ABSTRACT
The aim of this study was to compare the protective effects of curcumin, curcumin-β-cyclodextrin nanoparticle curcumin (BCD-CUR) and nanoliposomal curcumin (NLC) on unsymmetrical dimethylhydrazine (UDMH) induced poison in mice. Curcumin, BCD-CUR, and NLC were prepared and their properties of zeta potential, particle size, encapsulation efficiency, and loading capacity were characterized. Eighty-eight male ICR mice on normal chow diet were randomly divided into 11 groups, and intraperitoneally injected with UDMH alone, or together with different doses of curcumin, BCD-CUR or NLC daily for up to 10 d. Enzyme activities of serum alanine transaminase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) were analyzed by fully-automatic analyzer and neurotransmitter levels were determined with high performance liquid chromatography (HPLC). 150 mg/kg curcumin treatment alone significantly reduced levels of serum ALT and LDH that were induced by UDMH and markedly increased level of γ-amino butyric acid (GABA) that were reduced by UDMH in the hippocampus. 150 mg/kg BCD-CUR not only decreased significantly the increase of ALT, LDH and glutamate (Glu) but also recovered levels of AST and GABA. 150 mg/kg NLC recovered profoundly levels of AST and GABA while decreased remarkably the UDMH induced increase of ALT, LDH, Glu and 5-hydroxytryptamine (5-HT). In addition, treatments with all tested doses of NLC significantly reduced the UMDH induced dopamine (DA), the monoamine neurotransmitter. NLC had more profound protective effects against liver and central nervous system injury induced by UDMH than a suspension of BCD-CUR or curcumin did in mice.
Highlights
Unsymmetrical dimethylhydrazine (UDMH) treatment alone elevated levels of DA and 5-HT but decreased level of GABA in the hippocampus while increased Glu levels in serum of mice.
Treatment simultaneously with curcumin, or curcumin-β-cyclodextrin nanoparticle (BCD-CUR) or nanoliposomal curcumin (NLC) prevented UDMH induced poison in mice.
NLC displayed better protective effects against UMDH induced liver and central nervous system injury in mice than BCD-CUR or curcumin suspension did.
Introduction
Unsymmetrical dimethylhydrazine (UDMH), a liquid propellant for rockets, is widely utilized in launching rockets and spaceships because of its effective performance.Citation1,2 However, as a highly toxic substance, UDMH is clearly harmful to living organisms,Citation3 causing immunological, genetic,Citation4-6 and even carcinogenic,Citation4,7,8 changes. In addition, chronic poisoning by UDMH may result in mild fatty liver and an increase in alanine transaminase (ALT) levels.Citation1 Currently, drug therapy is the usual approach to acute UDMH poisoning, but this usually results in toxic side effects. Furthermore, dietary nutrition and some phytopreparations exhibit promising protective effects against UDMH toxicity.Citation6 Curcumin is a dietary polyphenol from the roots and rhizomes of spice curcumin longa, which has historically been widely used in Asia as a pigment, food additive, and condiment with a satisfactory safe profile.Citation9 Evidence has shown that it has anti-inflammatory,Citation10,11 antioxidant,Citation12-15 anti-cancer,Citation16-18 neuroprotective,Citation19 anti-HIV, and anti-mutagenic properties.Citation20,21 In addition, our previous studies have confirmed that pyridoxal (vitamin B6) can spontaneously react with UDMH at room temperature to diminish its damage.Citation22,23 The fact that both the curcumin and pyridoxal molecule structure contain an analogous carbonyl group made us speculate that curcumin may have protective effects against the toxic effects of UDMH. However, previous studies indicated that curcumin has low solubility, poor bioavailability, and does not cross the blood brain barrier easily or reach an effective concentration in the brain tissue.Citation19,24,25 On the other hand, other studies reported that when it is formulated with phosphatidylcholine,Citation26,27 or complexed with cyclodextrin,Citation28,29 or nanostructured into liposomes, curcumin has better absorbability, bioavailability and drug potency.Citation21,30 Therefore, in the present study, we prepared curcumin-β-cyclodextrin nanoparticle (BCD-CUR) and nanoliposomal curcumin (NLC) and compared their protective effects on UDMH poisoning in mice.
