ABSTRACT
Purpose
To overview the recent literature regarding the relationship between COVID-19 vaccines and glycemic control.
Methods
Data were extracted from text and tables of all available articles published up to September 2023 in PubMed Database describing glucose homeostasis data in subjects exposed to COVID-19 vaccines, focusing on patients with diabetes mellitus (DM).
Results
It is debated if the immune system impairment observed in diabetic patients makes them susceptible to lower efficacy of vaccines, but evidence suggests a possible improvement in immune response in those with good glycemic control. Despite their proven protective role lowering infection rates and disease severity, COVID-19 vaccines can result in diabetic ketoacidosis, new-onset diabetes, or episodes of hyper- or hypoglycemia.
Conclusions
Evidence with COVID-19 vaccines highlights the strong relationship existing between DM and immune system function. Clinicians should strive to achieve optimal glucose control before vaccination and promptly manage possible glucose homeostasis derangement following vaccine exposure.
Introduction
Vaccines are the most efficient and safe public health interventions, being able to decrease the incidence of certain infections and disease-related morbidity and mortality, with an excellent cost-benefit ratio.Citation1 Moreover, they offer “social shield” for vulnerable individuals who can’t receive vaccinations due to immunodeficiency.Citation2 The SARS-CoV-2 related disease (COVID-19) has been the largest epidemic event in the past decades and an extraordinary challenge for modern healthcare system. Although pneumonia and acute respiratory distress syndrome are the major concerns of COVID-19, a huge number of complication has been associated with this viral infection, possibly affecting multiple organs and systems, and leading to long-lasting consequences.Citation3 The endocrine systems is majorly targeted during the acute phase of COVID-19, hence many possible derangements may occur, including new-onset symptoms or worsening of previous endocrine disorders ().Citation4 From a pathophysiological perspective, a direct damage from the virus could be responsible. Indeed, the hypothalamus, the pituitary and many peripheral endocrine glands (e.g. thyroid, gonads, pancreatic islets) express the angiotensin-converting-enzyme-2 (ACE2) receptor, which is deemed to aid viral entrance through the cell surface.Citation4–6 To date, following COVID-19, there has been a reported increase of 30–50% in the incidence of type 2 DM (T2DM) compared with individuals without infection.Citation7,Citation8 A complex mutual relationship indeed exists between immune system activity and glucose homeostasisCitation9,Citation10 and it is established that DM subjects have worse infective disease outcomes,Citation11,Citation12 with higher hospitalization risk and up to 7-fold higher mortality rates compared with healthy subjects.Citation13 The COVID-19 health emergency raised the need of straightforward policies to protect DM individualsCitation14,Citation15 and the American Diabetes Association’s (ADA’s) recommendations suggest to prioritize and offer COVID-19 vaccines to people with diabetes.Citation16 Interestingly in this concern, beyond the possible effects directly related to the viral infection, some authors also reported endocrine-related clinical manifestations following COVID-19 vaccine administration, involving the thyroid, pituitary gland, adrenals, and also pancreatic islets and glucose homeostasis, suggesting a possible direct mechanism of disruption played by vaccine administration.Citation17 In this narrative mini-review, we aim to provide an up-to date overview of the literature concerning the interplay between COVID-19 vaccines and glycemic control in individuals with DM. For this purpose, we reviewed all available perspective, retrospective, and review articles published up to September 2023 in PubMed Database reporting data on either the impact of DM and glycemic control on the response to COVID-19 vaccination or the effects of COVID-19 vaccines on altered glucose homeostasis. The search terms used included “diabetes,” “COVID-19 vaccination,” “blood glucose,” “immune response.” Data were extracted from the main text and from the tables of the manuscripts retrieved. Only articles written in English were considered.
Table 1. Endocrine system-related clinical disorders observed after COVID-19 infection.
