ABSTRACT
We developed a new sludge reduction HA-A/A-MCO (Hydrolysis-Acidogenosis-Anaerobic/Anoxic -Multistep Continuous Oxic tank) process, which has improved phosphate (P) and nitrogen (N) removal. Its biological treatment unit uses an A2/O P & N removal process with hydrolysis acidification, multistep continuous aeration, and continuous flow, coupled with sidestream P removal by draining out anaerobic P-bearing wastewater. The process has advanced synchronization of P and N removal and sludge reduction. The improved performance is closely associated with the population structure of P-accumulating organisms (PAOs). This study investigated the relationship between P removal performance and the population structure of PAOs. The results show that the average effluent P content of HA-A/A-MCO process was only 0.44 mg/L, when the influent P concentration was 8∼12 mg/L. The effluent met the A standard set by GB18918-2002. PAOs were able to effectively release 1 mg of P and absorb 2.8 mg of P. The system removed P by draining out anaerobic P-rich wastewater, as P had been reduced in the aerobic absorption process. This reduced the need for excess P uptake ability of the PAOs. The bacterial pure culture method was applied to isolate 5 PAOs with typical P absorption and removel features. 16SrDNA amplification and sequence analysis revealed that Acinetobacter sp. and Lampropedia sp played dominant roles in anaerobic P-releasing process. Moreover, Devosia sp. and Bdellovibrio sp were the primary strains in the aerobic tank, and, they were the major stains for P absorption. Uncultured Bacterium and other uncultured strains were detected in the anoxic tank.
Introduction
Currently, sludge reduction methods are limited by low phosphate (P) and nitrogen (N) removal rates. It is difficult to effectively reduce sludge while simultaneously removing biological P. We developed the HA-A/A-MCO (hydrolysis-acidogenosis-Anaerobic/Anoxic-Multistep Continuous Oxic tank) process, which improved the removal of P and N and reduced sludge production. In this process, P was removed by draining out anaerobic P-rich wastewater.
The biological treatment unit of the HA-A/A-MCO uses an A2/O P & N removal process with hydrolysis acidification, multistage-series contact aeration, and continuous flow. This long-term study indicates that this process has advanced synchronization of P & N removal and sludge reduction.Citation1-3 Improved performance was closely associated with the population structure of P-accumulating organisms (PAOs). Because of this, we isolated and purified PAOs from sludge in tanks of various capacities by using the bacterial pure culture method. The population structure of PAOs was determined by both 16SrDNA sequence analysis and P uptake and release experiments. This study aimed to discover the relationship between P removal performance and PAOs.
Material and methods
Equipment and process
shows the HA-A/A-MCO process. It consists of a hydrolysis acidification tank, an anaerobic P-releasing tank, an anoxic tank, multistep continuous oxic tanks, a secondary sedimentation tank, a sidestream sedimentation tank, and a chemical P-removal tank. The equipment was made from PVC material. The effective volume of the hydrolysis acidification tank was 50 L and the corresponding hydraulic retention time (HRT) was 2.5 h. Both the anaerobic and anoxic tanks had 30 L of effective volume and 1.5 h of HRT. The multistep continuous oxic tanks were further divided into 3 independent parts. Tank 1, which was the bacterial dispersion area, had 15 L of effective volume with 0.5 h∼0.75 h of HRT. Tank 2, the protozoa growth region, had 30 L of effective volume and 1.5 h of HRT. Tank 3, the metazoan growth area, had 40 L of effective volume and 2 h of HRT. Oxygen was pumped into multistep continuous oxic tanks by an air compressor through microporous aeration tubes in the bottom of the tanks. The second and third tanks were filled to 40% of their capacity with combined biological filler. The sidestream sedimentation tank generated anaerobic P release supernatant, which was required by the chemical P removal tank. Its HRT was 1 h. The radial-flow method was applied to the secondary sedimentation tank, and the HRT was 1 h.
Figure 1. HA-A/A-MCO process. Influent Tank (1), hydrolysis acidification tank (2), anaerobic tank (3), anoxic tank (4), No. 1, 2 and 3 multistep continuous oxic tanks (5, 6 and 7), sidestream sedimentation tank (8), chemical P-removal tank (9), secondary sedimentation sank(10), anaerotic P release returning sludge (11), denitrification reflux (12), nitrification reflux, residue sludge reflux (14), flow control pump (15), air compressor (16), stirrer (17), efiller (18), high phosphorus sludge (19), effluent (20).