Materials and methods
Chemicals and reagents
All chemicals used in this study unless otherwise stated were of analytical grade. Curcumin (95%), sodium 1-octanesulfonate, liquid UDMH (98%), methanol (HPLC grade), ethanol, sulfosalicylic acid, citric acid, sodium tetraborate decahydrate, sodium acetate anhydrous, sodium sulfite anhydrous, perchloric acid and ethylenediamine tetraacetic acid disodium salt (Na2-EDTA) were purchased from National Chemicals Group Company of China. Standard curcumin, β-cyclodextrin, phosphatidylcholine, sodium carboxymethyl cellulose (CMC), dopamine, 3,4-dihydroxy-phenylacetic acid (DOPAC), homovanillic acid (HVA), 5-hydroxytryptamine (5-HT), 5-hydroxyindole-3-Acetic acid (5-HIAA), glutamate (Glu) and γ-Amino butylic acid (GABA) were purchased from Sigma (USA). 1,2-Phthalic dicarboxaldehyde (OPA) (analytical grade) was purchased from Acros Company of USA. Sodium 1-octanesulfonate (analytical purity) was purchased from Hitachi Chemical Co., Ltd. of Japan.
Preparation of curcumin formulation
Curcumin was dissolved in 0.5% CMC injection water and vortexed to get a curcumin suspension (designated as curcumin). BCD-CUR was prepared as a previous study with modifications.Citation28 Briefly, β-cyclodextrin (200 mg) was dissolved in 40 ml of deionised water and to which 8 mg curcumin in 10 ml ethanol was added. The solution was sonicated for 5 min and stirred with a magnetic stirrer at 200 rpm for 1 h. Then, the solution was centrifuged at 3000 rpm for 15 min. The supernatant containing BCD-CUR inclusion complexes was filtered through a 0.45 μm filter to remove any free CUR. The subsequent BCD-CUR inclusion complex was lyophilized and stored at 4°C until further use. NLC preparation adopted a modified ethanol-dripping method: lipid (phosphatidylcholine: cholesterol = 5:1, w/w), sodium deoxycholate and Tween-80 were added into proper volume ethanol as the ratio of 15:4:4 (w/w) under magnetic stirring at 1000 rpm for 30 min. For obtaining curcumin-loaded liposomes, curcumin was added under stirring. The final ethanol mixture ratio of lipid, sodium deoxycholate, Tween-80 and curcumin was 15:4:4:1 (w/w). The above ethanol mixture with or without curcumin was injected into mannitol solution (5.3 mg/ml), with high speed homogenization (A220-18G-S; Shanghai Angyi Instruments, Shanghai, China) at 10,000 rpm for 15 min. The ratio of the ethanolic-lipid phase to the auqueous phase was 1:5 (v/v) in the final suspension. Then, the suspension was centrifuged at 100,000 g for 90 min at 10°C and freeze dried to collect the NLC. Sephadex G-50 gel column elution was used to get narrow elution curves and confirm the nanoparticle formation for both BCD-CUR and NLC.
Determination of zeta potential, particle size, entrapment efficiency percentage (EE%) and loading capacity percentage (LC%) for BCD-CUR and NLC
Zeta potential determination
Zeta potential was determined by laser doppler electrophoresis using Zeta Potential Analyzer 2000(Malvern Instruments Ltd, UK). 1 mg BCD-CUR or NLC were dissolved with 0.05 mol/l phosphate buffer saline (PBS, pH = 7.0) to get a final concentration of 0.075%. The measurement temperature was 25 ± 0.1°C, and each sample was analyzed in triplicates.
Particle size measurement
Particle size was determined by dynamic light scattering using a Nano-ZS90 particle size analyzer (Malvern Instruments Ltd, UK). 1 mg BCD-CUR or NLC were dissolved with 0.05 mol/l phosphate buffer saline (PBS, pH = 7.0) to the final concentration of 0.025%. The measurement temperature was 25 ± 0.1°C and each sample was analyzed in triplicates.