Impact of DM and glycemic control on the immune response to COVID-19 vaccines
Clinical evidence
There is an ongoing debate concerning the potential impact of poor glycemic control on the antibody response to COVID-19 vaccines and their effectiveness in diabetic individuals. Adequate glycemic and metabolic control is indeed considered vital for achieving an optimal immune response post-vaccination. Findings from a meta-analysis of 43 studies suggest that glucose control plays a pivotal role in determining the antibody response to all vaccines recommended to adults with DM, particularly among elderly.Citation18 To date, few studies have explored this association within the context of COVID-19 vaccination in the diabetic population, yielding conflicting results. In a longitudinal cohort of healthcare professionals, Lustig et al. described a lower antibody response to COVID‐19 vaccination in individuals with DM compared with healthy controls; a similar effect was noted for older age, male gender and hypertension.Citation19 In another cohort of 374 Japanese health workers, Mitsunaga and colleagues found that older age, hypertension and serum glycosylated hemoglobin (HbA1c) ≥ 6.5% at the time of vaccination were independent predictors of a lower immune response, as evidenced by lower anti-SARS-CoV-2 spike-specific antibody titer observed 7–20 days post-vaccination.Citation20 In line with these observations, in a study of 555 older residents of long-term care facilities participating in the GeroCovid Vax study, Virgilio et al. found that residents with T2DM had a weaker antibody response to the first dose of COVID-19 vaccine compared with those without diabetes. However, no differences were observed between residents with T2DM using insulin and non-diabetics, suggesting that insulin therapy might buffer this effect and restore the humoral responseCitation21.In a cohort of 262 adult individuals (81 with T2DM and 181 non-diabetic subjects), Ali and coauthors found that both T2DM patients and non-diabetic subjects elicited a strong immune response after the second dose of COVID-19 mRNA based-vaccine. Nonetheless, individuals with T2DM showed lower levels of anti-SARS-CoV-2 IgG and neutralizing antibodies as compared with non-diabetics, while no significant differences were observed with regard to body mass index (BMI), age, gender, and hypertension status.Citation22 Consistently with these findings, in the Italian experience documented in the CAVEAT study, significantly reduced virus-neutralizing antibody capacity was observed in T2DM patients with serum HbA1c above 7% when compared to healthy normo-glycemic subjects and T2DM group with optimal glycemic control.Citation23 Interestingly, at 2 months follow-up, there was no improvement of immune response among those who continued to have poor glycemic control. However, those who achieved glycemic control after the initial assessment demonstrated a significant increase in immune response, suggesting that optimizing glucose control may enhance vaccine-induced response. Conversely, a prospective observational study by Papadokostaki and colleagues including 174 participants (14 with type 1 diabetes mellitus (T1DM), 44 with T2DM, and 116 without DM) showed that almost 17% of participants with DM did not develop adequate humoral immune response to the BNT162b2 mRNA-based vaccine after the first dose; however, the humoral immune response was high and similar between individuals with and without DM, especially after the second dose of the vaccine. Moreover, the authors found no significant correlation between anti-SARS-CoV-2 receptor-binding domain IgG titer and either age, DM duration or HbA1c levels.Citation24 In a prospective multicenter study conducted in Europe, no differences were observed in humoral responses to COVID-19 vaccination among 150 adults with DM (including T1DM and T2DM) and age-matched healthy subjects, regardless of whether they had good or poor glycemic control. Interestingly, the authors found that only age and poor renal function were associated with reduced immune responses following vaccination.Citation25 Also, in a cohort-study involving 81 adolescents with T1DM and moderate to poor glycemic control (median HbA1c: 8.6%), no differences were observed in humoral immune responses induced by vaccine administration when compared with 71 healthy control subjects. Furthermore, comparable occurrences of SARS-CoV-2 breakthrough infections were noted in both cohorts over a 6-month monitoring phase,Citation26 emphasizing the practical significance of these findings.
Pathogenetic hypotheses
As previously mentioned, impaired glycemic control is considered a crucial determinant of compromised immune system activity and function, partly explaining the higher risk of infection observed in the diabetic population.Citation27 In this context, a recent article investigated several factors that may account for this increased risk in people with DM.Citation28 According to the literature, there are three primary mechanisms through which diabetes can disrupt the functioning of the immune system:
–Alteration of the adaptive immune system, which includes a reduction in T-regulatory cells, γδ T cells with limited secretion of IFN-2, alterations in the polarization of TH1-TH2 cells, and a decreased ability of B-cells to produce the anti-inflammatory cytokine IL-10;Citation28–32
–Disruption of the innate immune system, characterized by endothelial dysfunction, reduced recruitment of neutrophils (resulting in decreased chemotaxis and phagocytosis capabilities), decreased NK lymphocytes, and increased production of pro-inflammatory cytokines;Citation33–37
–Impact on glucose metabolism, leading to oxidative stress that interferes with both cellular and humoral immunity, further exacerbating cytokine releaseCitation38,Citation39 Oxidative damage and inflammatory stress resulting from “cytokine storms” throughout one’s life can harm immune cells, contributing to the concept of “immunosenescence,” which explains the elevated risk of infection (and reduced innate response to vaccine antigens) frequently observed in older individuals.Citation40,Citation41 One could speculate that similarly to the age-related vulnerability to infectious disease reported in DM patients, a reduced response to vaccine antigens could be observed in this population, especially in the elderly.
