ABSTRACT
The objective of this study was to comparatively evaluate 3 different sinus lift tools, namely umbrella-shaped sinus lift curette YSL-04, our recently designed probe-improved sinus lift curettes, and our newly invented elevator 014, using our previous developed goat ex vivo models for direct visualizing the effectiveness of detaching sinus mucosa in real time. Goat ex vivo models for direct visualizing the effectiveness of detaching sinus mucosa in real time were generated according to our previously developed protocol. The effectiveness for each tool was evaluated through the length of sinus mucosa detached in mesial and distal directions or buccal and palatal directions, and the space volume created by detaching maxillary sinus mucosa in mesial, distal, buccal and palatal directions. The results showed that all 3 sinus lift tools could transcrestally detach the maxillary sinus mucosa and create extra space under the elevated sinus floor on the goat ex vivo sinus models. Moreover, our newly invented elevator 014 had advantages over the other 2 in term of the capability to detach the sinus mucosa. Our newly invented elevator 014 might be a promising tool for detaching maxillary sinus mucosa in transcrestal maxillary sinus floor elevation.
Introduction
Summers first proposed osteotome sinus floor elevation (OSFE), i.e. to elevate the maxillary sinus floor via the transcrestal approach using osteotome.Citation1,2 The advantages for this procedure over conventional lateral maxillary sinus lift include fewer traumas, simple and less invasive operation, and less postoperative discomfort. However, OSFE procedure has higher risk of maxillary sinus membrane perforations than lateral maxillary sinus lift due to visual limitations.Citation3 The small sized access hole prepared in OSFE procedure restrains the scale of detached sinus mucosa. Thus, the height achieved for elevated sinus floor is limited in order to avoid too much focused local stress to sinus mucosa.
To overcome the limitations of Summers' OSFE, various derivatives have been proposed and used to develop sinus lift tools for transcrestal sinus floor elevation.Citation4-15 The improved sinus lift tools for detaching the maxillary sinus mucosa could be classified into 2 types: rigid tools such as umbrella-shaped sinus lift curette YSL-04, and elastic tools such as balloons. Mazor et al.Citation16 gently detach maxillary sinus mucosa using balloons via the uniform pressure generated by injecting water into the balloon, which significantly reduced the perforation possibility for maxillary sinus mucosa and increased the height of elevated sinus floor. Chen et al.Citation17 utilized hydraulic pressure and pliable bone graft mixture to gently detach the soft tissue of sinus mucosa from sinus bone without the perforation risk. No perforation was observed due to the gentle pressure applied by hydraulic pressure and condensed grafting mixture instead of invading surgical instruments. Among the 1,557 implants, only 8 failures were observed during the 8-year study period.
Nevertheless, it has been well-known that special attention should be paid when maxillary sinus floor has to be elevated for more than 5 mm, and current techniques for transcrestal sinus floor elevation might not be suitable for those cases with severely insufficient heights of residual alveolar bones.Citation18-20 Moreover, the risk of perforation increases as larger tension is generated to the sinus mucosa during sinus floor elevation. Studies on dead heads showed that the rate for perforation of sinus mucosa was 24% when OSFE was used to elevate the sinus floor for a height of 4–8 mm.Citation21 Among these, 50% perforations were due to the continuous tension from the erupted mucosa. When perforation occurs, grafting materials could leak into the maxillary sinus if they are used to graft the elevated sinus floor. Thus, infection could happen in the maxillary sinus and even affect the healing around the implant, increasing the risk for the implant to fall off. Therefore, efforts are needed to resolve these challenges by improving the sinus lift tools and techniques.
In this study, we evaluated 3 tools for transcrestal sinus elevation using goat ex vivo sinus models. These tools were umbrella-shaped sinus lift curette YSL-04, our recently designed probe-improved sinus lift curettes, and our newly invented elevator 014. The effectiveness for each tool was evaluated based on the length of sinus mucosa detached and the space volume created after maxillary sinus lift. The results showed that all 3 sinus lift tools could transcrestally detach the maxillary sinus mucosa to some extents and create extra space under the elevated sinus floor. Moreover, our newly designed elevator 014 had apparent advantages over the other 2 in term of the capability to detach the sinus mucosa due to its great elasticity and shape-memory. This elevator 014 might be a promising tool for detaching maxillary sinus mucosa in the transcrestal maxillary sinus floor elevation.