HPLC determination of EE% and LC% for BCD-CUR and NLC
EE% and LC% for BCD-CUR and NLC were determined following previously reported methods,Citation10,21 Briefly 1 mg NLC or BCD-CUR was dissolved in 5 ml of anhydrous methanol. After centrifugation (11,000 rpm for 15 min), the supernatant was used for the HPLC to determine the total amount of curcumin. Equal volume of the suspension was dispensed into a 0.9% NaCl solution gfat a ratio of 5:1(v/v) to salt out the non-incorporated drug. After centrifugation (18,000 rpm for 30 min), the upper suspension was taken to determine the amount of curcumin encapsulated under the same HPLC conditions. EE% = amount of curcumin encapsulated in BCD-CUR or NLC/total amount of curcumin, and LC% = amount of curcumin encapsulated in BCD-CUR or NLC/total amount of carriers
Animals and treatments
88 healthy SPF grade ICR mice (male, 20 ± 2 g), obtained from the Department of Experiment Animals, Beijing University (Beijing, China), were randomly divided into 11 groups of 8 each: control group (administered 0.2 ml normal saline (NS) once a day); +UDMH group (intraperitoneal injection 78.873 mg/kg body weight/day UDMH; curcumin treatment groups (UDMH + intragastrically administered 75, 150 or 225 mg/kg body weight/day curcumin); BCD-CUR treatment groups (UDMH + intragastrically administered 75, 150 or 225 mg/kg body weight/day BCD-CUR); NLC treatment groups (UDMH + intragastrically administered 75, 150 or 225 mg/kg body weight/day NLC). Administrations were executed at the same time point each day and lasted for 10 d. All animal experiments were approved by the local animal ethics committee(AC No.2011013). An hour after the last administration, the mice were sacrificed and blood samples were collected from the orbital plexus to separate blood serum by centrifugation at 3000 g for 10 min. Meanwhile, double-side hippocampus and striatum were separated, frozen immediately in liquid nitrogen and stored at −80°C.
Measurement of serum biomarkers for injury
The enzyme activities of serum biomarkers for UDMH injury, such as alanine transaminase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) in serum of mice were determined on a fully automated biochemical analyzer (Beckman Coulter Chemistry Analyzer AU5811, USA).
Detection of monoamine neurotransmitters
The levels of γ-aminobutyric acid (GABA) and glutamate (Glu) in hippocampus were determined by HPLC. Striatum was accurately weighed and then mixed with 0.4 M perchloric acid solution (1 mg striatum adding 10 μl perchloric acid), homogenized in an ice-bath at 200 rpm for 30 seconds, and then spun at 12,000 rpm for 30 min at 4°C. The supernatant was kept at −70°C for HPLC analysis. The mobile phase preparation for monoamine neurotransmitter determination was as follow: 35.724 g citric acid (85 mM), 16.406 g sodium acetate anhydrous (100 mM), 0.1489 g Na2-EDTA (0.2 mM), and 0.3894 g sodium 1-octanesulfonate (0.9 mM) were dissolved in 1.7 L ultrapure water, at pH 3.7 with concentrated hydrochloric acid (HCl), followed by addition of 300 ml methanol (15%), and then vacuum filtered for later use.
Analysis of samples was performed using a Agilent1100 HPLC work station with a HP 1049 A electrochemical detector (glassycarbon working electrode, voltage+0.70v). The following HPLC conditions were maintained: column, ODS C18 reversed phase chromatographic column (5 μm, 150 × 4.6 mm); mobile phase, citric acid - sodium acetate buffer system (see the mobile phase preparation below); injection volume, 50 μl for monoamine neurotransmitters determination, or 25 μl for amino acid neurotransmitters determination (25 μl sample was diluted 100 fold, 25 μl was pipetted, adding OPA to react for 10 min); column temperature 25°C; flow rate 0.9 ml/min; run time 15 min.