Impact of COVID-19 vaccines on glucose homeostasis
Clinical evidence
Despite previous evidence reassuring about vaccines’ neutral effect on glycemic control,Citation42,Citation43 a rising number of recent reports highlights potential disruptions linked to COVID-19 vaccines. The most commonly reported adverse events after COVID-19 vaccination are local reactions (e.g. pain and the tenderness at the injection site), followed by mild to moderate systemic side effects such as headache, fatigue, and muscle pain.Citation44 Nevertheless, severe adverse events in both adults and children have also been described, including anaphylaxis,Citation45 myocarditis/pericarditisCitation46 and thrombosis.Citation47 Interestingly, some potentially severe vaccine-induced effects on glucose homeostasis have been reported in either subjects with DM, or in subjects without history of DM, suggesting a possible direct mechanism disrupting glucose homeostasis. A case-series by Edwards and colleaguesCitation48 described 3 cases of acute and severe hyperglycemia arising shortly after the first administration of adenovirus-vectored COVID-19 (ChAdOx1) vaccine, occurred in obese subjects and in adult men with prediabetes. Cases of glycemic derangement have also been described following administration of COVID-19 mRNA vaccines. These include a case of severe diabetic ketoacidosis (DKA) in a young girl with T1DM,Citation49 and 3 cases (1 with new onset T2DM) of hyperglycemic hyperosmotic state (HHS) appeared within 2–10 days of vaccine exposure.Citation50 Moreover, Sasaki et al.Citation51 reported the new onset of T1DM 8 weeks after the 2nd administration of mRNA vaccine, which was associated with a marked anti-COVID-19 immunization. Similarly, Aydoğan and colleaguesCitation52 reported 4 cases of adult-onset T1DM occurring 2–8 weeks after mRNA vaccine in previously euglycemic subjects. DKA after the first administration of mRNA vaccine was also reported in a previously healthy 50 years old Chinese man, who was lately found to harbor genetic predisposition to fulminant diabetes (HLA: DQB1 × 02:03/03:03 and DRB1 × 09:01/09:01).Citation53 In a retrospective experience from two Italian centers, the authors found no significant difference of time in range (TIR), total daily insulin and bolus insulin requirement in 39 adolescents and young adults with T1DM treated with either advanced hybrid closed-loop (AHCL) insulin delivery system or multiple daily insulin injections (MDI) using a flash glucose monitoring (FGM) system.Citation54 Similar results were observed in a study carried out in Greece on 70 young adolescents with T1DM,Citation55 in a small Italian series of 35 subjects with T1DM using a continuous glucose monitoring (CGM) device,Citation56 in another Italian cohort-study involving 150 T1DM adults,Citation57 and in a Chinese cohort of subjects with T2DM.Citation58 Similarly, a retrospective study of 74 individuals with T1DM and T2DM using CGM enrolled in the multicenter COVAC-DM Study also showed no significant difference in TIR around the COVID-19 vaccination. However, a deterioration of glucose levels, represented by a decrease of TIR and an increase of time above range (TAR), was observed in T1DM patients on days on which typical vaccine-related side effects were present.Citation59 Conversely, in a retrospective analysis of 97 adult patients with T1DM using a FGM system, 58% of subjects experienced major perturbations of glucose control, with 30% and 10% of individuals showing a decrease of TIR of over 10% and 20%, respectively, in the 7 days after the first COVID-19 vaccination. Interestingly, this effect was more pronounced when HbA1c was lower and in patients taking oral hypoglycemic agents (metformin/dapagliflozin) in addition to basal – bolus insulin regimen. Moreover, there was no difference between mRNA-based and viral vector-based vaccines in relation to their metabolic effect.Citation60 Similar results were demonstrated in a smaller cohort of 20 adults with T1DM, in which the greatest fall in the % of glucose levels on target was reported in older patients, those taking metformin/dapagliflozin + basal – bolus insulin, and those with higher BMICitation61 In both these studies, there was no significant change in glucose variability in the 7 days post- COVID-19 vaccination compared with the previous week.Citation60,Citation61 On the other hand, in the largest T1DM adult cohort so far described, the “PRO-VACS” study, conducted on 454 subjects, the authors found a higher glycemic variability without significant difference of glycemic control parameters after mRNA-1273 COVID-19 vaccine administrations.Citation62 An analysis of the European reporting frequency of impaired glucose metabolism events among COVID-19 vaccines in 2021 also confirmed the finding of a larger glycemic variability, especially in subjects who received mRNA-based vaccines.Citation63 In particular, mRNA vaccines were associated with a higher reporting frequency of both T1DM and T2DM, hyperglycemia, hypoglycemia, and inadequate control of DM compared to viral vector-based vaccines.Citation63 Interestingly, while no similar result had been previously described, in a recent retrospective Chinese T2DM cohort, Lin et al. observed that the use of SGLT2i was independently associated with a reduced risk of glycemic control worsening after vaccination.Citation64
Pathogenetic hypotheses
Although a possible association between COVID-19 vaccination and the onset of hyperglycemia and related complications has been widely reported in both young and adult populations,Citation7,Citation65 their frequency and underlying mechanisms remain largely speculative. In this context, three main pathogenic hypotheses have been proposed:
- Direct vaccine-mediated pancreatic damage. It is well established that SARS-CoV-2 virus can infect and replicate inside human pancreas.Citation66 Despite the heterogeneity of its expression on β-cell surface, the presence of the ACE-2 receptor is believed to facilitate viral entry.Citation67 Consistently, viral RNA has been found in β-cells of patients with COVID-19, possibly leading to β-cell dysregulation and consequent hyperglycemia.Citation68 Intriguingly, cases of pancreatic injury, acute pancreatitis, and even recurrent pancreatitis have been documented post-COVID-19 vaccinationCitation69,Citation70 even in individuals without concurrent SARS-Cov-2 infection, suggesting a potential direct vaccine-related mechanism of harm.
