ABSTRACT
Treating non-small-cell lung cancer (NSCLC) has gained increased importance in recent years due to the high mortality rate and dismal five-year survival rate. Immune checkpoint inhibitors (ICI) are a promising approach with exceptional outcomes in NSCLC thanks to the antigenic nature of cells. Conversely, immune system over-stimulation with ICI is a double-edged sword that can lead to various negative effects ranging from mild to life-threatening. This review explores current breakthroughs in nanoparticle-based ICI and their limitations. The PubMed, Scopus and Web of Science were examined for relevant publications. Thirty-eight trials (N = 16,781) were included in the analyses. The mixed effects analyses on quantifying the treatment effect contributed significantly to the subgroups within studies for ICI treatment effect. Models confirmed ICI’s higher impact on treatment effectivity and the decrease in respondents’ mortality compared to conventional treatment regiments. ICI might be used as first-line therapy due to their proven effectiveness and safety profile.
Background
Lung cancer has the highest rates of morbidity and mortality, with an estimated 2.3 million diagnoses and 1.8 million deaths in 2021.Citation1,Citation2 Non-small cell lung cancer (NSCLC) is its most common histological type, with a 5-year survival rate of 2.8%; having a poor prognosis, since most afflicted persons are diagnosed at an advanced stage, making it difficult to treat.Citation3–5
Immune checkpoint inhibitors (ICI) are perspective innovative immunotherapeutic drugs that work by blocking the programmed cell death protein 1/ligand 1 (PD-1/PD-L1) or CTLA-4 pathways to reactivate T-lymphocytes targeting tumors. An accumulating amount of evidence shows that ICI may improve clinical outcomes in patients with advanced NSCLC.Citation6,Citation7 ICI provide long-lasting responses in an increasing percentage of patients with metastatic cancer and are widely employed in (neo)adjuvant situations. Based on these results, the United States Food and Drug Administration (FDA) approved ICI as first-line therapies for advanced NSCLC. When used as first-line therapy, ICI have a 5-year survival rate of 23% compared to other treatments with survival below 16%.Citation8
However, immunostimulants can cause targeted hyperactivation of the immune response, which may lead to normal tissue damage.Citation9 While acute toxicities are more prevalent, chronic immune-related adverse events (irAEs) are becoming more recognized, as they may occur in up to 40% of patients.Citation10 Other long-term concerns include fatal irAEs (FAEs), occurring in 0.4%–1.2% of patients.Citation10 The occurrence of irAEs like rashes, colitis, hepatitis, myocarditis, endocrinopathies, and pneumonitis has been regularly documented.Citation11 Although FAEs are uncommon, they may occur due to overwhelming autoinflammation resistant to steroids and/or other immunosuppressive agents.Citation10 When deciding treatment, oncologists should evaluate the FAEs possibility.
Pneumonitis, also known as checkpoint inhibitor pneumonitis (CIP), is one of the most serious irAEs.Citation12–14 Immune checkpoint inhibitors cause immune dysregulation, which is associated with CIP, despite their unclear mechanism of action. Even while CIP is documented to occur in 3% to 5% of the population, it is associated with a mortality rate of 10% to 17%; conversely, CIP morbidity varies from 7% to 13% in NSCLC patients.Citation12–15–Citation17 CIP is most frequent in the first 6 months after a course of therapy.Citation12,Citation18 Although most irAEs are moderate (grade 1 or 2), in less than 10% of patients treated with immune checkpoint inhibitors, severe (grade 3 or 4) and possibly life-threatening irAEs (grade 5) may occur.Citation19 Meta-analyses indicate they occur at a modest incidence, ranging from 0.4% with monotherapy to 1.2% with combination anti- regimens.Citation10 It is possible that growing levels of pre-existing autoantibodies are responsible for the development of immune-related adverse events.Citation20 However, particular antibodies related to CIP are now being investigated in more depth than either of these conditions. Additionally, a rise in inflammatory cytokines is associated with the irAEs development. NSCLC patients after receiving atezolizumab who developed CIP exhibited greater C-reactive protein and interleukin-6 (IL-6) levels than those who did not develop CIP.Citation21 Cytokines may also be used as predictors of negative outcomes, and their elevated expression is linked with significant ICI toxicity.Citation22–24 While patients receiving chemotherapy had a greater risk of mortality due to myelosuppression and infection, those receiving immunotherapy faced a greater risk of death due to CIP.Citation25–27
Survival, efficiency, together with irAEs are the most relevant markers for the safe and sufficient NSCLC treatment when comparing the ICI risk versus benefit. Thus, the aim of this systematic review was to explore and compare the effectiveness and safety profile of ICI alone or in combination with conventional agents against an active comparator or placebo between and across clinical trials and moderated by the grades of Common Terminology Criteria for Adverse Events (CTCAE). Furthermore, objective response rate of ICI was to validate on the treatment effect and the absolute number of deaths across study arms subgroups.
Methods
Sources of data and search
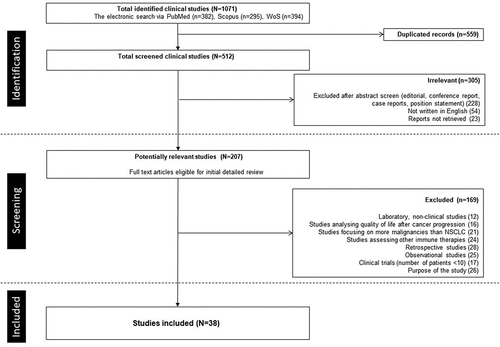
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020. We examined PubMed, Scopus and Web of Science (WoS) from January 2015 to December 2021 for relevant articles published in English. The preliminary results were checked by ClinicalTrials.gov. Non-small lung cancer, adverse event, immune checkpoint inhibitors (anti-programmed cell death 1, anti-programmed cell death ligand 1, anti-cytotoxic T-lymphocyte associated antigen 4), and particular ICI medication names were included in the search approach (ipilimumab, selumetinib, tremelimumab, nivolumab, pembrolizumab, atezolizumab, cemiplimab, durvalumab). We further evaluated possibly relevant papers (N = 512) after a preliminary review of titles and abstracts.