Determination of amino acid neurotransmitters
The levels of dopamine (DA) and 5-HT in hippocampus were determined by HPLC. Double-side hippocampuses were added to 1 ml of 10% sulfosalicylic acid solution, homogenized in ice-bath at 200 rpm for 30 seconds, and centrifuged at 14,000 rpm for 20 min at 4°C. The supernatant was stored at −70°C for HPLC. The mobile phase preparation for amino acid neurotransmitters determination was as follow: 35.724 g citric acid (85 mM), 16.406 g sodium acetate anhydrous (100 mM), 0.1489 g Na2-EDTA (0.2 mM), and 0.39 g sodium 1-octanesulfonate (0.9 mM) were dissolved in 1.8 l ultra pure water, followed by addition of 200 ml methanol (10%) at pH 3.65 with concentrated hydrochloric acid (HCl) and finally vacuum filtered for use.
Statistical analysis
Statistical analysis of data was performed using SPSS 16.0 software and a statistically significant difference was determined at a minimal level of significance of p < 0.05. All results were expressed as mean ± standard error of the mean.
Results and discussion
Particle characterization for BCD-CUR and NLC
BCD-CUR and NLC were successfully prepared by β-cyclodextrin inclusion and ethanol dripping method, respectively, and the particle characteristics determined were shown in .
Table 1. Characteristics of BCD-CUR and NLC.
The mean particle size and zeta potential for β-cyclodextrin inclusion method (BCD-CUR) were 133.49 ± 7.72 nm and −31.76± 0.19 mV, respectively, while those for the NLC were, 121.81 ± 9.78 nm and −7.9 ± 0.11 mV, respectively. The loading capacity for BCD-CUR was 19.73 ± 1.12, which was much higher than that for NLC (4.13 ± 0.06). Therefore, BCD-CUR possessed higher absolute value of zeta potential than that for NLC. The EE% of BCD-CUR was 76.6 ± 1.3, which was lower than that of NLC (88.2 ± 1.2).
Effects of curcumin, BCD-CUR, and NLC on enzyme activity levels of ALT, AST, and LDH in mice after UDMH administration
The changes of enzyme activities of ALT, AST, and LDH in serum of mice after the administration with UDMH alone, or together with curcumin, BCD-CUR and NLC were shown in .
Figure 1. Effect of curcumin, BCD-CURs and NLCs on the enzyme activities of ALT(a), AST(b), LDH(c) in mice blood serum. Results were expressed as mean ± SD, n = 8. a: Values of p < 0.05, b : p < 0.01, in comparison with the UDMH group. c : p < 0.01, in comparison with the control group. d: p < 0.05,in comparison with the 75 mg/kg Curcumin+UDMH group. ALT, alanine transaminase; AST, aspartate aminotransferase; LDH, lactic dehydrogenase; UDMH, unsymmetrical dimethyl hydrazine; BCD-CURs, curcumin-β-cyclodextrin nanoparticle; NLCs, nanoliposomal curcumin.