- Exaggerated vaccine-induced inflammatory response causing pancreatic injury. Beyond the hypothesis of a direct insult to the islets, the most reliable hypothesis to explain SARS-CoV-2 related pancreatic damage seems to be strictly related to the exaggerated immune response induced by the viral infection, often resulting in massive release of pro-inflammatory factors (IL-1, IL-6, TNF) known as “cytokine storm.” Some recent evidence suggests that this hyperinflammation process might be partially mediated by mast cell activation.Citation71 Eventually, a strong glycol-metabolic disruption seems to be associated with this paradoxical immune system activation, resulting in decreased pancreatic blood flow, increased oxidative stress, reduction of insulin production rate, and a peripheral reduction in insulin-sensitivity.Citation72,Citation73 Genetic susceptibility to abnormal cytokine release after vaccination has been described.Citation74 Although a similar release of cytokines has never been documented in vivo after vaccination, some authors speculate that, due to molecular mimicry, vaccine excipients, viral vectors (eg. Covishield vaccine) or the SARS-CoV-2 spike protein derived from mRNA vaccines could trigger an abnormal immune response, favoring pancreatic inflammation, β-cell dysfunction, and ultimately leading to acute hyperglycemia ().Citation75
Figure 1. Potential mechanism of post-COVID-19 vaccination hyperglycemia and related acute complications. Created with Biorender.com.
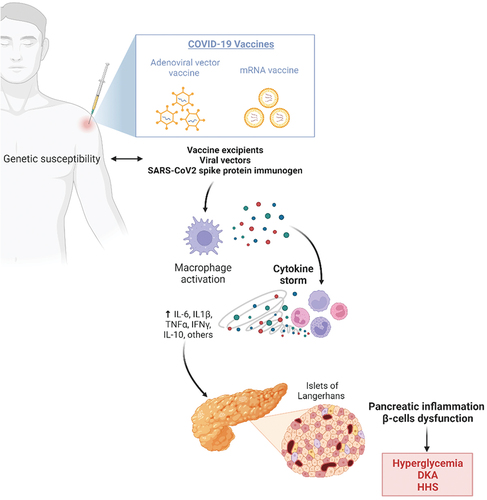
– Autoimmune damage to the islet triggered by immunization. β-cell dysfunction could be triggered by auto-immune damage following development of islet autoimmunity, which has been found to transiently increase in individuals with high genetic risk of T1DM after COVID-19.Citation76,Citation77 Growing evidence suggests that certain COVID-19 vaccines may also trigger the onset or exacerbations of autoimmune diseases by molecular mimicry, including T1DM.Citation78,Citation79
Conclusions
The experience with COVID-19 vaccines highlights the intricate mutual relationship between DM and immune system function, bearing practical implications for the clinical management of diabetic patients. Vaccination can trigger the immune system and favor inflammation, mast cell activation, and exaggerated cytokine release, potentially disrupting β-cell function, increasing oxidative stress, and altering insulin sensitivity. These factors may impact short-term blood sugar control, potentially resulting in DKA, new-onset diabetes, or episodes of hyper- or hypoglycemia. Therefore, prompt management of adverse glycemic events associated with vaccination is essential. Nevertheless, given the higher risk of viral infections in the diabetic population, vaccination remains the safest, most effective, and cost-effective public health measure, therefore strongly recommended for diabetic individuals. Furthermore, optimal glycemic control appears to enhance vaccine-induced immune responses, emphasizing the importance of striving for it to maximize the protective effects of vaccination.
Author contribution statement
All authors contributed to the manuscript conception and design.
ACB, WV and NB conceptualized the article and performed literature research.
ACB, WV and NB drafted the article.
AGL, GM, MM, SP and AN critically revised the work.
All authors have read and approve the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kim JJ. The role of cost-effectiveness in US vaccination policy. N Engl J Med. 2011;365(19):1760–7. doi:10.1056/NEJMp1110539.
- Meng H, Mao J, Ye Q. Booster vaccination strategy: necessity, immunization objectives, immunization strategy, and safety. J Med Virol. 2022;94(6):2369–75. doi:10.1002/jmv.27590.
- Desai AD, Lavelle M, Boursiquot BC, Wan EY. Long-term complications of COVID-19. Am J Physiol Cell Physiol. 2022;322(1):C1–c11. doi:10.1152/ajpcell.00375.2021.
- Mirza SA, Sheikh AAE, Barbera M, Ijaz Z, Javaid MA, Shekhar R, Pal S, Sheikh AB. COVID-19 and the endocrine system: a review of the current information and misinformation. Infect Dis Rep. 2022;14(2):184–97. doi:10.3390/idr14020023.
- Lisco G, De Tullio A, Stragapede A, Solimando AG, Albanese F, Capobianco M, Giagulli VA, Guastamacchia E, De Pergola G, Vacca A. COVID-19 and the endocrine system: a comprehensive review on the theme. J Clin Med. 2021;10(13):2920. doi:10.3390/jcm10132920.