Selection of studies with quality control
Due to the absence of specific safety data and first-hand clinical results, conference abstracts, posters, reviews, position statement, not written in English and reports not retrieved, case studies, and editorials were eliminated (n = 305). Additionally, laboratory, non-clinical studies, papers on more malignancies than NSCLC, and findings on quality of life, as well as studies assessing other immune therapies on a summary, but did not provide data on site, organ, or system-level toxicity, retrospective and observational studies, and purposes of the study were omitted (n = 169). Only randomized controlled studies in individuals with NSCLC comparing therapies that included an ICI medicine or comparing an ICI agent to conventional monotherapy were eligible. Additionally, studies comparing various dosages or administration locations (pharmaceutical forms, effectiveness, and administration sites) of a single ICI medication were considered. In addition, high-quality single-arm and placebo-controlled studies were chosen to form a validation group. The ongoing study with preliminary results on survival or results on irAEs was also included into analyses. Finally, studies were controlled with a technique provided by the Cochrane Collaboration Handbook, which evaluated the bias risk from the original research.Citation28,Citation29 The appropriateness of five areas was evaluated: the production of random sequences, the concealment of allocations, the blinding procedure, the evaluation of outcomes, and the reporting of findings.Citation28 Each element was given a risk of bias assessment index assessed as yes, no, or uncertain based on the bias likelihood. With the use of the modified Jadad scale, it was possible to statistically measure the investigations’ method quality (high quality, four points and over; or low quality, three points and under).Citation30 Every opinion difference about the selection of studies, data extraction, and control of the data quality was handled via discussion to reach a common understanding. Only scores three and up were chosen for final analyzed models. In total, 38 studies were included in the definite analyses. (Please, see for detailed information.)
Data extraction
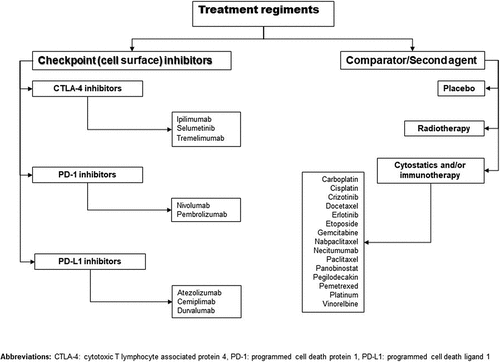
Despite the fact that irAEs were assumed to represent the result of interest, it was discovered that this phrase was inadequate for describing the toxicity associated with standard treatment. In line with that, some AE may not be due solely to immunotherapy, but to other potential causes. It was decided that all adverse events would be utilized if treatment-related adverse events (TEAEs) were not available in research. We chose TEAEs as the main outcome, along with survival data, because they were consistent with most studies and considered a viable alternative to immune-related adverse events. The primary text was analyzed to conduct a comprehensive and thorough data extraction procedure on the clinical trials database. The original articles represented in a single trial were built-in the mixed effects analyses. However, TEAE was retrieved that had provided the most comprehensive report. The other data were used to augment rudimentary information. Data were extracted as well as summarized based on the following information from the studies reports: authors, ClinicalTrials.gov number, phase of clinical trial, year of publication, follow-up time, total number of patients, number of patients in safety analysis, treatment regimens, survival up to 12 months, overall survival and hazard ratio as an indicator of treatment effect, absolute number of deaths, TEAEs, the presence of CIP, and the CTCAE. Treatment regiments of studies papers were divided into three basic groups: i) immune checkpoint inhibitors, ii) active comparator, like cytostatics and immunotherapies (monoclonal antibodies, biological therapy, mRNA vaccine) as well as radiotherapy, and iii) placebo. Moreover, ICI were split up by using their target. (Please, see for detailed information).
The standardized CTCAE is the most widely used instrument in clinical practice for irAEs severity assessing, due to including a grading system and explicit descriptions for each irAE category.Citation31 All grades of side events imply total, severe, and life-threatening toxicity, according to their severity.Citation31 The CTCAE displays grades 1 through 5 with unique clinical descriptions of severity for each immune-related adverse event: grade 1: mild, asymptomatic, or mild symptoms; clinical or diagnostic observations only; intervention not indicated; grade 2: moderate, minimal, local, or noninvasive intervention indicated; grade 3: severe or medically significant, but not immediately life-threatening, hospitalization or prolongation of hospitalization indicated; grade 4: life-threatening consequences, urgent intervention indicated; grade 5: death related to irAEs.Citation31 Presence of CIP and CTCAE Grade were defined by more than or equal to 10% of the study sample, significant prevalence in cohorts, and/or as positive presentation in the analyzed paper.
Involvement of patients and the general public
Patients were not involved in the formulation of the review question or results evaluation. No patients were contacted for input on the interpretation or writing up of the data. The outcomes will not be shared with research participants or the relevant patient group. All studies included into analyses were in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments, or comparable ethical standards in the informed consents for clinical trials included into this review.
Data synthesis and statistical analysis
Frequencies, durations, percentages, total numbers, medians, means, and standard deviations were calculated for the descriptions. Two different perspectives evaluated TEAEs: overview and detail, based on the total number of all TEAEs and the number of each specific TEAE, respectively. Regardless of the irAE grading, general safety was used to indicate the TEAEs overview without distinguishing between their specific classifications. Next, grades (1–5) of CTCAE were stratified into two groups: G-I: low (grade 1 + 2) and G-II: serious (grade 3 + 4); grade 5 was only confirmed in 4 studies; therefore, it was allied to the serious stratified group (G-II). Study subgroups were stratified according to the study arms: interventional drug versus comparator. Firstly, two studies with more than 3 study arms were split into 3 subgroups; secondly, arms were divided in two divisions: interventional and conventional therapy. Finally, we performed mixed effects analyses. A random effects model was used to combine studies within each subgroup, and a fixed effect model was used to combine subgroups and yield the overall effect. Regardless of the study arms, subgroup analyses were performed for the total number of TEAEs, survival with the presence of checkpoint inhibitor pneumonitis, hazard ratio, as well as the absolute number of deaths. So far, analyzed models were moderated by G-I versus G-II of CTCAE. Forrest plots were generated depicting the risk ratio, and 95% confidence interval of study models using mixed effects analyses and p-values lower than or equal to 0.01 were considered significant. GraphPad Prism 9.3.0 and Comprehensive Meta Analysis version 3.0 were used for statistical analyses.