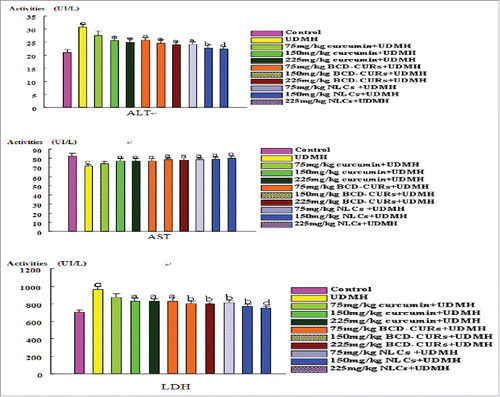
Compared with NS control, I.P. administration of UDMH alone resulted in higher levels of ALT and LDH but lower level of AST. In general, the changes in the levels of ALT, AST, and LDH reflect the changes of liver function and ALT is taken as the gold standard for liver injury evaluation. UDMH increased levels of ALT and LDH, and decreased levels of AST, indicating that UDMH exerts adverse effects on liver function, which was consistent with a previous observation that mild fatty liver occurs frequently in occupation workers who often come in contact with UDMH.Citation6 75 mg/kg curcumin dosing had no obvious protective effects on UDMH (p > 0.05), but both 150 mg/kg and 225 mg/kg curcumin dosing had significant protective effects (p < 0.05). 150 mg/kg curcumin dosing significantly reduced (p < 0.05) the levels of ALT (25.62 ± 1.29 IU/L versus 30.63 ± 1.35 IU/L), LDH (830.75 ± 32.84 IU/L vs. 962.55 ± 33.06 IU/L), and increased the level of AST (76.62 ± 3.51 IU/L versus 71.07 ± 2.10 IU/L) in the serum. 225 mg/kg curcumin dosing seemed to have better effect than 150 mg/kg curcumin doing did, but there was no statistical difference between the 2 higher dosages. These results suggested that the protective effects of curcumin increased with increasing the doses, but when the quantity of curcumin reached some extent, its protective effects was saturated. This result was in agreement with the earlier reports,Citation31-33 perhaps because of its low water solubility and low systematical bioavailability. However, all dosages of BCD-CUR or NLC displayed strong protective effects. 150 mg/kg BCD-CUR decreased remarkably the levels of ALT to 24.58 ± 1.24 IU/L and that of LDH to 801.46 ± 25.71 IU/L, but increased the level of AST to 78.21 ± 2.18 IU/L. 150 mg/kg NLC decreased the levels of ALT further to 22.74 ± 1.21 IU/L and that of LDH further to 769.76 ± 21.59 IU/L (p < 0.01), and increased the level of AST up to 79.05 ± 2.32 IU/L. With increasing doses of BCD-CUR or NLC, the protective effects seemed to increase in parallel, which was likely related to the improvement in the BCD-CUR or NLC, compared with curcumin suspension.Citation19 In fact, 150 or 225 mg/kg NLC dosing showed significant efficiency in the decrease of ALT levels (p < 0.01). In terms of the protective effects against UDMH induced liver injury, NLC had better effects than BCD-CUR, which suggested that NLC had better drug potency.
Effect of curcumin, BCD-CUR and NLC on amino acid neurotransmitter levels in mice after I.P. administration of UDMH
As shown in , UDMH administration decreased sharply the level of GABA (251.7 ± 6.4 ng/g vs. 364.6 ± 16.8 ng/g), while increased markedly the levels of Glu, DA and 5-HT, as compared with the NS control group. Whereas GABA and Glu are important amino acid neurotransmitters, DA and 5-HT are important monoamine neurotransmitters. Convulsion resulting from UDMH-induced central nervous system injury was closely relative to the decrease of GABA and increase of Glu and DA.Citation30 Since curcumin has therapeutic effect in a small number of neurodegenerative diseases, it could protect nerve cells in hippocampus after cerebral ischemia reperfusion injury.Citation34 In this study, 75 mg/kg curcumin had no significant effects, but 150 mg/kg curcumin significantly prevented the decrease of GABA in the hippocampus caused by UDMH administration, and 225 mg/kg curcumin had better protective effects. Both levels of GABA were significantly different (p < 0.05) from that the UDMH group.
Table 2. Effect of curcumin, BCD-CUR and NLC on the levels of Glu and GABA in hippocampus of mice.
However, all BCD-CUR groups and NLC groups had significant protective effects (p < 0.01) against UDMH induced injury. The most effective administrations were higher dosage of BCD-CUR groups (150 mg/kg and 225 mg/kg) (p < 0.05) and all the NLC groups (p < 0.01). 150 mg/kg BCD-CUR recovered the level of GABA to 304.7 ± 17.7 ng/g (p < 0.01) and decreased Glu levels to 2108 ± 34 ng/g (p < 0.05), while 150 mg/kg NLC recovered GABA further to 324.1 ± 13.8 ng/g (p < 0.01) and decreased Glu further to 2045 ± 33 ng/g (p < 0.01) and 5-HT to 0.83 ± 0.01 ng/g (p < 0.01). This result showed that BCD-CUR or NLC had better protective effects than curcumin suspension, and the performance of NLC was more than excellent.