- Lazartigues E, Qadir MMF, Mauvais-Jarvis F. Endocrine significance of SARS-CoV-2’s reliance on ACE2. Endocrinology. 2020;161(9). doi:10.1210/endocr/bqaa108.
- Rathmann W, Kuss O, Kostev K. Incidence of newly diagnosed diabetes after COVID-19. Diabetologia. 2022;65(6):949–54. doi:10.1007/s00125-022-05670-0.
- Choi JH, Kim KM, Song K, Seo GH. Risk for newly diagnosed type 2 diabetes mellitus after COVID-19 among Korean adults: a nationwide matched cohort study. Endocrinol Metab (Seoul). 2023;38(2):245–52. doi:10.3803/EnM.2023.1662.
- Daryabor G, Atashzar MR, Kabelitz D, Meri S, Kalantar K. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front Immunol. 2020;11:1582. doi:10.3389/fimmu.2020.01582.
- Joshi SR, Shaw AC, Quagliarello VJ. Pandemic influenza H1N1 2009, innate immunity, and the impact of immunosenescence on influenza vaccine. Yale J Biol Med. 2009;82:143–51.
- Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341(25):1906–12. doi:10.1056/NEJM199912163412507.
- Muller LM, Gorter KJ, Hak E, Goudzwaard WL, Schellevis FG, Hoepelman AI, Rutten GEHM. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41(3):281–8. doi:10.1086/431587.
- Fang M, Ishigami J, Echouffo-Tcheugui JB, Lutsey PL, Pankow JS, Selvin E. Diabetes and the risk of hospitalisation for infection: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia. 2021;64(11):2458–65. doi:10.1007/s00125-021-05522-3.
- Mirani M, Favacchio G, Carrone F, Betella N, Biamonte E, Morenghi E, Mazziotti G, Lania AG. Impact of comorbidities and glycemia at admission and dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes with COVID-19: a case series from an academic hospital in Lombardy, Italy. Diabetes Care. 2020;43(12):3042–9. doi:10.2337/dc20-1340.
- Corona G, Pizzocaro A, Vena W, Rastrelli G, Semeraro F, Isidori AM, Pivonello R, Salonia A, Sforza A, Maggi M. Diabetes is most important cause for mortality in COVID-19 hospitalized patients: systematic review and meta-analysis. Rev Endocr Metab Disord. 2021;22(2):275–96. doi:10.1007/s11154-021-09630-8.
- Standards of care in diabetes-2023 abridged for primary care providers. Clin Diabetes. 2022;41(1):4–31. doi:10.2337/cd23-as01.
- Zhao Y, Wu X. Influence of COVID-19 vaccines on endocrine system. Endocrine. 2022;78(2):241–6. doi:10.1007/s12020-022-03119-3.
- Almasri L, Holtzclaw BJ. Assessing vaccine protection for older adults with diabetes: a systematic review. West J Nurs Res. 2022;44(6):582–97. doi:10.1177/01939459211005710.
- Lustig Y, Sapir E, Regev-Yochay G, Cohen C, Fluss R, Olmer L, Indenbaum V, Mandelboim M, Doolman R, Amit S. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9(9):999–1009. doi:10.1016/S2213-2600(21)00220-4.
- Mitsunaga T, Ohtaki Y, Seki Y, Yoshioka M, Mori H, Suzuka M, Mashiko S, Takeda S, Mashiko K. The evaluation of factors affecting antibody response after administration of the BNT162b2 vaccine: a prospective study in Japan. PeerJ. 2021;9:e12316. doi:10.7717/peerj.12316.
- Virgilio E, Trevisan C, Abbatecola A, Malara A, Palmieri A, Fedele G, Stefanelli P, Leone P, Schiavoni I, Maggi S. Diabetes affects antibody response to SARS-CoV-2 vaccination in older residents of long-term care facilities: data from the GeroCovid vax study. Diabetes Care. 2022;45(12):2935–42. doi:10.2337/dc22-1255.
- Ali H, Alterki A, Sindhu S, Alahmad B, Hammad M, Al-Sabah S, Alghounaim M, Jamal MH, Aldei A, Mairza MJ. Robust antibody levels in both diabetic and non-diabetic individuals after BNT162b2 mRNA COVID-19 vaccination. Front Immunol. 2021;12:752233. doi:10.3389/fimmu.2021.752233.
- Marfella R, D’Onofrio N, Sardu C, Scisciola L, Maggi P, Coppola N, Romano C, Messina V, Turriziani F, Siniscalchi M. Does poor glycaemic control affect the immunogenicity of the COVID-19 vaccination in patients with type 2 diabetes: the CAVEAT study. Diabetes Obes Metab. 2022;24(1):160–5. doi:10.1111/dom.14547.
- Papadokostaki E, Tentolouris A, Anastasiou IA, Psichogiou M, Iliaki E, Eleftheriadou I, Hatzakis A, Tentolouris N. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in people with diabetes: a prospective observational study. Vaccines. 2022;10(3):382. doi:10.3390/vaccines10030382.