Materials and methods
The tools and instruments used included bone saw (Tianjin Yutong Medical Instruments, China), umbrella-shaped sinus lift curette (model #: YSL-04, MTC, Korea, ), osteotome instruments (Kelor, Germany), NewTom QR-DVT9000 computed tomography (CT), CV MM-II portable dental micromotor (Fushan, China), emery grinding needle (Sharp peak diamond abrasives company, China), probe-improved sinus lift curettes (designed and fabricated by the corresponding author, ), shape-memory Ni/Ti alloy wire containing tube elevator (elevator 014) invented and fabricated by the corresponding authors (International patent application: PCT/CN2015/089842) (),Citation4 MNT-M070 hammer (Shanghai, China), extraction forceps (Weirong, China), and ruler.
Figure 1. (A) The umbrella-shaped sinus lift curette YSL-04 (MCT company). Inserted images showed the tips; (B) The probe-improved sinus lift curettes (designed and fabricated by the corresponding author of this paper). Inserted images showed the tips (b1 and b2 were used for detaching sinus mucosa in mesiodistal directions; b3 and b4 were used for detaching sinus mucosa in buccal-palatal directions); (C) The shape-memory Ni/Ti alloy wire containing tube elevator (elevator 014, invented and fabricated by the corresponding author of this paper) showing the primary detaching knife (the part between the 2 red lines in the picture).
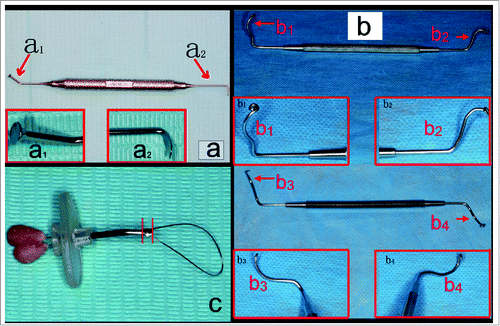
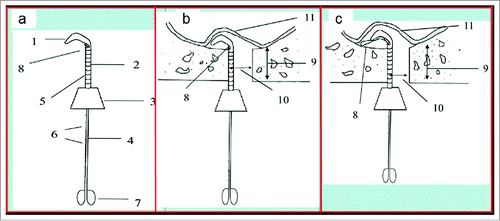
Figure 2. (A) The sketch of elevator 014 showing the main components (1: primary detaching knife; 2: tube; 3: holder; 4: spring wire; 5: scale; 6: scales; 7: stopper; 8: shape-memory Ni/Ti alloy wire.); (B) The sketch of primary detaching (10: implant hole or transcrestal access; 11: maxillary sinus mucosa.); and (C) The sketch of secondary detaching (9: residual alveolar bone).
Seventy one 1.5–2 y old goats (male or female, 20–30 kg weight) were purchased from the Animal Experimental Center at the First Affiliated Hospital of PLA General Hospital. They were healthy with fully developed maxilla and without maxillary sinus diseases. They were housed for one week before being sacrificed for the experiments. The protocol was approved by Institutional Animal Care and Use Committees.
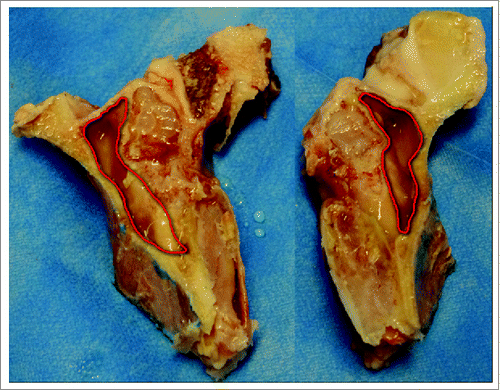
The goats were sacrificed in the animal laboratory to obtain the entire heads. The heads were cleaned, and then scanned to examine and locate the maxillary sinus floors. After CT scans, the maxillofacial soft tissues were separated from the hard tissues. The mandibles were removed using bone saw. Four mark lines were drawn on each goat skull according to our previously developed protocol (manuscript submitted). Cuts were made along these marking lines using bone saw to obtain the primary goat ex vivo models (). The maxillary pre-molar or molar root was extracted from the primary model, and a 4 mm transcrestal hole was made as the transcrestal access to the maxillary sinus floor through the standard clinical steps used in OSFE. The resulting models for real-time direct visualization of detaching sinus mucosa during sinus floor elevation were preserved in saline at 4°C and used within 24 hours for the following experiments.