Effect of curcumin, BCD-CUR and NLC on monoamine neurotransmitter levels in mice after I.P. administration of UDMH
As shown in . The administration of UDMH significantly increased the levels of DA and 5-HT as compared with NS group (6.15 ± 0.31 ng/g versus 4.11 ± 0.23 ng/g for DA, and 1.05 ± 0.02 ng/g vs. 0.59 ± 0.03 ng/g for 5-HT). Adding curcumin or BCD-CUR to the UDMH administration had no significant effects on the level of DA, while adding NLC to the UDMH administration did (5.64 ± 0.25 ng/g, 5.51 ± 0.19 ng/g, and 5.49 ± 0.21 ng/g, respectively, p < 0.05). The adding of 150 mg/kg and 225 mg/kg BCD-CUR into UDMH administration significantly reduced the increase of 5-HT induced by UDMH, however, there were no statistical differences between these 2 BCD-CUR doses, suggesting that the protective effects did not increase after the dose of BCD-CUR reached 150 mg/kg. The UDMH induced increase of DA and 5-HT were significantly reduced by 150 mg/kg NLC, to 5.51 ± 0.19 ng/g (p < 0.05) and 0.83 ± 0.01 ng/g (p < 0.01), respectively. Adding various doses of curcumin or BCD-CUR into UDMH had no obvious recovery effects on DA, while adding all doses of NLC into UDMH significantly recovered the level of DA (p < 0.05). Therefore, as shown in , the protective effects of NLC, BCD-CUR, and curcumin suspension when prioritized are: NLC > BCD-CUR > curcumin suspension with marked dosage-effects. The group of Thiyagarajan and Sharma reported that the neuroprotective and anti-oxidant effect of curcumin was dosage- dependent only when intraperitoneal dosing reached 200∼300 mg/kg.Citation19,35-37 Better protective effects of the BCD-CUR or NLC than the curcumin suspension may be attributed to their greater oral absorbility and permeability through the blood brain barrier, higher concentration time curve (AUC0–∞),Citation24,26 and targeting capacity of liposome.Citation10,25 But excessive dosing might have side-effects on the neurons, which needs further investigation.
Table 3. Effect of curcumin, BCD-CUR and NLC on the levels of DA and 5-HT in the brain striatum.
Conclusion
UDMH resulted in increased monoamine neurotransmitter levels of DA and 5-HT, decreased the level of the amino acid neurotransmitter GABA, and increased level of Glu. Curcumin, BCD-CUR and NLC had notable protective effects on UDMH induced poisoning in mice. Among the 3 formulations, NLC had by far the greatest effect, and BCD-CUR was superior to curcumin suspension. This effect was likely related to their differences of these 3 formulations in oral absorbability, bioavailability and permeability through the blood brain barrier. This study shows that utilizing NLC is a promising approach as a functional food additive against UDMH poisoning.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by a grant from the Research and Development Foundation of Wuhan Economic College, China (RDFEC 2011021), Doctoral Scientific Research Foundation of Hubei University of Technology (337/383), Foundation of Hubei Province (2015CFB678), Young Talent Project Hubei Provincial Education Department (Q20151412) and Startup, Foundation for Doctors of Hubei University of Technology (Grant No. BSQD10036).
References
- Li W, Hu J, Zheng ZJ. Effects of melatonin on neurotransmitter levels caused by 1- 1-dimethylhydrazine in mouse brain. Acta Nutrimenta Sinica 2012; 34:2.189-92.