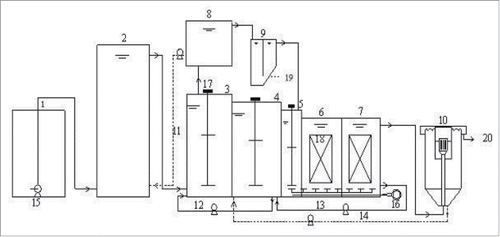
Wastewater and a small amount of anaerobic P release sludge were transported into the hydrolysis acidification tank to complete the VFA transformation and sludge reduction. Then, after anoxic N removal, VFA-rich supernatant entered the anaerobic tank together with the denitrification reflux flow. VFA stimulated PAOs to release P and generate liquid with a high P concentration. In accordance with the P concentration in anaerobic P release solutions, P release supernatant with 13% of inflow was imported into the chemical P removal tank for chemical fixation. The resulting chemical sludge was used for P recycling. After P release, the mixed liquid was transferred into the anoxic tank together with an aerobic nitrification liquid and the sludge was returned to complete N removal by denitrification. Together with the P removal supernatant, the obtained N removal mixture entered the multistep continuous oxic tanks for aerobic P absorption, carbon oxidation, N ammonification, and N nitrification. Each tank had a different organic matter concentration gradient, HRT, dissolved oxygen concentration, and filling rate of biological filler.
The range of a number of factors was controlled in order to enhance the growth intensity of the advanced microorganisms and to extend the food chain. The amount of sludge produced was reduced by the predation effect of microfauna. The generated mixture was discharged through the secondary sedimentation tank.
During the process, the influent flow was 20 L/h. The DO of each section in the multistep continuous oxic tanks was controlled within a range of 0.5–1.0 mg/L for Tank 1, 1.0–1.5 mg/L for Tank 2, and 1.0–1.5 mg/L for Tank 3. The reflux ratio of sludge, nitrification liquid, denitrification N removal liquid, and anaerobic P release mixture was 40%, 150%, 100%, and 2%, respectively. The sludge retention time (SRT) was ∼60 d. The mixed liquor suspended solids (MLSS) was 5100–5800 mg/L. The sludge load was 0.18–0.21 kgCOD/kgMLSS.d.
Sample collection
Samples were collected from November 10th to 25th, 2009 at different time points when the HA-A/A-MCO system was operating. Activated sludge was taken from the middle sites of the anaerobic tank, anoxic tank, and aerobic tanks 1, 2 and 3.
Experimental water quality and determination methods
Wastewater used in the experiment was made of Chongqing University campus sewage and tap water, supplemented with starch, glucose, milk powder, NH4Cl, KH2PO4, and anhydrous Na2CO3. The water quality and related analysis methods are listed in .
Table 1. Quality indexes of wastewater and analysis methods.
Isolation and purification of P-accumulating organismsCitation4,5
The activated sludge samples were put into sterile centrifuge tubes containing a few sterilized glass beads with a diameter of 2–3 mm. The tubes were oscillated in a vortex mixer for 10 min in order to evenly disperse sludge. The coating method was used to separate and purify strains. The enrichment culture medium (pH 6.8–6.9) contained 1.2 g of casein, 0.3 g of yeast extract, 1.0 g of CH3COONa·3H2O, 30 mg of CaCl2·2H2O, 100 mg of MgSO4·7H2O, 100 mg of NaCl, 20 mg of KH2PO4, 0.6 mL of trace element solution, and 1000 mL of distilled water. The trace element solution consisted of 1.5 g of FeCl3·6H2O, 0.03 g of KI, 0.03 g of CuSO4·5 H2O, 0.06 g of Na2MoO4·2H2O, 0.15 g of H3BO3, 0.12 g of MnCl2·4H2O, 0.12 g of ZnSO4·7H2O, and 0.15 g of CoCl2·2H2O.
P uptake and release test of purified strainsCitation4,5
The strains obtained by the coating method were inoculated into 100 mL of enrichment culture medium. The culture that was generated was centrifuged at 4000 rpm for 10 min using a low speed refrigerated centrifuge. It was washed once with synthetic wastewater medium. The bacterial culture was transferred into 100 mL of synthetic wastewater medium with continuous N supplement. It was cultured in a 30°C water bath for 3 h. Samples were taken every 1 h and immediately centrifuged at 10000 rpm for 10 min. The P content in the obtained supernatant was determined. Then, the bacterial liquid was cultured in a 30°C shaker (200 rpm) for aerobic growth for 6–10 h. Samples were taken every 1 h and immediately centrifuged at 10000 rpm for 10 min. The P content of the obtained supernatant was determined.