The resulting 142 ex vivo goat sinus models (each goat provided 2 sinus models) were randomly divided into 9 groups: group A (18 models) for detaching the sinus mucosa in mesial and distal directions using umbrella-shaped sinus lift curette (); group B (18 models) for detaching the sinus mucosa in buccal direction and palatal directions using umbrella-shaped sinus lift curette; group C (11 models) for detaching the sinus mucosa in both mesiodistal direction and buccal-palatal direction using umbrella-shaped sinus lift curette; group D (18 models) for detaching the sinus mucosa in mesial and distal directions using probe-improved sinus lift curette with tips b1 and b2(); group E (18 models) for detaching the sinus mucosa in buccal direction and palatal direction using probe-improved sinus lift curette with tips b3 and b4; and group F (11 models) for detaching the sinus mucosa in both mesiodistal direction and buccal-palatal direction using both probe-improved sinus lift curettes; group G (18 models) for detaching the sinus mucosa in mesial and distal directions using elevator 014 (); group H (18 models) for detaching the sinus mucosa in buccal direction and palatal direction using elevator 014; and group I (11 models) for detaching the sinus mucosa in both mesiodistal direction and buccal-palatal direction using elevator 014.
In particular, the sinus mucosa in groups A and B was detached according to group assignment using umbrella-shaped sinus lift curette YSL-04 through the transcrestal access (i.e., implant socket) prepared on the maxilla. The operation of detaching sinus mucosa was stopped when the curette could not be further forced along the particular directions through the implant socket. In group A, the lengths of the detached mucosa were measured in the mesial direction and distal direction. In group B, the lengths of the detached mucosa were measured in the buccal direction and palatal direction. In group C, the mucosa (n = 11) was mesiodistally, buccally and palatally detached using the umbrella-shaped sinus lift curette to visualize the space volumes created after sinus lift. The operation of detaching sinus mucosa was stopped when the curette could not be further forced through the implant socket.
The sinus mucosa in groups D and E was detached according to group assignment using probe-improved sinus lift curettes through transcrestal access (i.e. implant socket) prepared on the maxilla. The operation of detaching sinus mucosa was stopped when the curette could not be further forced along the particular directions through the implant socket. In group D, the lengths of the detached mucosa were measured in the mesial direction and distal direction. In group E, the lengths of the detached mucosa were measured in the buccal direction and palatal direction. In group F, the mucosa (n = 11) was mesiodistally, buccally and palatally detached using the probe-improved sinus lift curettes to visualize the space volumes created after sinus lift. The operation of detaching sinus mucosa was stopped when the curette could not be further forced through the implant socket.
To detach sinus mucosa in groups G and H, the sinus mucosa was initially detached using the primary detaching knife in elevator 014 () in the desired directions according to the group assignment, followed by detaching the sinus mucosa in the directions using the shape-memory wire in elevator 014 (). The wire was used to detach the sinus mucosa via the movements of protruding, followed by rotation and turnover. During the detaching, the most front end of the wire was ensured to be closely against the sinus floor bone. The operation was gently performed to avoid perforation to the sinus mucosa during the detaching. When the most front end of the wire reached the farthest position or the mucosa was perforated, the length of the detached sinus mucosa in that particular direction was recorded after the detaching operation was stopped. In group I, the mucosa (n = 11) was mesiodistally, buccally and palatally detached using elevator 014. The operation of detaching sinus mucosa was stopped when the most front end of the wire reached the farthest position in every direction or the mucosa was perforated.
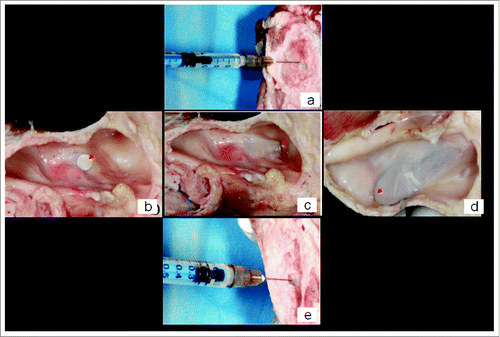
Before and after detaching sinus mucosa in groups C, F and I, saline was injected into the space under the sinus mucosa () to obtain the space volume under the initial sinus mucosa (V1) and the space volume under the elevated sinus mucosa (V2). The space volume created by transcrestal sinus lift was calculated by subtracting V1 from V2.
Figure 4. The determination of space volume created under the elevated maxillary sinus mucosa: (A) adding saline into the space before detaching maxillary sinus mucosa through the implant socket; (B) detaching maxillary sinus mucosa ex vivo using umbrella-shaped sinus lift curette YSL-04 with the tip of YSL-04 being seen under the sinus mucosa (red arrow); (C) detaching maxillary sinus mucosa ex vivo using probe-improved sinus lift curette with the tip being seen under the sinus mucosa (red arrow); (D) detaching maxillary sinus mucosa ex vivo using elevator 014 with the shape-memory Ni/Ti alloy wire being seen under the sinus mucosa (red arrow); (E) adding saline into the space after detaching maxillary sinus mucosa through the implant socket.