- Sridhar S, Susheela G, Jayasimha G. Crosslinked chitosan membranes: characterization and study of dimethylhydrazine dehydration by pervaporation. Polym Int 2011; 50.1156-61 http://dx.doi.org/10.1002/pi.761
- Belysheva TV, Kazachkov EA, Bogovtseva LP. Electrophysical properties of In2O3 and WO3-based gas-sensitive semiconducting films as sensors for unsymmetrical dimethylhydrazine in air. J Anal Chem 2006; 61(7):683-6; http://dx.doi.org/10.1134/S1061934806070148
- Bauer RM, Tarr MJ, Olsen RG. Effect of 1, 1-Dimethylhydrazine on lymphoproliforation and interleukin 2 immunoregulatory function. Arch Environ Contam Toxicol 1990; 19(1):148-53; PMID:2331149; http://dx.doi.org/10.1007/BF01059824
- Savelev YV, Veselov VY, Robota LP. Utilization of unsymmetrical dimethylhydrazine in the production of polyurethanes: synthesis, structure, and properties of new products. Russ J Appl Chem 2006; 79(4):665-71; http://dx.doi.org/10.1134/S1070427206040318
- Kolumbaeva SZ, Shalakhmetova TM, Begimbetova DA. Mutagenic effect of the rocket fuel component unsymmetrical dimethylhydrazine on rats of different age. Russ J Genet 2007; 43(6):608-12; http://dx.doi.org/10.1134/S1022795407060038
- Shamil C, Vladimir A, Lioudmila A. Gas chromatography-mass spectrometric determination of unsymdimethylhydrazine in soil and water by derivatization with aromatic aldehydes. Can J Chem Eng 1998; l(76):680-4.
- Roe FJ, Grant GA, Millican DM. Carcinogenicity of hydrazine and 1,1-Dimethylhydrazine for mouse lung. Nature(London) 1967; 216:375-6; http://dx.doi.org/10.1038/216375a0
- Zhang YL, Han JQ, Lu CH. Extract ion and antiseptic effect of curcumin on foods. Trans IonsChinese Agr Eng 2005; 21(2):144-8.
- Purusotam B, Haider H, Ingunn T. Liposomal delivery system enhances anti-inflammatory properties of curcumin. J Pharma Sci 2012; 101:2. 598-609; PMID:21989712; http://dx.doi.org/10.1002/jps.22785
- Luo C, Li Y, Zhou B. A monocarbonyl analogue of curcumin, 1,5-bis(3-hydroxyphenyl)-1,4-pentadiene-3-one (Ca 37). Exhibits potent growth suppressive activity and enhances the inhibitory effect of curcumin on human prostate cancer cells. Apoptosis 2014; 19:542-53.
- Wu B, Li XZ, Yang GE. Research progress in antioxidant property and antioxidant mechanism of curcumin. Acta AgrJiangxi 2009; 21(1):121-3.
- Tuba A, Tihami G. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact 2008; 174(1):27-37; PMID:18547552; http://dx.doi.org/10.1016/j.cbi.2008.05.003
- Reddy AC, Lokesh BR. Effect of dietary turmeric (Curcuma longa) on iron - induced lip id peroxidation in the rat liver. Food Chem Toxicol 1994; 32(3):279-83; PMID:8157223; http://dx.doi.org/10.1016/0278-6915(94)90201-1
- Jayaprakasha GK, Jaganmohan RL, Sakariah KK. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem 2006; 98:720-4; http://dx.doi.org/10.1016/j.foodchem.2005.06.037
- Kazim S, Cemal O, Mehmet T. Comparative in vivo evaluations of curcumin and its analog difluorinated curcumin against cisplatin-induced nephrotoxicity. Biol Trace Elem Res 2014; (157):156-63; PMID:24415068; http://dx.doi.org/10.1007/s12011-014-9886-x
- Cheng L, Yan L, Bo B. A monocarbonyl analogue of curcumin, 1,5-bis(3-hydroxyphenyl)-1,4-pentadiene-3-one (Ca 37), exhibits potent growth suppressive activity and enhances the inhibitory effect of curcumin on human prostate cancer cells. Apoptosis 2014; 19:542-53; PMID:24297639; http://dx.doi.org/10.1007/s10495-013-0947-y
- Bisht S, Feldman G, Soni S. Polymeric nanoparticle-encapsulated curcumin(“nanocurcumin”): A novel strategy for human cancer therapy. J Nanobiotechonol 2007; 5:1-18; http://dx.doi.org/10.1186/1477-3155-5-1
- Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Epilepsy Res 1999; 35:183-9; PMID:10413314; http://dx.doi.org/10.1016/S0920-1211(99)00013-3
- Liu ZF, Ming C, Jiang W. Enhancement of curcumin oral absorption and pharmacokinetics ofcurcuminoids and curcumin metabolites in mice. Cancer Chemother Pharmacol 2012; 69:679-89; PMID:21968952; http://dx.doi.org/10.1007/s00280-011-1749-y
- Kurien BT, Souza AD, Scofield RH. Heat-solubilized curry spice curcumin inhibits antibody–antigen interaction in in vitro studies: A possible therapy to alleviate autoimmune disorders. Mol Nutr Food Res 2010; 54:1202-9; PMID:20146265
- Hu J, Li W, Li DY, Eliminating effects of vitamin B6 and vitamin C on UDMH and their chemical mechanism. Adv Mater Res 2012; 3(47)344-8.