The medium for the P uptake and release test contained 0.925 g of CH3COONa·3H2O 0.925 g, 0.1 g of peptone, 0.01 g of yeast extract, 0.05 g of NaCl, 65.51 g of KH2PO4·3H2O, 0.075 g of NaHCO3,153.7 mg of MgSO4·7H2O, and 1 mg of CaCl2·2H2O 33.1.
16SrDNA sequencingCitation4,5
To prepare the PCR templates single colonies were selected using sterile toothpicks and transferred into 30 μL of sterile water. The samples were heated at 98°C for 5 min using a PCR machine. The solution was transferred into 1.5 mL tubes and centrifuged at 10000 rpm for 5 min. The supernatant was collected and used as DNA templates for PCR.
The forward and reverse primers for 16SrDNA amplification were 8F- AGAGTTTGATCCTGGCTCAG and 1495R-CTACGGCTACCTTGTTACGA, respectively. The forward and reverse primers matched the 8–27 bps and 1514–1495 bps of the E.coli 16SrRNA gene, respectively. The primers were synthesized by Sanbo Zhiyuan Gene Technology Co. Ltd.
The 25 μL PCR reaction system contained 2.5 μL of 10xbuffer(Mg2+), 1 μL of each primer (10 pmol/L), 0.5 μL of 10 mmol/L dNTP, 2 μL of DNA templates, and 2.5U TaqDNA polymerase. TE was used as a negative control.
The PCR program was 95°C for 5 min, followed by 35 cycles of 94°Cfor 1 min, 56°C for 1 min, and 72°C for 1 min 30 s, and 72°C for 10 min. 5 μl of PCR products was used for electrophoresis. The results were detected and recorded using a gel imaging system.
PCR products were sent to Shanghai Yingjun Biological Technology Co., Ltd for sequencing.
Results and discussion
Phosphorus removal efficiency
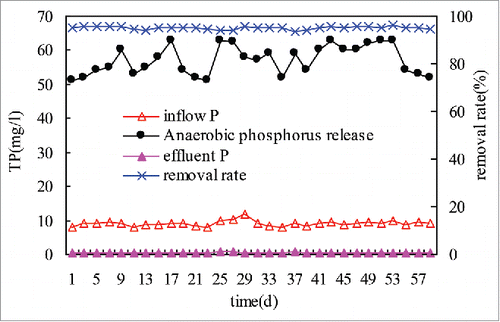
During the stable operating period of the HA-A/A-MCO system, the TP removal efficiency was detected by determing the TP concentration of influent, effluent, and the anaerobioc supernatant in the sidestream sedimentation tank once every other day. ().
As shown in , when the influent TP content was 8∼12 mg/L with an average of 9.2 mg/L, the TP content after anaerobic P release reached 50∼63 mg/L with an average of. After aerobic P uptake occurred through the No 1, 2 and 3 multistep continuous oxic tanks, the final effluent TP concentration was 0.37∼0.6 mg/L with an average of 0.44 mg/L. The average TP removal rate reached 95.2%. The effluent P content met the A standard of “Standards for discharged pollutants from urban sewage treatment factories” (GB18918-2002).
Aerobic phosphorus uptake potential
The basis for biological P removal is the superb P uptake ability of PAOs. There are 2 types of P uptake. In Type I P uptake, the organisms can absorb more P than biomatrix and they form P-accumulating granules. In Type II aerobic P uptake is greater than anaerobic P uptake. Type II P uptake directly affects water quality. Many studies confirm that a greater anaerobic P release results in better aerobic P.Citation6 However, only a small amount of P in urban influent wastewater can be aerobically absorbed. Therefore, some scientists suggest that the efficiency of anaerobic P release is not critical for the P removal effect of urban wastewater.Citation7 When the concentration of anaerobic P release liquid is 10–20 mg/L a good biological P removal effect is still available.Citation8
In the present study, we found that the HA-A/A-MCO system effectively absorbed 42 mg/L of P and released 47.56 mg/L of P.Citation9 The amount of absorbed and released P was similar. Numerous studies demonstrate that the P released by PAOs at the anaerobic stage was 1 mg, while the absorbed P was 2–3 mg at the aerobic stage.Citation10 Therefore, HA-A/A-MCO technology does not have a noteworthy P uptake ability.