All the detaching operations were conducted firmly, evenly, and gently by the same surgeon. The data were reported as mean ± standard deviation and analyzed by the commercially available statistics software SPSS 17.0. p < 0.05 indicated significant differences.
Results and discussion
We have recently established goat ex vivo sinus models for direct visualization of transcrestal detaching maxillary sinus mucosa in real time (Manuscript submitted). In this study, we used goat ex vivo models () to comparatively evaluate 3 sinus lift tools shown in for detaching goat maxillary sinus mucosa. In particular, in groups A, B, D, E, G and H, the sinus mucosa was detached in the mesial and distal directions or buccal and palatal directions, followed by measurement of the length of sinus mucosa detached. In groups C, F and I, the space volume created under the detached sinus mucosa was determined () after the sinus mucosa was detached in the mesial, distal, buccal and palatal directions. The tool used could be directly observed under the sinus mucosa during the detaching operation ().
summarized the results for detaching goat maxillary sinus mucosa ex vivo. YSl-04 could detach the goat maxillary sinus mucosa for 3.7 ± 2 .1 mm in the mesial or distal direction, and 2.8 ± 2.1 mm in the buccal or palatal direction. Our recently designed probe-improved sinus lift tools could detach the goat maxillary sinus mucosa for 5.6 ± 2 .5 mm in the mesial or distal direction, and 5.1 ± 3 .1 mm in the buccal or palatal direction. The elevator 014 could detach the goat maxillary sinus mucosa for 22.9 ± 2 .5 mm in the mesial or distal direction, and 17.4 ± 4 .3 mm in the buccal or palatal direction. An average space volume of 52.7 ± 20 .2 µl was created after detaching goat maxillay sinus mucosa in both mesial/distal direction and buccal/palatal direction using YSL-04; the average space volume created using our recently designed probe-improved sinus lift tools was 83.6 ± 32 .6 µl; while the average space volume created using elevator 014 was 1.8764 ± 0 .2366 ml.
Table 1. The efficacy of ex vivo detaching goat maxillary sinus mucosa for 3 different sinus lift tools. Data were reported as mean ± standard deviation.
When the 3 tools were used to detach the sinus mucosa in mesial/distal directions, we found significant differences among groups A, D and G in the length of sinus mucosa detached (p<0.01). The data in each group fitted the normal distribution. Our probe-improved sinus lift curette with tips b1 and b2 could detach maxillary sinus mucosa 0.5 times longer than umbrella-shaped sinus lift curette YSL-04 in mesial or distal direction; while elevator 014 could make the detachment 5 times longer than umbrella-shaped sinus lift curette YSL-04.
When the 3 tools were used to detach the sinus mucosa in buccal/palatal directions, we found significant differences among groups B, E and H in the length of sinus mucosa detached (p<0.01). The data in each group fitted the normal distribution. Our probe-improved sinus lift curette with tips b3 and b4 could detach maxillary sinus mucosa 0.8 times longer than umbrella-shaped sinus lift curette YSL-04 in buccal or palatal direction; while elevator 014 could make the detachment 5 times longer than umbrella-shaped sinus lift curette YSL-04.
When the 3 tools were used to detach the sinus mucosa in both mesiodistal and buccal-palatal directions, we found significant differences among groups C, F and I in the space volume created under the elevated sinus mucosa (p < 0.001). The data in each group fitted the normal distribution. Our probe-improved sinus lift curettes could create a space volume 0.5 times larger than umbrella-shaped sinus lift curette YSL-04 under the elevated sinus floor; while elevator 014 could create a more than 34 times larger volume than umbrella-shaped sinus lift curette YSL-04.
Great efforts have been taken to improve existing sinus lift tools or invent new sinus lift tools to achieve optimized clinical efficacy.Citation4-6,22 The length of maxillary sinus mucosa detached has been used for evaluating the effectiveness of a sinus lift tool. The transcrestal access made to the maxillary sinus floor is generally limited by the size of the implant hole prepared in the maxilla. Although umbrella-shaped sinus lift curettes similar to YSL-04 are frequently used in the clinics to detach maxillary sinus mucosa, our results showed that YSL-04 had limited detaching range as demonstrated in the length of sinus mucosa detached (). This tool was even more inefficient to detach maxillary sinus mucosa on a highly sloped sinus floor (i.e., buccal-palatal directions). Thus, this type of tools could only be used at the very beginning.