Results
Systematic review and characteristics of the reviewed studies
After checking all inclusion and exclusion criteria, 38 papers (N = 16,781) published between January 2015 and December 2021 were included into analyses definitely (please, see ). Studies duration occurred from 3 to 53 months (29 ± 9). Survival up to 12 months varied from 18% to 69% (28 ± 8%) and median of overall survival between 6.0 and 20.2 months (9.1 ± 2.2). 94.7% (36/38) of included studies were in advanced (III-IV) stage of disease and consisted of 21 different active drugs with different doses and cycles as well as different combinations; 6 studies evaluated one interventional treatment with one dose without any comparator, 9 trials had one study arm with solo ICI or not only ICI, 2 studies were controlled by combination with placebo, and 1 study was compared with solo placebo. So far, monotherapy with Nivolumab (n = 2332) was controlled by six comparators, Atezolizumab (n = 2006) by 8, Pembrolizumab (n = 2078) by 6, Durvalumab (n = 1473) by 8, CTLA-4 inhibitors (Tremelimumab, Selumetinib) (n = 60) by 3, combination of 2 ICI (n = 1253) by 3, and combination of ICI with conventional therapy (n = 2313) by 10 comparators. Conventional therapy (n = 3150) was linked to 14 ICI (monotherapy or its combinations), two or more conventional therapies (n = 1308) to 6 ICI combinations and pooled conventional therapy with placebo (n = 808) was connected with ICI 4-times. Mixed effects analyses based on total number of TEAEs was firstly conducted to investigate the comparability of TEAEs. In the consistency model, we found non-significant differences in the total number of TEAEs between studies independent of stages of NSCLS. The results obtained using the consistency model fit well with the results using the inconsistency model. These results showed that the safety of all investigated and compared drugs in the studies was similar. (Please, see for detailed information of the studies characterization).
Table 1. Characteristic of included studies.
Analysis of drug-based treatments and treatment-related adverse effects
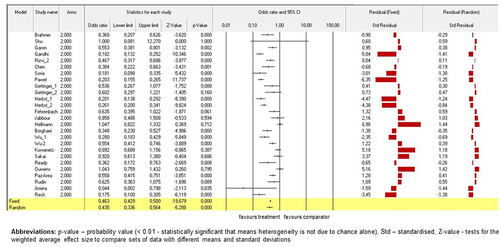
For all studies (n = 27; N = 14,609) in these mixed effects analyses, NSCLC patients were randomized according to their treatment subgroups. The study by Ready et al.Citation57 (p < .01) contributed significantly to random as well as fixed models on the subgroup analysis for total number of TEAEs. Moreover, risk ratio (RR) of trial by Ready et al.Citation57 for the interventional arms when compared to the conventional comparators was lower than 0.1. All other studies also showed that RR for the interventional arms versus the conventional comparators were lower than or equal to 1.0; however, they did not contribute significantly to the model. Standardised residual clarified no contrast in results between the fixed and random models. (Please, see for detailed information of the models). The similar summary for the subgroup with two study arms and the summary across all studies were obtained when the mixed effects analysis was grouped by study arms subgroups. (Please, see for detailed information of the models).
Figure 3. The model of drug-based network and TEAEs. Fixed-effect and randomised-effect models on the study arms subgroups containing the total number of TEAEs. Forrest plots depict the risk ratio of the favourable treatments versus comparators. The standardised residuals of both models are displayed.
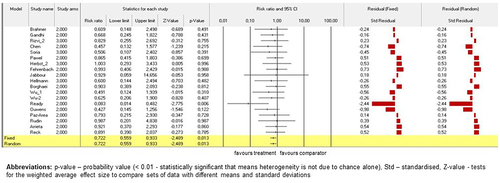
Figure 4. The model of drug-based network and TEAEs. Fixed-effect and randomised-effect models on the study arms subgroups grouped by these subgroups containing the total number of TEAEs. Forrest plots depict the risk ratio of the favourable treatments versus comparators. The standardised residuals of both models are displayed.
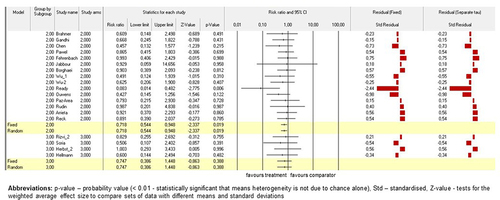
Next, CTCAE Grades I vs II were defined as a categorical moderator variable; thus, the model was grouped by that variable and heterogeneity values was provided because of an analysis being run within each grade and an overall analysis across grades as well as within groups and between groups. As a result, the study by Ready et al.Citation57 (p < .01) contributed significantly to random and fixed models on the subgroup analysis for total number of TEAEs in Grade II of CTCAE as well as in overall model. RR for all studies across groups were lower or equal to 1.0 for the interventional arms versus the conventional comparators. Standardised residual clarified no contrast in results between models, like it was in the previous analysis. (Please, see for detailed information of the models).
Figure 5. The model of drug-based network and TEAEs. Fixed-effect and randomised-effect models on the study arms subgroups containing the total number of TEAEs were moderated by CTCEA Grade (G-I against G-II). The models were grouped by CTCEA Grade and heterogeneity values was provided because of an analysis being run within each grade and an overall analysis across these grades as well as within groups and between groups. Forrest plots depict the risk ratio of the favours treatments versus comparators. The standardised residuals of both models are displayed.
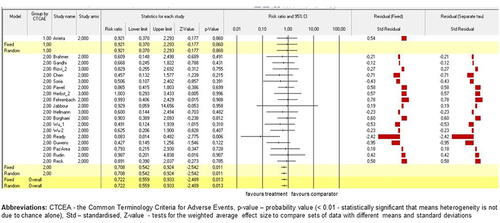
Analysis based on the survival rate and general life prosperity of NSCLC patients
Firstly, the analysis across groups for survival involved all studies (n = 38) and 16,781 patients. Secondly, all included trials compared their survival (in months) with one common comparator that is the most serious and fatal immune-related adverse event in clinical practice, such as checkpoint inhibitor pneumonitis, which has been the common comparator in many trials. Finally, only study by Ready et al.Citation57 (p < .001) contributed significantly to random and fixed models on survival analysis compared by CIP. Standardised residual clarified the contrast in results between models. (Please, see for detailed model information).
Figure 6. Model on survival compared by CIP between studies. Fixed-effect and randomised-effect models on clinical trials (n=38) containing survival were compared by the presence of CIP. Forrest plots depict the risk ratio of the favours treatments versus comparators. The standardised residuals of both models are displayed.