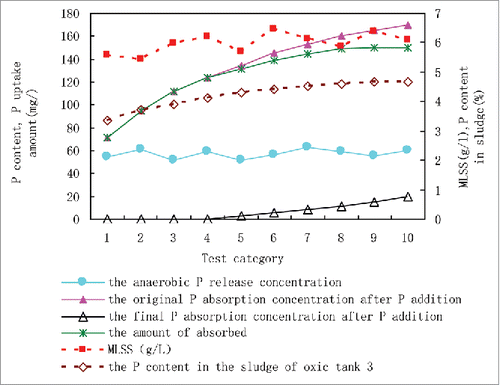
To investigate the P uptake ability of activated sludge in the HA-A/A-MCO system, we performed an anaerobic P release experiment using the experimental water described in section 1.3. When P release was completed, we added phosphate manually to provide enough P for excess absorption. The P uptake results are shown in .
As shown in , the P content in aerobic sludge was 3.35∼4.66%, which is much higher than the P content in the biological matrix. The general P content in regular activated sludge was 1.5∼2%. The data show that the activated sludge in the HA-A/A-MCO system has Type I P-absorbing capacity. In addition to complete absorption of the original phosphate that resulted from anaerobic P release and was carried by the wastewater, the sludge showed advanced P uptake ability during aerobic P absorption process. The amount of absorbed P beyond the released P was ∼108 mg/L. The system effectively released 1 mg of P and was able to uptake about 2.8 mg P. Thus, the HA-A/A-MCO system has extensive P absorption ability.
Furthermore, the P content of sludge in the HA-A/A-MCO system with SRT characteristics increased along with the available P. Hence, SRT is not the most important factor affecting sludge P content. The system has great P storage potential when PAOs are activated. However, during its special operation process, the HA-A/A-MCO system does not need to perform the Type II P uptake in order to complete P absorption. This is related to the order in which P is removed from the system and is dependent on the anaerobic and aerobic processes.
The traditional P removal order is anaerobic P release→aerobic P uptake→P removal by discharging sludge.
The P removal order of the HA-A/A-MCO system is anaerobic P release→P removal by discharging water→aerobic P uptake.
Thus, the final water quality of the sludge reduction P removal system strongly depends on how much P can be aerobically absorbed by PAOs beyond the amount of anaerobically released P. P has been eliminated in the aerobic P uptake process in the HA-A/A-MCO system used to remove P in P-rich wastewater. Therefore, the water quality can be guaranteed, as long as the PAOs absorb the same amount of released P. This method decreases the need for the excess P uptake ability of PAOs. The PAOs can show great P removal effect as long as they maintain P-uptake and P-release abilities for inducing the enrichment of low concentration P in urban wastewater.
Analysis of P removal bacterial population
The excellent P removal ability of this system is related to its P removal bacterial population. In this study, the method of bacterial pure culture (see Section 1.4) was applied to isolate and purify P removal strains from activated sludge in various tanks. The phosphorus absorption and release test of pure strains (see section 1.5) was used to further identify the P removal population. In the end, the population structure of P removal bacteria was analyzed and defined by 16SrDNA sequencing assays (see Section 1.6).
Phosphorus absorption and release test of separated and purified strains
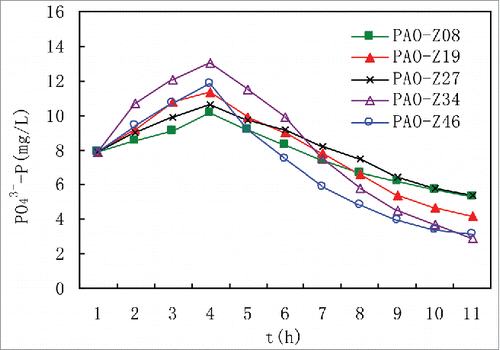
We isolated and purified strains by using the coating method. In total, 48 strains were obtained from activated sludge in various reaction tanks. The P absorption and release ability of each strain was tested. The dissolved oxygen concentration was < 0.3 mg/L at the anaerobic stage, while the average was 1.3∼2.5 mg/L at the aerobic stage. Five strains carrying typical characteristics of anaerobic phosphorus release and aerobic phosphorus uptake were identified. They were labeled PAO-Z08, PAO-Z19, PAO-Z27, PAO-Z34, and PAO-Z46. The P absorption and release features of these 5 strains are shown in .