- Sourij C, Tripolt NJ, Aziz F, Aberer F, Forstner P, Obermayer AM, Kojzar H, Kleinhappl B, Pferschy PN, Mader JK. Humoral immune response to COVID-19 vaccination in diabetes is age-dependent but independent of type of diabetes and glycaemic control: the prospective COVAC-DM cohort study. Diabetes Obes Metab. 2022;24(5):849–58. doi:10.1111/dom.14643.
- Emeksiz HC, Hepokur MN, Şahin SE, Şirvan BN, Çiçek B, Önder A, Yıldız M, Aksakal DK, Bideci A, Ovalı HF. Immunogenicity, safety and clinical outcomes of the SARS-CoV-2 BNT162b2 vaccine in adolescents with type 1 diabetes. Front Pediatr. 2023;11:1191706. doi:10.3389/fped.2023.1191706.
- Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2016;4(2):148–58. doi:10.1016/S2213-8587(15)00379-4.
- Erener S. Diabetes, infection risk and COVID-19. Mol Metab. 2020;39:101044. doi:10.1016/j.molmet.2020.101044.
- Zeng C, Shi X, Zhang B, Liu H, Zhang L, Ding W, Zhao Y. The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: relationship with metabolic factors and complications. J Mol Med (Berl). 2012;90(2):175–86. doi:10.1007/s00109-011-0816-5.
- Xia C, Rao X, Zhong J. Role of T lymphocytes in type 2 diabetes and diabetes-associated inflammation. J Diabetes Res. 2017;2017:1–6. doi:10.1155/2017/6494795.
- DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, Markham D, Strissel KJ, Watkins AA, Zhu M. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci USA. 2013;110(13):5133–8. doi:10.1073/pnas.1215840110.
- Kim JH, Park K, Lee SB, Kang S, Park JS, Ahn CW, Nam, JS Relationship between natural killer cell activity and glucose control in patients with type 2 diabetes and prediabetes. J Diabetes Investig. 2019;10(5):1223–8. doi:10.1111/jdi.13002.
- Marhoffer W, Stein M, Maeser E, Federlin K. Impairment of polymorphonuclear leukocyte function and metabolic control of diabetes. Diabetes Care. 1992;15(2):256–60. doi:10.2337/diacare.15.2.256.
- Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997;14(1):29–34. doi:10.1002/(SICI)1096-9136(199701)14:1<29:AID-DIA300>3.0.CO;2-V.
- Huang J, Xiao Y, Zheng P, Zhou W, Wang Y, Huang G, Xu A, Zhou Z. Distinct neutrophil counts and functions in newly diagnosed type 1 diabetes, latent autoimmune diabetes in adults, and type 2 diabetes. Diabetes Metab Res Rev. 2019;35(1):e3064. doi:10.1002/dmrr.3064.
- Lecube A, Pachón G, Petriz J, Hernández C, Simó R, Sesti G. Phagocytic activity is impaired in type 2 diabetes mellitus and increases after metabolic improvement. PLOS ONE. 2011;6(8):e23366. doi:10.1371/journal.pone.0023366.
- Alexandraki KI, Piperi C, Ziakas PD, Apostolopoulos NV, Makrilakis K, Syriou V, Diamanti-Kandarakis E, Kaltsas G, Kalofoutis A. Cytokine secretion in long-standing diabetes mellitus type 1 and 2: associations with low-grade systemic inflammation. J Clin Immunol. 2008;28(4):314–21. doi:10.1007/s10875-007-9164-1.
- Morris G, Gevezova M, Sarafian V, Maes M. Redox regulation of the immune response. Cell Mol Immunol. 2022;19(10):1079–101. doi:10.1038/s41423-022-00902-0.
- Pitocco D, Tesauro M, Alessandro R, Ghirlanda G, Cardillo C. Oxidative stress in diabetes: implications for vascular and other complications. Int J Mol Sci. 2013;14(11):21525–50. doi:10.3390/ijms141121525.
- Ventura MT, Casciaro M, Gangemi S, Buquicchio R. Immunosenescence in aging: between immune cells depletion and cytokines up-regulation. Clin Mol Allergy. 2017;15(1):21. doi:10.1186/s12948-017-0077-0.
- Pera A, Campos C, López N, Hassouneh F, Alonso C, Tarazona R, Solana R. Immunosenescence: implications for response to infection and vaccination in older people. Maturitas. 2015;82(1):50–5. doi:10.1016/j.maturitas.2015.05.004.
- Dorrell L, Hassan I, Marshall S, Chakraverty P. Clinical and serological responses to an inactivated influenza vaccine in adults with HIV infection, diabetes, obstructive airways disease, elderly adults and healthy volunteers. Int J STD AIDS. 1997;8(12):776–9. doi:10.1258/0956462971919264.
- Feery BJ, Hartman LJ, Hampson AW, Proietto J. Influenza immunization in adults with diabetes mellitus. Diabetes Care. 1983;6(5):475–8. doi:10.2337/diacare.6.5.475.
- Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, Sudre CH, Nguyen LH, Drew DA, Merino J. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–49. doi:10.1016/S1473-3099(21)00224-3.
- Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US—December 14, 2020-January 18, 2021. JAMA. 2021;325(11):1101–2. doi:10.1001/jama.2021.1967.
- Goddard K, Lewis N, Fireman B, Weintraub E, Shimabukuro T, Zerbo O, Boyce TG, Oster ME, Hanson KE, Donahue JG. Risk of myocarditis and pericarditis following BNT162b2 and mRNA-1273 COVID-19 vaccination. Vaccine. 2022;40(35):5153–9. doi:10.1016/j.vaccine.2022.07.007.
- See I, Lale A, Marquez P, Streiff MB, Wheeler AP, Tepper NK, Woo EJ, Broder KR, Edwards KM, Gallego R. Case series of thrombosis with thrombocytopenia syndrome after COVID-19 Vaccination—United States, December 2020 to August 2021. Ann Intern Med. 2022;175(4):513–22. doi:10.7326/M21-4502.
- Edwards AE, Vathenen R, Henson SM, Finer S, Gunganah K. Acute hyperglycaemic crisis after vaccination against COVID-19: a case series. Diabet Med. 2021;38(11):e14631. doi:10.1111/dme.14631.
- Zilbermint M, Demidowich AP. Severe diabetic ketoacidosis after the second dose of mRNA-1273 COVID-19 vaccine. J Diabetes Sci Technol. 2022;16(1):248–9. doi:10.1177/19322968211043552.
- Lee HJ, Sajan A, Tomer Y. Hyperglycemic emergencies associated with COVID-19 vaccination: a case series and discussion. J Endocr Soc. 2021;5(11):bvab141. doi:10.1210/jendso/bvab141.
- Sasaki H, Itoh A, Watanabe Y, Nakajima Y, Saisho Y, Irie J, Meguro S, Itoh H. Newly developed type 1 diabetes after coronavirus disease 2019 vaccination: a case report. J Diabetes Invest. 2022;13(6):1105–8. doi:10.1111/jdi.13757.
- Aydoğan B, Ünlütürk U, Cesur M. Type 1 diabetes mellitus following SARS-CoV-2 mRNA vaccination. Endocrine. 2022;78(1):42–6. doi:10.1007/s12020-022-03130-8.
- Tang X, He B, Liu Z, Zhou Z, Li X. Fulminant type 1 diabetes after COVID-19 vaccination. Diabetes Metab. 2022;48(2):101324. doi:10.1016/j.diabet.2022.101324.
- Piccini B, Pessina B, Pezzoli F, Casalini E, Toni S. COVID-19 vaccination in adolescents and young adults with type 1 diabetes: glycemic control and side effects. Pediatr Diabetes. 2022;23(4):469–72. doi:10.1111/pedi.13326.
- Gouda N, Dimitriadou M, Sotiriou G, Christoforidis A. The impact of COVID-19 vaccination on glycaemic control in children and adolescents with type 1 diabetes mellitus on continuous glucose monitoring. Acta Diabetol. 2022;59(12):1609–14. doi:10.1007/s00592-022-01968-y.
- D’Onofrio L, Coraggio L, Zurru A, Carlone A, Mignogna C, Moretti C, Maddaloni, E, Buzzetti, R. Short-term safety profile of sars-Cov2 vaccination on glucose control: continuous glucose monitoring data in people with autoimmune diabetes. Diabetes Res Clin Pract. 2021;179:109022. doi:10.1016/j.diabres.2021.109022.
- D’Addio F, Sabiu G, Usuelli V, Assi E, Abdelsalam A, Maestroni A, Seelam AJ, Ben Nasr M, Loretelli C, Mileto D. Immunogenicity and Safety of SARS-CoV-2 mRNA vaccines in a cohort of patients with type 1 diabetes. Diabetes. 2022;71(8):1800–6. doi:10.2337/db22-0053.
- Lu D, Gao Y, Qi X, Li A, Zhang J. The COVID-19 vaccination hesitancy among Chinese individuals with diabetes and the impact on glycemic control of vaccination: a questionnaire study. BMC Endocr Disord. 2022;22(1):329. doi:10.1186/s12902-022-01201-5.
- Aberer F, Moser O, Aziz F, Sourij C, Ziko H, Lenz J, Abbas F, Obermayer AM, Kojzar H, Pferschy PN. Impact of COVID-19 vaccination on glycemia in individuals with type 1 and type 2 diabetes: substudy of the COVAC-DM study. Diabetes Care. 2022;45(2):24–6. doi:10.2337/dc21-1563.
- Heald AH, Stedman M, Horne L, Rea R, Whyte M, Gibson JM, Anderson SG, Ollier W. The change in glycaemic control immediately after COVID-19 vaccination in people with type 1 diabetes. Diabet Med. 2022;39(4):e14774. doi:10.1111/dme.14774.
- Heald AH, Rea R, Horne L, Metters A, Steele T, Leivesley K, Whyte MB, Stedman M, Ollier W. Analysis of continuous glucose tracking data in people with type 1 diabetes after COVID-19 vaccination reveals unexpected link between immune and metabolic response, augmented by adjunctive oral medication. Int J Clin Pract. 2021;75(12):e14714. doi:10.1111/ijcp.14714.