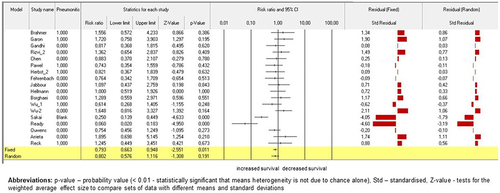
Next, 27 studies (N = 14,609) in this analysis on quantifying the treatment effect across studies using survival data was the log hazard rate. The study by Brahmer et al.Citation6 (p < .001), Gandhi et al.Citation34 (p < .001), von Pawel et al.Citation42 (p < .01), Herbst et al.Citation46 (p = .01), Borghaei et al.Citation52 (p < .01), both studies by Wu et al.Citation53,Citation54 (p < .01), Ouwens et al.Citation58 (p < .001), Paz-Ares et al.Citation61 (p < .001), Arrieta et al.Citation64 (p < .001), and Reck et al.Citation7 (p < .001) contributed significantly to random as well as fixed models on the subgroups within studies for ICI treatment effect. Standardised residual clarified the contrast in results random versus fixed models. (Please, see for detailed information of the models).
Figure 7. Model on survival compared by ICI treatment effect. Fixed-effect and randomized-effect models containing quantifying the ICI treatment effect across studies using survival data by the log hazard rate. Forrest plots depict the risk ratio of the favorable treatments versus comparators. The standardized residuals of both models are displayed.
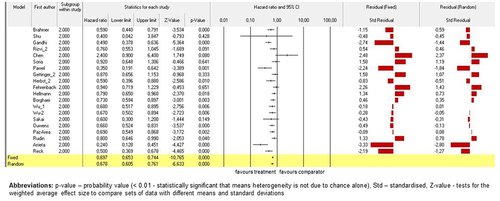
In line with the previous finding, analysis on mortality was conducted to investigate the risk of death in patients underwent ICI therapy versus conventional drugs. Majority of investigated trials on ICI showed significant decreasing of total number of deaths in patients who underwent ICI therapy. Standardised residual clarified the contrast in results between random and fixed models. (Please, see for detailed information of the models).
Figure 8. Models on survival compared by mortality across studies. Fixed-effect and randomised-effect models on mortality across studies investigated the total number of deaths in ICI subgroup against conventional drugs. Forrest plots depict the risk ratio of the favours treatments versus comparators. The standardised residuals of both models are displayed.
Discussion
This systematic review examined recent advances in nanoparticle-based immune checkpoint therapy, as well as the limits of this therapeutic approach. To commence, the total number of irAEs was undertaken to determine the comparability of irAEs between reviewed trials. We confirmed no variations in the safety of all investigated and conventional therapy between reviewed trials. This finding is an important demonstration for comparable safety of investigated and causal therapy according to actual European Society for Medical Oncology (ESMO) Clinical Practice Guidelines on diagnosis, treatment and follow-up for NSCLC,Citation67 which depend on asymptomatic or symptomatic process, staging with risk assessment, efficacy by evaluations of genes and biomarkers, or treatment response and potential follow-up therapy.
The next step of analyses across study arms subgroups on the total number of irAEs and grouping by CTCEA Grades because of an analysis was run within each grade and an overall analysis across grades as well as within subgroups and between CTCEA grades showed that only study by Ready et al.Citation57 decreased significantly of immune-related adverse events. Moreover, the sole substantial difference on survival compared with the most serious and fatal immune-related adverse events, such as checkpoint inhibitor pneumonitis, across study arms subgroups was also confirmed only by trial of Ready et al.Citation57 This studyCitation57 described a first-line therapy for advanced and/or metastatic NSCLC, nivolumab with low-dose ipilimumab proved efficacious and tolerated. Tumors with PD-L1 expression of 1% or greater and tumors with less than 1%, have been identified as potentially relevant cutoffs for nivolumab plus ipilimumab therapy, with the total number of mutations concentrations of more than ten being associated with improved response and prolonged progression-free survival.Citation57 The similar combination and dosages were studied by Gettinger et al.Citation44 in advanced NSCLC; however, their patients were pre-treated with standard platinum-based chemotherapy, and they used different scheme for treatment cycles (every two versus every six weeks). The next reason why outcomes of trial by Ready et al.Citation57 seem to be safer might be data on checkpoint inhibitor pneumonitis. While the presence of CIP was stated in respondents of Gettinger’s studyCitation44 sufficiently (approximately equals to 10% of participants), CIP in Ready’s trialCitation57 was presented insufficiently (less than 10% of responders). This should be in line with revealing that CIP is associated with a mortality rate of 10% to 17%.Citation12–15–Citation17 Moreover, CIP patients’ bronchoalveolar lavage samples showed increased lymphocytosis, which was mostly composed of CD4+ T-cells.Citation14,Citation68 This indicates NSCLC has been progressing due to extensive underlying lung cancer with lymphangitic spread and alveolar damage.Citation14–17–Citation68
In terms of the effectiveness and treatment impact on survival or plasticity reduction, we verified a substantial difference between immune checkpoint inhibitor and conventional therapy, which is corroborated by the treatment effectivity and decrease in respondents’ mortality in the subgroups with interventional checkpoint (cell surface) inhibitors against comparator agents. We confirmed that receiving ICI therapy had a greater probability of survival because of treatment effect and plasticity reduction (11/27) and dying less (15/27) often. These findings are in line with the latest guidelines on metastatic non-small cell lung cancer.Citation67 Definitely, since benefit was shown with PD-1 blockade in lung cancers, three PD-1 inhibitors and PD-L1 inhibitors have been approved by the United States Food and Drug Administration and the European Medicines Agency in the second-line setting.Citation69–71 The three approved agents in the immunotherapy-naive, second-line setting include atezolizumab, nivolumab and pembrolizumab.Citation67 They have been approved on the basis of phase 3 trials demonstrating improved overall survival in comparison with docetaxel.Citation6,Citation37,Citation42,Citation53 Improving survival under ICI therapy might be explained by showing a link between the development of irAEs and increased survival in NSCLC and other malignancies, as well as a link between longer immune checkpoint inhibitor treatment duration and the development of irAEsCitation50,Citation72–74 The outcomes of Shankar et al. established that multisystem irAEs are related to better overall survival and/or progression free survival, and that this connection becomes stronger as the number of irAEs increases.Citation74 Although patients with multisystem irAEs experienced a longer ICI treatment course, the link between immune-related adverse event count and increased survival remains after correcting for ICI duration, indicating that patients obtain a stronger therapeutic impact, yet are at a higher risk of irAEs.Citation74