The results of the P absorption and release test () indicate that these 5 strains showed significant characteristics of anaerobic P release and aerobic excess P uptake. When the strains had the same P concentration (7.9 mg/L) in the synthetic wastewater medium, PAO-Z34 showed the most obvious anaerobic phosphorus release and aerobic excess phosphorus uptake characteristics. The highest concentration of released P was 13 mg/L. The lowest phosphorus concentration was 2.9 mg/L at the end of the aerobic phosphorus uptake stage. The removal rate reached 63.3%. The P removal ability of the remaining 4 strains was reduced in an order of PAO-Z46>PAO-Z19>PAO-Z08 > PAO-Z27. The ability to absorb and release P was also gradually weakened. The P absorption and release test results of the purified strains show that the 5 strains that were isolated and purified from activated sludge in each reaction tank had typical and apparent features of absorbing and releasing P. We preliminarily defined these 5 strains as PAOs.
16SrDNA amplification of P-accumulating organisms
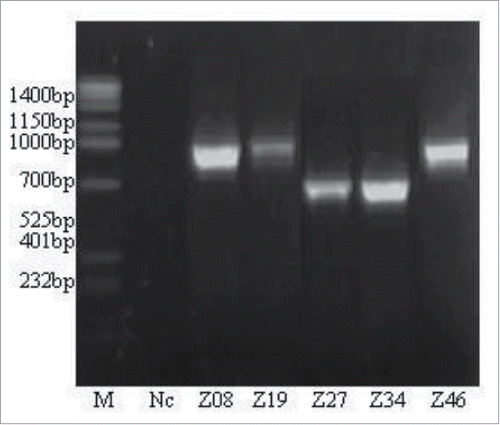
16SrDNA amplification was performed on the 5 strains (PAO-Z08, PAO-Z19, PAO-Z27, PAO-Z34, and PAO-Z46) identified as PAOs ().
As shown in , the PCR bands were intense, bright, and specific for each of these 5 strains. There was no smear. Additionally, no bands were observed in the negative control lane, indicating good PCR amplification. The 5 strains were divided into 2 groups according to the size of PCR products. One group contained the PAO-Z27 and PAO-Z34 strains whose PCR products were around 700 bp. The other group had PAO-Z08, PAO-Z19, and PAO-Z46 strains with the band size of 1000 bp.
It has been reported that Proteobacteria is one of the dominant microbial groups in enhanced biological P removal systems, including α-Proteobacteria, β-Proteobacteria, γ-Proteobacteria and other subgroups (Seviour, Takashi, and Motoharu 2003). Among these, the size of PCR amplification products was 1000 bp for α-Proteobacteria and 700 bp for β-Proteobacteria.
Our observations suggest that the operation conditions of each reaction tank in the HA-A/A-MCO system provide a suitable growing and breeding environment for α-Proteobacteria and β-Proteobacteria. The growth and metabolism of these 2 bacterial populations plays a key role in P removal. They are the main P removal populations in the system.
Sequencing of 16SrDNA in P-accumulating organisms
PCR amplification products of the 5 strains were sent to Shanghai Yingjun Biotechnology Co. Ltd. for sequencing. The sequencing results were aligned with available sequences in the National Center for Biotechnology Information Library (Genbank) and GenBank/European Molecular Biology Laboratory/DNA DataBank of Japan database. Their species were then determined. The results are shown in .
Table 2. 5 Sequence analysis of 16SrDNA in tested strains.
Known species that have the highest homology with the 5 strains were identified. The homology of 4 strains with registered bacterial species was greater than 99%.
The sequencing results in show that in the HA-A/A-MCO system, microbial populations whose dominant P removal strains include α-Proteobacteria and β-Proteobacteria include Acinetobacter sp., Devosia sp., Lampropedia sp., and Bdellovibrio sp, among others. Other species have not yet been identified and studied.
