaccine hesitancy - a systematic review. Vaccine. 2015;33(34):4180–12. doi:10.1016/j.vaccine.2015.04.040.
- Brown KF, Shanley R, Cowley NA, van Wijgerden J, Toff P, Falconer M, Ramsay M, Hudson MJ, Green J, Vincent CA. et al. Attitudinal and demographic predictors of measles, mumps and rubella (MMR) vaccine acceptance: development and validation of an evidence-based measurement instrument. Vaccine. 2011;29(8):1700–9. doi:10.1016/j.vaccine.2010.12.030.
- Betsch C, Schmid P, Heinemeier D, Korn L, Holtmann C, Bohm R. Beyond confidence: Development of a measure assessing the 5C psychological antecedents of vaccination. PLOS ONE. 2018;13(12):e0208601. doi:10.1371/journal.pone.0208601.
- Opel DJ, Mangione-Smith R, Taylor JA, Korfiatis C, Wiese C, Catz S, Martin DP. Development of a survey to identify vaccine-hesitant parents: the parent attitudes about childhood vaccines survey. Hum Vaccin. 2011;7(4):419–25. doi:10.4161/hv.7.4.14120.
- Sarathchandra D, Navin MC, Largent MA, McCright AM. A survey instrument for measuring vaccine acceptance. Prev Med. 2018;109:1–7. doi:10.1016/j.ypmed.2018.01.006.
- Shapiro GK, Tatar O, Dube E, Amsel R, Knauper B, Naz A, Perez S, Rosberger Z. The vaccine hesitancy scale: Psychometric properties and validation. Vaccine. 2018;36(5):660–7. doi:10.1016/j.vaccine.2017.12.043.
- Frew PM, Murden R, Mehta CC, Chamberlain AT, Hinman AR, Nowak G, Mendel J, Aikin A, Randall LA, Hargreaves AL. et al. Development of a US trust measure to assess and monitor parental confidence in the vaccine system. Vaccine. 2019;37(2):325–32. doi:10.1016/j.vaccine.2018.09.043.
- Gilkey MB, Magnus BE, Reiter PL, Al M, Dempsey AF, Brewer NT. The Vaccination Confidence Scale: a brief measure of parents’ vaccination beliefs. Vaccine. 2014;32(47):6259–65. doi:10.1016/j.vaccine.2014.09.007.
- Gilkey MB, Al M, Magnus BE, Reiter PL, Dempsey AF, Brewer NT. Vaccination confidence and parental refusal/delay of early childhood vaccines. PLOS ONE. 2016;11(7):e0159087. doi:10.1371/journal.pone.0159087.
- Shapiro GK, Kaufman J, Brewer NT, Wiley K, Menning L, Leask J, Abad N, Betsch C, Bura V, Correa G. et al. A critical review of measures of childhood vaccine confidence. Curr Opin Immunol. 2021;71:34–45. doi:10.1016/j.coi.2021.04.002.
- World Health Organization. Understanding the behavioural and social drivers of vaccine uptake WHO position paper - May 2022. Wkly Epidemiol Rec. 2022;97(20):209–24.
- World Health Organization. Behavioural and social drivers of vaccination: tools and practical guidance for achieving high uptake. 2022.
- Bonner C, Tuckerman J, Kaufman J, Costa D, Durrheim DN, Trevena L, Thomas S, Danchin M. Comparing inductive and deductive analysis techniques to understand health service implementation problems: a case study of childhood vaccination barriers. Implement Sci Commun. 2021;2(1):100. doi:10.1186/s43058-021-00202-0.
- Collins D. Cognitive interviewing: origin, purpose and limitations. In: Collins D. editor. Cognitive interviewing practice. London: SAGE Publications Ltd; 2015. p. 3–27.
- Leask J, Kinnersley P, Jackson C, Cheater F, Bedford H, Rowles G. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12(1):154. doi:10.1186/1471-2431-12-154.
- Costa-Pinto JC, Willaby HW, Leask J, Hoq M, Schuster T, Ghazarian A, O’Keefe J, Danchin MH. Parental immunisation needs and attitudes survey in paediatric hospital clinics and community maternal and child health centres in Melbourne, Australia. J Paediatr Child Health. 2018;54(5):522–9. doi:10.1111/jpc.13790.
- Hair JF, Babin BJ, Anderson R, Black WC. Multivariate data analysis. 8th ed. (UK): Cengage; 2019.
- Australian Bureau of Statistics. Socio-economic indexes for areas. 2022. https://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa#:~:text=Socio%2DEconomic%20Indexes%20for%20Areas%20(SEIFA)%20is%20a%20product,from%20the%20five%2Dyearly%20Census.