Regardless of the activity of PD-1/PD-L1 inhibitors in NSCLC, only 20% of responders ultimately react to treatment.Citation75 Therefore, patients with a PD-L1 tumor percentage score of 50% or above had a 5-year survival rate of 29.6%.Citation8 The similar results were showed in the phase 3 KEYNOTE-024 study that established the pembrolizumab as first-line treatment in NSCLC individuals with untreated, advanced NSCLC and tumor characterized by PD-L1 expression more than 50% in absence of the epidermal growth factor receptor mutation and anaplastic lymphoma kinase translocation.Citation7,Citation37,Citation67 Furthermore, results of the phase 3 trials KEYNOTE-189 showed in patients with previously untreated metastatic NSCLC without epidermal growth factor receptor mutation and/or anaplastic lymphoma kinase mutations, the addition of pembrolizumab to pemetrexed and cisplatin or carboplatin resulted in significantly better treatment effect and longer overall survival and progression-free survival than solo chemotherapy.Citation34,Citation37,Citation67 In line with that, CTLA-4 and PD-1 commonly seen on activated T-cells are the most reliable targets for the treatment of cancer.Citation76 Gianchecchi and FierabracciCitation77 reported an increase in central memory T-cells and a decrease in the expression of CTLA-4 and PD-1 in the Treg population. PD-1+ and CTLA-4+ Tregs have a detrimental effect on CD8+ T-cells, conventional T-cells (such as Tcms), and macrophage proinflammatory responses in various ways.Citation77 In this way, increasing the number of activated alveolar T-cells while lowering the anti-inflammatory Treg phenotype may lead to dysregulation of T-cell activity. It is also possible that anti-CTLA-4 antibodies directly bind to CTLA-4 expressed on normal tissues, such as the pituitary gland, which could also lead to immune-related adverse events. This might explain why pituitary inflammation is a well-documented adverse effect of anti-CTLA-4 antibody treatment.Citation78 It is necessary to study and verify other potential mechanisms. Updating guidelines on early and locally advanced NSCLC have to focus on treatment recommendations, including follow-up and survivorship. The latest studies have started to accumulate evidence for ICI in elderly responders with advanced NSCLC. Although no studies dedicated to elderly patients were reported yet, it can be inferred that overall response rates as well as survival are not different between patients lower than 65 years and those with age higher than 65 years, based on subgroup analysis of the randomized second-line trials.Citation67 This is one of the many important demonstrations,Citation67 which call for new personalized medicine recommendations and new treatment algorithms that should be updated as soon as possible, in accordance with approving of their safety and efficacy. The extension of ICIs from advanced NSCLC to earlier stages in NSCLC has resulted in benefits for long-term responders.Citation52
Strengths and limitations
The strengths of this systematic review are based on electronic sources from January 2015 to December 2021 and mixed effects analyses of included clinical trials results, and precise specified inclusion criteria, such as minimum responders in the study sample, type of therapy (including conventional monotherapy and/or placebo), as well as comparing various dosages or administration locations and data on immune-related adverse events, survival, and mortality. These inclusion criteria were chosen to prevent bias because of improving ICI guidelines over studied decade. Moreover, this review aggregated effects from several studies and yielded an average treatment effect, with its efficacy and safety more precise than the individual study results.
On the other hand, there are several weaknesses. Not realizing analyses on different drugs, variety of dosage across identical agents as well as across different drugs between studies and timing of the change in dosage due to difficult statistical elaboration with potential bias in outcomes. Moreover, certain trials have insufficient data and information about the variables and comorbidities under investigation, which could potentially affect the risk of adverse events, morbidity, and mortality. Some AEs may not be due solely to immunotherapy, but to other potential causes. This might be a potential confounding factor. Therefore, we decided to use all adverse events in research if TEAEs were unavailable. Because it was consistent with the majority of studies and considered a viable alternative to immune-related adverse events, treatment-related adverse events (TEAEs) were chosen as the main outcome, together with data on survival.
Implications and further research
Importantly, prevention the first. After undergoing an early diagnosis procedure, the most essential is to compile a comprehensive toxicity profile, a toxicity spectrum, and a rating of the ICI safety used in NSCLC therapy. Except for optimal supportive care or experimental therapy in clinical trials, there are no conventional therapies for such individuals. Thus, novel molecules with higher treatment effect and lower number of irAEs are urgently required. The goal of future researches and their analyses should be to determine best practice and areas of uncertainly in the diagnosis, treatment and follow-up of NSCLC. Additionally, researchers must be able to optimize the design of future studies, with an emphasis on antitumour efficacy and safety as two critical success factors.
Conclusions
We verified no variations in the safety profiles of all investigated and conventional agents across this review. Our finding demonstrated the comparability of investigated and causal therapy according to actual guidelines for NSCLC treatment recommendations. Only one study significantly contributed to the total number of immune-related adverse events across study arms and CTCEA grades within each grade, and an overall analysis across grades. Additionally, the difference in survival compared with checkpoint inhibitor pneumonitis across studies subgroups was confirmed in the same study. Its interventional focus was based on advanced and/or metastatic NSCLC with dual ICI agents as the first-line therapy in a longer period between treatment cycles. Conducted mixed effects analyses showed that ICI might have a greater impact on treatment efficacy and the decrease in respondents’ mortality compared to conventional treatment regiments. These findings suggest patients with NSCLC should undergo relevant clinical and laboratory assessment followed by targeted and personalized treatment to reduce their high risk of mortality. Immune checkpoint inhibitors were confirmed as the first-line therapy due to their proved effectiveness and safety profile.
Authors’ contributions
Sara Maria Majernikova wrote the main manuscript text, did the analyses and created all Figures and Tables.
Availability of data and materials
We examined the PubMed, Scopus and Web of Science from January 2015 to December 2021 for relevant articles and conferences meetings published in English. Non-small lung cancer, lung cancer, biomarkers, immune checkpoint inhibitors (anti-programmed cell death 1, anti-programmed cell death ligand 1, anti-cytotoxic T-lymphocyte associated antigen 4), and particular ICI medication names were included in the search approach (ipilimumab, selumetinib, tremelimumab, nivolumab, pembrolizumab, atezolizumab, cemiplimab, durvalumab).
Ethics approval and consent to participate
No patients were contacted for input on the interpretation or writing up of the data. All studies included into analyses were in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments, or comparable ethical standards in the informed consents for clinical trials included into this review.
No third party participated in this study
The datasets analyzed during the current study available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article.