Among the 5 strains, PAO-Z27 was determined to be Acinetobacter sp., and PAO-Z19 was identified as Lampropedia sp. These 2 populations played a major role in the anaerobic P release process in the HA-A/A-MCO system. Rhee et al.,Citation11 for the first time, separated one strain of Acinetobacter sp from activated sludge in the EBPR system. He proposed that it was the primary PAO. However, Stante et al.Citation12 found that Lampropedia sp. has the ability to absorb carbon sources and release phosphate under anaerobic conditions while uptaking phosphorus under aerobic conditions. This is one kind of bacteria, carrying basic PAO metabolism characteristics. In addition, Bdellovibrio sp, represented by the PAO-Z08 strain and Devosia sp., represented by PAO-Z46, appeared extensively in the aerobic tank. These were the dominant bacteria for aerobic P uptake in the system. PAO-Z34 was 98.8% similar to Uncultured Bacterium, which has not yet been cultured and investigated. It was only determined to be Proteus sp., and its species is unknown. This population was detectable in the anoxic tank in the system.
The PAOs mentioned above provide a solid foundation for the stable and excellent P removal performance of the HA-A/A-MCO system.
Conclusions
When the influent TP was 8∼12 mg/L, the average effluent P concentration was only 0.44 mg/L. The average removal rate of TP reached 95.2%. The effluent P concentration met the GB18918-2002 A standard.
The PAOs in the system effectively released 1 mg of P and absorbed 2.8 mg of P. The excess P uptake potential is massive. The HA-A/A-MCOse process removed P by discharging anaerobic P-rich wastewater. P was eliminated in the aerobic P uptake process. This reduces the requirements for the excess P uptake ability of PAOs.
This study adopted the bacterial pure culture method to separate and purify 5 POAs from the activated sludge in various reaction tanks. These 5 POAs have the typical characteristics of P absorption and release. 16SrDNA amplification and sequencing analysis of the 5 strains were performed, and the results show that Acinetobacter sp. and Lampropedia sp played dominant roles in anaerobic P release. Devosia sp. and Bdellovibrio sp mainly appeared in aerobic tanks. They were the dominant bacteria for aerobic P uptake. Uncultured Bacterium was detected in the anoxic tank of the system.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Supported by National Natural Science Foundation of China (No: 51408082), the Science and Technology Project of Chongqing Education Commission (No: kj1400320), and the Fund of Chongqing Science & Technology Commission (No: cstc2015jcyjA30010).
References
- Zuo N, Bai X, Ji F Y. Analysis on bacterial community structure in hydrolysis acidification tank in advanced HA-A/A-MCO sludge reduction process. Water Wastewater 2012; 28:112-6
- Zuo N, Ji F Y. Effect of SRT on phosphorus removal and sludge characteristics in HA-A/A-MCO sludge reduction process. Chin. Water Wastewater 2012; 28:30-4
- Ji FY, Zuo N, Huang LY, Zong SA. Study on operation performance of hydrolysis acidification-A2O sludge reduction process. Chin. J. Environ. Eng. 2010; 4:5-10
- Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev 1995; 59:143-69; PMID:7535888
- Wang TJ. Analysis on microbial community structure of SUFR reactor and isolation of phosphorous accumulating organisms. 2007; Chongqing: Southwest University
- Henze M, Harrenmoes P, Jansen JLC, Arvin E. Wastewater Treatment: Biological and Chemical Processes (2nd Edition). 1997; New York: Springer
- Bi XJ, Gao TY. Study of removing nitrogen and phosphorus in anoxic-anaerobic-aerobic process. Shanghai Environ Sci 1999; 18:19-21
- Zhang B, Gao T Y. Principle and characteristics of reversed A2/O process. Chin Water Wastewater 2000; 16:11-5
- Ji FY, Zuo N, Huang LY, Zong SA. Characteristics of phosphorus removal and phosphorus recovery in advanced sludge reduction process HA-A/A-MCO. Chin. Water Wastewater 2009; 25:29-33
- Zheng XC, Li YX. Nitrogen and Phosphorus Removal Technology in Sludge Reduction Process. 1998; Beijing: China Building Industry Press
- Rhee G Y, Fuhs G W. Wastewater denitrification with qne-carbon compounds as energy source. J Water Pollut Control Federat 1978; 50:2111-9
- Stante L, Cellamare CM, Malaspina F, Bortone G, Tilche A. Biological phosphorus removal by pure culture of lampropedia spp. Water Res 1997; 31:1317-24; http://dx.doi.org/10.1016/S0043-1354(96)00351-X