- Dicembrini I, Vitale V, Cosentino C, Cresci B, Pala L, Pieri M, Yannas D, Vannucci M, Zago E, Romani A. Interstitial glucose monitoring, type 1 diabetes and COVID-19 vaccine: the patient-reported outcomes and vaccine-associated changes in glucose and side effects (PRO-VACS). Acta Diabetol. 2022;59(3):435–8. doi:10.1007/s00592-021-01837-0.
- di Mauro G, Mascolo A, Longo M, Maiorino MI, Scappaticcio L, Bellastella G, Esposito K, Capuano A. European safety analysis of mRNA and viral vector COVID-19 vaccines on glucose metabolism events. Pharmaceuticals (Basel). 2022;15(6):677. doi:10.3390/ph15060677.
- Lin CW, Hung SY, Chen IW. A study of glycemic perturbations following two doses of COVID-19 vaccination for patients with diabetes: the impacts of vaccine type and anti-diabetes drugs. Diabetol Metab Syndr. 2023;15(1):81. doi:10.1186/s13098-023-01059-0.
- Karavanaki K, Rodolaki K, Soldatou A, Karanasios S, Kakleas K. Covid-19 infection in children and adolescents and its association with type 1 diabetes mellitus (T1d) presentation and management. Endocrine. 2023;80(2):237–52. doi:10.1007/s12020-022-03266-7.
- Müller JA, Groß R, Conzelmann C, Krüger J, Merle U, Steinhart J, Weil T, Koepke L, Bozzo CP, Read C. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3(2):149–65. doi:10.1038/s42255-021-00347-1.
- Fignani D, Licata G, Brusco N, Nigi L, Grieco GE, Marselli L, Overbergh L, Gysemans C, Colli ML, Marchetti P. SARS-CoV-2 receptor angiotensin I-converting enzyme type 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature. Front Endocrinol (Lausanne). 2020;11:596898. doi:10.3389/fendo.2020.596898.
- Clarke SA, Abbara A, Dhillo WS. Impact of COVID-19 on the endocrine system: a mini-review. Endocrinology. 2022;163(1). doi:10.1210/endocr/bqab203.
- Boskabadi SJ, Ala S, Heydari F, Ebrahimi M, Jamnani AN. Acute pancreatitis following COVID-19 vaccine: Aa case report and brief literature review. Heliyon. 2023;9(1):e12914. doi:10.1016/j.heliyon.2023.e12914.
- Cieślewicz A, Dudek M, Krela-Kaźmierczak I, Jabłecka A, Lesiak M, Korzeniowska K. Pancreatic injury after COVID-19 vaccine—a case report. Vaccines. 2021;9(6):576. doi:10.3390/vaccines9060576.
- Salvucci F, Codella R, Coppola A, Zacchei I, Grassi G, Anti ML, Nitisoara N, Luzi L, Gazzaruso C. Antihistamines improve cardiovascular manifestations and other symptoms of long-COVID attributed to mast cell activation. Front Cardiovasc Med. 2023;10:1202696. doi:10.3389/fcvm.2023.1202696.
- Lim S, Bae JH, Kwon HS, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17(1):11–30. doi:10.1038/s41574-020-00435-4.
- Shi J, Fan J, Su Q, Yang Z. Cytokines and abnormal glucose and lipid metabolism. Front Endocrinol (Lausanne). 2019;10:703. doi:10.3389/fendo.2019.00703.
- Murata K, Nakao N, Ishiuchi N, Fukui T, Katsuya N, Fukumoto W, Oka H, Yoshikawa N, Nagao T, Namera A. Four cases of cytokine storm after COVID-19 vaccination: case report. Front Immunol. 2022;13:967226. doi:10.3389/fimmu.2022.967226.
- Samuel SM, Varghese E, Triggle CR, Büsselberg D. COVID-19 vaccines and hyperglycemia—is there a need for postvaccination surveillance? Vaccines. 2022;10(3):454. doi:10.3390/vaccines10030454.
- Lugar M, Eugster A, Achenbach P, von Dem Berge T, Berner R, Besser REJ, Casteels K, Elding Larsson H, Gemulla G, Kordonouri O. SARS-CoV-2 infection and development of islet autoimmunity in early childhood. JAMA. 2023;330(12):1151–60. doi:10.1001/jama.2023.16348.
- Khunti K, Valabhji J, Misra S. Diabetes and the COVID-19 pandemic. Diabetologia. 2023;66(2):255–66. doi:10.1007/s00125-022-05833-z.
- Chen Y, Xu Z, Wang P, Li XM, Shuai ZW, Ye DQ, Pan H-F. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165(4):386–401. doi:10.1111/imm.13443.
- Patrizio A, Ferrari SM, Antonelli A, Fallahi P. A case of graves’ disease and type 1 diabetes mellitus following SARS-CoV-2 vaccination. J Autoimmun. 2021;125:102738. doi:10.1016/j.jaut.2021.102738.