List of abbreviations
| ATE | = | Atezolizumab |
| AUC | = | area under the curve |
| BID | = | twice a day |
| CBP | = | Carboplatin |
| CEM | = | Cemiplimab |
| CIP | = | checkpoint inhibitor pneumonitis |
| CIS | = | Cisplatin |
| CTCAE | = | Common Terminology Criteria for Adverse Events |
| CTLA-4 | = | anti-cytotoxic T-lymphocyte-associated protein 4 |
| DUR | = | Durvalumab |
| EP | = | Etoposide and platinum |
| ERL | = | Erlotinib |
| FAE | = | fatal immune-related adverse event |
| FDA | = | the United States Food and Drug Administration |
| GEM | = | Gemcitabine |
| ICI | = | Immune checkpoint inhibitors |
| irAE | = | immune-related adverse event |
| IV | = | intravenous |
| NA | = | not applicable |
| NIVO | = | Nivolumab |
| No | = | number |
| NP | = | Nabpaclitaxel |
| NSCLC | = | non-small-cell lung cancer |
| PD-1/PD-L1 | = | the programmed cell death protein 1/ligand 1 |
| PEM | = | Pembrolizumab |
| PEME | = | Pemetrexed |
| PO | = | peroral |
| PTX | = | Paclitaxel |
| QD | = | once a day |
| QxW | = | each x week |
| SBRT | = | Stereotactic body radiotherapy |
| TEAEs | = | treatment-related adverse events |
| TERM | = | Tremelimumab |
| TRT | = | Thoracic radiotherapy |
| TXT | = | Docetaxel |
| VIN | = | Vinorelbine |
khvi_a_2365771_sm6519.docx
Download MS Word (172.2 KB)Acknowledgments
Go to Dr. Dean Willis (Department of Neuroscience, Physiology and Pharmacology, Division of Biosciences, Faculty of Life Sciences, University College London, London, United Kingdom) for his suggestions regarding Methods.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–14. doi:10.3322/caac.21492.
- The Cancer Atlas. ed. Lung cancer. 3rd ed. The American Cancer Society, the International Agency for Research on Cancer, and the Union for International Cancer Control; 2021. https://canceratlas.cancer.org/the-burden/lung-cancer/.
- Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, Varella-Garcia M, Franklin WA, Aronson SL, Su PF, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. doi:10.1001/jama.2014.3741.
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50. doi:10.1056/NEJMoa061884.
- Herbst R, Heymach J, Lippman S. Lung cancer. N Engl J Med. 2008;359(13):1367–80. doi:10.1056/NEJMra0802714.
- Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–35. doi:10.1056/NEJMoa1504627.
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33. doi:10.1056/NEJMoa1606774.
- Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C, Horn L, et al. Five-year overall survival for patients with advanced non‒small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019;37(28):2518–27. doi:10.1200/JCO.19.00934.
- Ma K, Lu Y, Jiang S, Tang J, Li X, Zhang Y. The relative risk and incidence of immune checkpoint inhibitors related pneumonitis in patients with advanced cancer: a meta-analysis. Front Pharmacol. 2018;9:1430–42. doi:10.3389/fphar.2018.01430.
- Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19(4):254–67. doi:10.1038/s41571-022-00600-w.
- Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2(10):1346–53. doi:10.1001/jamaoncol.2016.1051.
- Suresh K, Naidoo J, Zhong Q, Xiong Y, Mammen J, de Flores MV, Cappelli L, Balaji A, Palmer T, Forde PM, et al. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J Clin Invest. 2019;129(10):4305–15. doi:10.1172/JCI128654.
- Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, Postow MA, Wolchok JD. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375–91. doi:10.1093/annonc/mdv383.
- Suresh K, Naidoo J, Lin CT, Danoff S. Immune checkpoint immunotherapy for non-small cell lung cancer: benefits and pulmonary toxicities. Chest. 2018;154(6):1416–23. doi:10.1016/j.chest.2018.08.1048.
- Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, Stevenson J, Pennell NA, Velcheti V. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152(2):271–81. doi:10.1016/j.chest.2017.04.177.
- Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–8. doi:10.1001/jamaoncol.2018.3923.
- De Velasco G, Je Y, Bossé D, Awad MM, Ott PA, Moreira RB, Schutz F, Bellmunt J, Sonpavde GP, Hodi FS, et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res. 2017;5(4):312–8. doi:10.1158/2326-6066.CIR-16-0237.
- Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709–17. doi:10.1200/JCO.2016.68.2005.
- Conde-Estévez D, Monge-Escartín I, Ríos-Hoyo A, Monzonis X, Echeverría-Esnal D, Moliner L, Duran-Jordà X, Taus Á, Arriola E. Prognostic factors and effect on survival of immune-related adverse events in patients with non-small-cell lung cancer treated with immune checkpoint blockage. J Chemother. 2021;33(1):32–9. doi:10.1080/1120009X.2020.1849488.
- Zeng DQ, Yu YF, Ou QY, Li XY, Zhong RZ, Xie CM, Hu QG. Prognostic and predictive value of tumor-infiltrating lymphocytes for clinical therapeutic research in patients with non-small cell lung cancer. Oncotarget. 2016;7(12):13765–81. doi:10.18632/oncotarget.7282.
- Lin X, Deng H, Yang Y, Wu J, Qiu G, Li S, Xie X, Liu M, Xie Z, Qin Y, et al. Peripheral blood biomarkers for early diagnosis, severity, and prognosis of checkpoint inhibitor-related pneumonitis in patients with lung cancer. Front Oncol. 2021;11:698832–43. doi:10.3389/fonc.2021.698832.
- Hommes JW, Verheijden RJ, Suijkerbuijk KPM, Hamann D. Biomarkers of checkpoint inhibitor induced immune-related adverse events-A comprehensive review. Front Oncol. 2021;10:585311–27. doi:10.3389/fonc.2020.585311.
- Mangan BL, McAlister RK, Balko JM, Johnson DB, Moslehi JJ, Gibson A, Phillips EJ. Evolving insights into the mechanisms of toxicity associated with immune checkpoint inhibitor therapy. Brit J Clin Pharma. 2020;86(9):1778–89. doi:10.1111/bcp.14433.
- Catacchio I, Scattone A, Silvestris N, Mangia A. Immune prophets of lung cancer: the prognostic and predictive landscape of cellular and molecular immune markers. Transl Oncol. 2018;11(3):825–35. doi:10.1016/j.tranon.2018.04.006.
- Geng Y, Shao Y, He W, Hu W, Xu Y, Chen J, Wu C, Jiang J. Prognostic role of tumor-infiltrating lymphocytes in lung cancer: a meta-analysis. Cell Physiol Biochem. 2015;37(4):1560–71. doi:10.1159/000438523.