- Cao YP, Li J, Cheng W. Investigation of effects of several kinds of ultrasound on the extract ion yield of curcum in and total flavanone from curcum. Chinese Sci Technol Food Ind 2008; 29(1):131-4.
- Min F, Yin J, Wei B. In vitro characterization and in vivo evaluation of nanostructured lipid curcumin carriers for intragastric administration. Int J Nanomed 2012; 7:5395-404; PMID:23091382
- Yang RL, Zhang S, Kong DL. Biodegradable polymer-curcumin conjugate micelles enhance the loading and delivery of low-potency curcumin. Pharm Res 2012; 39:3512-25.
- Marczylo TH, Verschoyle RD, Cooke DN. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol 2007; 60:171-7; PMID:17051370; http://dx.doi.org/10.1007/s00280-006-0355-x
- Sahin K, Orhan K, Tuzcu M. Comparative in vivo evaluations of curcumin and its analog difluornate2 d iCurcumin against cisplatin-induced nephrotoxicity. Biol Trace Elem Res 2014; 157:156-63; PMID:24415068; http://dx.doi.org/10.1007/s12011-014-9886-x
- Heni R, Citra AE, Rachmat M. Molecular inclusion complex of curcumin–β-cyclodextrin nanoparticle to enhance curcumin skin permeability from hydrophilic matrix gel. AAPS PharmSciTech 2013; 14(4)1303-12.
- Marczylo TH, Verschoyle RD, Cooke DN. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol 2007; 60:171-7; PMID:17051370; http://dx.doi.org/10.1007/s00280-006-0355-x
- Zou HB, Ding W, Zhou G. Synthesis of dinitrophenyl-hydrazine derivative from curcumin in and preevaluation of its activity. J Southwest Agr Univ 2006; 28(1):58-65.
- Lin H, Xu J, Wen C. Protective effect of curcumin solid-dispersity on experimental chronic liver injury in mice. Chinese Herbs Pharmacol Clinic 2007; 23(2):26-30.
- Fan CM, Liu XW. Study on the stability of water-soluble curcuminpigment. China Condiment 2012; 37(3):108-10.
- Hsu CH, Cheng AL. Clinical studies with curcumin. AdvExp Med Biol 2007; 595:471-80; PMID:17569225; http://dx.doi.org/10.1007/978-0-387-46401-5_21
- Wu JY, Zhang CL, Li J. Effect of curcumin on the expression of Bax and bcl-2 during cerebral ischemia in rat hippocampus. ACTA Academiae Medicame XuZhou 2007; 27(11):707-9.
- Shaik JD, Ankola D, Beniwal V. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci 2009; 37:223-30; http://dx.doi.org/10.1016/j.ejps.2009.02.019
- Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Meta Dispos 1999; 27:486-94; PMID:10101144
- Vareed SK, Kakarala M, Ruffin MT. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev 2008; 7:1411-7; http://dx.doi.org/10.1158/1055-9965.EPI-07-2693