- Australian Government Department of Health and Aged Care. National immunisation program schedule 2022. https://www.health.gov.au/topics/immunisation/when-to-get-vaccinated/national-immunisation-program-schedule.
- Opel DJ, Taylor JA, Zhou C, Catz S, Myaing M, Mangione-Smith R. The relationship between parent attitudes about childhood vaccines survey scores and future child immunization status: a validation study. JAMA Pediatr. 2013;167(11):1065–71. doi:10.1001/jamapediatrics.2013.2483.
- Delbecq AL, Van de Ven AH. A group process model for problem identification and program planning. J Appl Behav Sci. 1971;7(4):466–91. doi:10.1177/002188637100700404.
- Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika. 1991;78(3):691–2. doi:10.1093/biomet/78.3.691.
- Hensher DA, Rose JM, Greene WH. Applied choice analysis: a primer. (UK): University Press, Cambridge; 2005.
- Peterson RA, Albaum G, Beltramini RF. A meta-analysis of effect sizes in consumer behavior experiments. J Consum Res. 1985;12(1):97–103. doi:10.1086/209039.
- Schamberger T, Schuberth F, Henseler J. Confirmatory composite analysis in human development research. Int J Behav Dev. 2023;47(1):89–100. doi:10.1177/01650254221117506.
- Schuberth F, Henseler J, Dijkstra TK. Confirmatory composite analysis. Front Psychol. 2018;9:2541. doi:10.3389/fpsyg.2018.02541.
- Henseler J, Schuberth F. Using confirmatory composite analysis to assess emergent variables in business research. J Bus Res. 2020;120:147–56. doi:10.1016/j.jbusres.2020.07.026.
- Hu LT, Bentler PM. Fit indices in covariance structure modeling: sensitivity to underparameterized model misspecification. Psychol Methods. 1998;3(4):424–53. doi:10.1037/1082-989X.3.4.424.
- IBM Corp. IBM SPSS statistics for windows. 28.0 ed. Armonk (NY): IBM Corp.; 2021.
- Muthén LK, Muthén BO. Mplus User’s Guide. Seventh ed. Los Angeles (CA): Muthén & Muthén; 1998-2012.
- Abd Halim H, Abdul-Razak S, Md Yasin M, Isa MR. Validation study of the parent attitudes about childhood vaccines (PACV) questionnaire: the Malay version. Hum Vaccines Immunother. 2020;16(5):1040–9. doi:10.1080/21645515.2019.1674112.
- Ghazy RM, Elkhadry SW, Abdel-Rahman S, Taha SHN, Youssef N, Elshabrawy A, Ibrahim SA, Al Awaidy S, Al-Ahdal T, Padhi BK. et al. External validation of the parental attitude about childhood vaccination scale. Front Public Health. 2023;11:1146792. doi:10.3389/fpubh.2023.1146792.
- ElSayed DA, Bou Raad E, Bekhit SA, Sallam M, Ibrahim NM, Soliman S, Abdullah R, Farag S, Ghazy RM. Validation and cultural adaptation of the parent attitudes about childhood vaccines (PACV) questionnaire in Arabic language widely spoken in a region with a high prevalence of COVID-19 vaccine hesitancy. Trop Med Infect Dis. 2022;7(9):234. doi:10.3390/tropicalmed7090234.
- Olarewaju VO, Jafflin K, Deml MJ, Zimmermann C, Sonderegger J, Preda T, Staub H, Kwiatkowski M, Kloetzer A, Huber BM. et al. Application of the parent attitudes about childhood vaccines (PACV) survey in three national languages in Switzerland: Exploratory factor analysis and Mokken scale analysis. Hum Vaccin Immunother. 2021;17(8):2652–60. doi:10.1080/21645515.2021.1894894.
- Mutlu M, Cayir Y, Kasali K. Validity and reliability of the Turkish version of the parent attitudes about childhood vaccines (PACV) scale. J Healthc Qual Res. 2023;38(1):11–19. doi:10.1016/j.jhqr.2022.06.002.
- Kaufman J, Ryan R, Glenton C, Lewin S, Bosch-Capblanch X, Cartier Y, Cliff J, Oyo-Ita A, Ames H, Muloliwa AM. et al. Childhood vaccination communication outcomes unpacked and organized in a taxonomy to facilitate core outcome establishment. J Clin Epidemiol. 2017;84:173–84. doi:10.1016/j.jclinepi.2017.02.007.