- Yu X, Zhang X, Yao T, Zhang Y, Zhang Y. Fatal adverse events associated with immune checkpoint inhibitors in non–small cell lung cancer: a systematic review and meta-analysis. Front Med. 2021;8:1–9. doi:10.3389/fmed.2021.627089.
- Chen B, Li H, Liu C, Xiang X, Wang S, Wu A, Shen Y, Li G, Lee M-C. Prognostic value of the common tumour-infiltrating lymphocyte subtypes for patients with non-small cell lung cancer: a meta-analysis. PLOS ONE. 2020;15(11):0242173–92. doi:10.1371/journal.pone.0242173.
- Higgins JPT GS. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 ed. The Cochrane Collaboration; 2012. www.handbook.cochrane.org.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928–d5928. doi:10.1136/bmj.d5928.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi:10.1016/0197-2456(95)00134-4.
- National Institutes of Health and National Cancer Institute. Common terminology criteria for adverse events (CTCAE) version 6.0. 2021. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm.
- Shu C, Gainor J, Awad M, Chiuzan C, Grigg C, Pabani A, Garofano R, Stoopler M, Cheng S, White A, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):786–95. doi:10.1016/S1470-2045(20)30140-6.
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al. KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28. doi:10.1056/NEJMoa1501824.
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al. KEYNOTE-189 Investigators. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. doi:10.1056/NEJMoa1801005.
- Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–65. doi:10.1016/S1470-2045(15)70054-9.
- Rizvi N, Cho B, Reinmuth N, Lee K, Luft A, Ahn M, van den Heuvel M, Cobo M, Vicente D, Smolin A, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non–small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 2020;6(5):661–74. doi:10.1001/jamaoncol.2020.0237.
- Planchard D, Reinmuth N, Orlov S, Fischer JR, Sugawara S, Mandziuk S, Marquez-Medina D, Novello S, Takeda Y, Soo R, et al. ARCTIC: durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Ann Oncol. 2020;31(5):609–18. doi:10.1016/j.annonc.2020.02.006.
- Soria JC, Fülöp A, Maciel C, Fischer JR, Girotto G, Lago S, Smit E, Ostoros G, Eberhardt WEE, Lishkovska P, et al. SELECT-2: aphase II, double-blind, randomized, placebo-controlled study to assess the efficacy of selumetinib plus docetaxel as a second-line treatment of patients with advanced or metastatic non-small-cell lung cancer. Ann Oncol. 2017;28(12):3028–36. doi:10.1093/annonc/mdx628.
- Vergnenegre A, Monnet I, Bizieux A, Bernardi M, Chiapa AM, Léna H, Chouaïd C, Robinet G. Open-label phase II trial to evaluate safety and efficacy of second-line metronomic oral vinorelbine-atezolizumab combination for stage-IV non-small-cell lung cancer - VinMetAtezo trial, (GFPC‡ 04-2017). Future Oncol. 2020;16(4):5–10. doi:10.2217/fon-2019-0730.
- Chiara Garassino M, Cho B-C, Kim J-H, Mazières J, Vansteenkiste J, Lena H, Corral Jaime J, Gray JE, Powderly J, Chouaid C, et al. Final overall survival and safety update for durvalumab in third- or later-line advanced NSCLC: the phase II ATLANTIC study. Lung Cancer. 2020;147:137–42. doi:10.1016/j.lungcan.2020.06.032.
- Felip E, Ardizzoni A, Ciuleanu T, Cobo M, Laktionov K, Szilasi M, Califano R, Carcereny E, Griffiths R, Paz-Ares L, et al. CheckMate 171: a phase 2 trial of nivolumab in patients with previously treated advanced squamous non-small cell lung cancer, including ECOG PS 2 and elderly populations. Eur J Cancer. 2020;127:160–72. doi:10.1016/j.ejca.2019.11.019.
- von Pawel J, Bordoni R, Satouchi M, Fehrenbacher L, Cobo M, Han JY, Hida T, Moro-Sibilot D, Conkling P, Gandara DR, et al. Long-term survival in patients with advanced non-small-cell lung cancer treated with atezolizumab versus docetaxel: results from the randomised phase III OAK study. Eur J Cancer. 2019;107:124–32. doi:10.1016/j.ejca.2018.11.020.
- Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, Powderly JD, Heist RS, Carvajal RD, Jackman DM, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004–12. doi:10.1200/JCO.2014.58.3708.
- Gettinger S, Redman M, Bazhenova L, Hirsch F, Mack P, Schwartz L, Bradley J, Stinchcombe T, Leighl N, Ramalingam S, et al. Nivolumab plus ipilimumab vs nivolumab for previously treated patients with stage IV squamous cell lung cancer: the lung-MAP S1400I phase 3 randomized clinical trial. JAMA Oncol. 2021;7(9):1368–77. doi:10.1001/jamaoncol.2021.2209.
- Herbst R, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios C, Morise M, Felip E, Andric Z, Geater S. et al. Atezolizumab for first-line treatment of PD-L1–selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–39. doi:10.1056/NEJMoa1917346.
- Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–50. doi:10.1016/S0140-6736(15)01281-7.
- Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, et al. POPLAR study group. atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–46. doi:10.1016/S0140-6736(16)00587-0.
- Jabbour SK, Lee KH, Frost N, Breder V, Kowalski DM, Pollock T, Levchenko E, Reguart N, Martinez-Marti A, Houghton B, et al. Pembrolizumab plus concurrent chemoradiation therapy in patients with unresectable, locally advanced, stage III non-small cell lung cancer: the phase 2 KEYNOTE-799 nonrandomized trial. JAMA Oncol. 2021;7(9):1–9. doi:10.1001/jamaoncol.2021.2301.
- Peters S, Felip E, Dafni U, Belka C, Guckenberger M, Irigoyen A, Nadal E, Becker A, Vees H, Pless M, et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer-the ETOP NICOLAS trial. lung cancer. Lung Cancer. 2019;133:83–7. doi:10.1016/j.lungcan.2019.05.001.
- Hellmann M, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim S, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora Jimenez E, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020–31. doi:10.1056/NEJMoa1910231.
- Besse B, Garrido P, Cortot AB, Johnson M, Murakami H, Gazzah A, Gil M, Bennouna J. Efficacy and safety of necitumumab and pembrolizumab combination therapy in patients with stage IV non-small cell lung cancer. Lung Cancer. 2020;142:63–9. doi:10.1016/j.lungcan.2020.02.003.
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. doi:10.1056/NEJMoa1507643.
- Wu Y-L, Lu S, Cheng Y, Zhou C, Wang J, Mok T, Zhang L, Tu H-Y, Wu L, Feng J, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol. 2019;14(5):867–75. doi:10.1016/j.jtho.2019.01.006.
- Wu YL, Zhang L, Fan Y, Zhou J, Zhang L, Zhou Q, Li W, Hu C, Chen G, Zhang X, et al. Randomized clinical trial of pembrolizumab vs chemotherapy for previously untreated Chinese patients with PD-L1-positive locally advanced or metastatic non–small-cell lung cancer: KEYNOTE-042 China study. Int J Cancer. 2021;148(9):2313–20. doi:10.1002/ijc.33399.
- Kowanetz M, Zou W, Gettinger SN, Koeppen H, Kockx M, Schmid P, Kadel EE 3rd, Wistuba I, Chaft J, Rizvi NA, et al. Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti-PD-L1). Proc Natl Acad Sci USA. 2018;115(43):10119–26. doi:10.1073/pnas.1802166115.
- Sakai K, Tsuboi M, Kenmotsu H, Yamanaka T, Takahashi T, Goto K, Daga H, Ohira T, Ueno T, Aoki T, et al. Tumor mutation burden as a biomarker for lung cancer patients treated with pemetrexed and cisplatin (the JIPANG-TR). Cancer Sci. 2020;112(1):388–96. doi:10.1111/cas.14730.
- Ready N, Hellmann MD, Awad MM, Otterson GA, Gutierrez M, Gainor JF, Borghaei H, Jolivet J, Horn L, Mates M, et al. First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol. 2019;37(12):992–1000. doi:10.1200/JCO.18.01042.
- Ouwens M, Darilay A, Zhang Y, Mukhopadhyay P, Mann H, Ryan J, Dennis PA. Assessing the influence of subsequent immunotherapy on overall survival in patients with unresectable stage III non-small cell lung cancer from the PACIFIC study. Curr Ther Res Clin Exp. 2021;95:100640–50. doi:10.1016/j.curtheres.2021.100640.
- Provencio M, Nadal E, Insa A, García-Campelo MR, Casal-Rubio J, Dómine M, Majem M, Rodríguez-Abreu D, Martínez-Martí A, De Castro Carpeño J, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. The Lancet Oncology. 2020;21(11):1413–22. doi:10.1016/S1470-2045(20)30453-8.
- Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, Rodriguez C, Cambridge L, Rizvi H, Wolchok JD, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol. 2017;28(3):583–9. doi:10.1093/annonc/mdw640.
- Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E, Juan-Vidal O, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211. doi:10.1016/S1470-2045(20)30641-0.
- Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, Narwal R, Steele K, Gu Y, Karakunnel JJ, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17(3):299–308. doi:10.1016/S1470-2045(15)00544-6.
- Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, Cheema PK, Rodriguez-Abreu D, Wollner M, Yang JC, et al. KEYNOTE-604 Investigators. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369–79. doi:10.1200/JCO.20.00793.
- Arrieta O, Barrón F, Ramírez-Tirado LA, Zatarain-Barrón ZL, Cardona AF, Díaz-García D, Yamamoto Ramos M, Mota-Vega B, Carmona A, Peralta Álvarez MP, et al. Efficacy and safety of pembrolizumab plus docetaxel vs docetaxel alone in patients with previously treated advanced non-small cell lung cancer: the PROLUNG phase 2 randomized clinical trial. JAMA Oncol. 2020;6(6):856–64. doi:10.1001/jamaoncol.2020.0409.
- Stratigos AJ, Sekulic A, Peris K, Bechter O, Prey S, Kaatz M, Lewis KD, Basset-Seguin N, Chang ALS, Dalle S, et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: an open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021;22(6):848–57. doi:10.1016/S1470-2045(21)00126-1.
- Middleton G, Brock K, Savage J, Mant R, Summers Y, Connibear J, Shah R, Ottensmeier C, Shaw P, Lee SM, et al. Pembrolizumab in patients with non-small-cell lung cancer of performance status 2 (PePS2): a single arm, phase 2 trial. LancetLancet Respir Med. 2020;8(9):895–904. doi:10.1016/S2213-2600(20)30033-3.
- Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, et al. CLINICAL PRACTICE GUIDELINES metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Updated version published 15 September 2020 by the ESMO guidelines committee and this publication supersedes the previously published version. Ann Oncol. 2018;29(4):iv192–237. doi:10.1093/annonc/mdy275. 2020.
- Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, Kelly RJ, Hann CL, Levy B, Feliciano JL, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: Incidence and risk factors. J Thorac Oncol. 2018;13(12):1930–9. doi:10.1016/j.jtho.2018.08.2035.
- The United Kingdom medicines & healthcare products regulatory agency. 〈https://Www.gov.uk/government/organisations/medicines-and-healthcare-products-regulatory-agency〉.
- European medicines agency. cluster activities.〈https://Www.ema.europa.eu/en/partners-networks/international-activities/cluster-activities〉.
- Zaim R, Redekop K, Uyl-de Groot CA. Immune checkpoint inhibitors for the treatment of non-small cell lung cancer: a comparison of the regulatory approvals in Europe and the United States. J Cancer Policy. 2022;33:100346–52. doi:10.1016/j.jcpo.2022.100346.
- Das SJD. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):306–17. doi:10.1186/s40425-019-0805-8.
- Ellen Maher V, Fernandes LL, Weinstock C, Tang S, Agarwal S, Brave M, Ning Y-M, Singh H, Suzman D, Xu J, et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol. 2019;37(30):2730–7. doi:10.1200/JCO.19.00318.
- Shankar B, Zhang J, Naqash AR, Forde PM, Feliciano JL, Marrone KA, Ettinger DS, Hann CL, Brahmer JR, Ricciuti B, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. 2020;6(12):1952–6. doi:10.1001/jamaoncol.2020.5012.
- Herzberg B, Campo MJ, Gainor JF. Immune checkpoint inhibitors in non-small cell lung cancer. The Oncologist. 2017;22(1):81–8. doi:10.1634/theoncologist.2016-0189.
- Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38(1):255–67. doi:10.1186/s13046-019-1259-z.
- Gianchecchi E, Fierabracci A. Inhibitory receptors and pathways of lymphocytes: the role of PD-1 in treg development and their involvement in autoimmunity onset and cancer progression. Front Immunol. 2018;9:2374–86. doi:10.3389/fimmu.2018.02374.
- Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6(230):230–45. doi:10.1126/scitranslmed.3008002.