Therefore, we designed 2-piece probe-improved sinus lift curettes from metal probes used in the clinics by bending, grinding and polishing (). Each piece had 2 working tips as showed in . One piece was specially designed to meet the low slope of 0° to 30° when detaching maxillary sinus mucosa in the mesial/distal direction, while the second was specially designed to meet the high slope of 60° to 90° when detaching maxillary sinus mucosa in the buccal/palatal direction. Both had a long and complicated bent working tip at each end (). These special structures were able to move along the sloped sinus bone floor to detach the sinus mucosa. However, the length of sinus mucosa detached using them was not significantly increased compared to umbrella-shaped sinus lift curette YSL-04 (). Indeed, there was no significant difference between groups A and D, or between groups B and E in the average length of sinus mucosa detached. Moreover, no significant difference was observed in the space volumes created in group C and F. In the clinical practice, a space volume of 0.67 ± 0.17 ml has been created using the inflatable balloon technique via the transcrestal approach to elevate the maxillary sinus floor.Citation12 Pommer et al. was able to produce a space volume of 0.9–3.1 ml under the lifted sinus mucosa in fresh human skulls using the gel-pressure technique via the transcrestal approach.Citation8 Although it would be impossible to compare our results with those reported in the literatures due to the differences between human beings and goats, the space volume created in groups C and F should not be enough to provide sufficient bone argumentation for stable dental implantation.
As demonstrated in this study, it is difficult to detach the sinus mucosa to a large scale using rigid tools such as the umbrella-shaped sinus lift curette like YSL-04, and probe-improved sinus lift curettes, thus limiting the volume generated and the height of the elevated sinus mucosa. In general, it has been recognized that transcrestal maxillary sinus floor elevation using direct contacting tools is limited to those cases which need only to lift the sinus floor for a height of 3 mm or less due to safety issues. However, if better direct contacting sinus lift tools could be used to detach the maxillary sinus to much larger scales, this height limit could be lifted further. Thus more clinical cases could be treated using the transcrestal approach to elevate the maxillary sinus floor using direct contacting tools.
We designed shape-memory Ni/Ti alloy wire containing tube elevator consisted of primary detaching tool and secondary detaching tool as shown in . Primary detaching tool was composed of tube and detaching knife at the front end, while the secondary detaching tool was a shape-memory Ni/Ti alloy wire with a diameter of 0.014 inch. The primary detaching tool was able to detach the sinus mucosa right around the implant hole to open some gaps between the sinus bone and the sinus mucosa (). The secondary tool would be used to further detach sinus mucosa far away from the implant hole after some gaps were created around the implant hole using the primary tool (). This wire could detach the sinus mucosa via the movements of protruding, rotation and turnover, thus extending the detached sinus mucosa to a much larger scale. The length of sinus mucosa detached with elevator 014 was significantly increased in both mesiodistal and buccal-palatal directions (). Meanwhile, much larger space volume could be easily made by using elevator 014 to detach the sinus mucosa ex vivo in goat. Unlike the rigid tips in umbrella-shaped sinus lift curette and probe-improved sinus lift curette, the elastic and shape memory Ni/Ti alloy wire avoided the high risk of perforation to sinus mucosa. Therefore, elevator 014 had the capability of adopting the slope of sinus floor due to its excellent elasticity and shape-memory.
Conclusions
All three sinus lift tools could detach the maxillary sinus mucosa to different extents and create extra space under the elevated sinus floor on goat ex vivo sinus models. Moreover, elevator containing shape-memory Ni/Ti alloy wire with a diameter of 0.014 inch had advantages over the other 2 tools for the capability to detach maxillary sinus mucosa. The newly invented elevator 014 might be a promising tool for detaching maxillary sinus mucosa in transcrestal maxillary sinus floor elevation.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This study was funded by the Clinical Support of PLA General Hospital (2014FC-SXYY-1003) and the Capital's Special Health Development Research (2014-4-5022).
References
- Summers RB. A new concept in maxillary implant surgery: The osteotome technique. Compendium (Newtown, Pa) 1994; 15:152, 154–156, 158 passim; quiz 162; PMID:8055503
- Emmerich D, Att W, Stappert C. Sinus floor elevation using osteotomes: A systematic review and meta-analysis. J Periodontol 2005; 76:1237-51; PMID:16101354; https://doi.org/10.1902/jop.2005.76.8.1237
- Garbacea A, Lozada JL, Church CA, Al-Ardah AJ, Seiberling KA, Naylor WP, Chen JW. The incidence of maxillary sinus membrane perforation during endoscopically assessed crestal sinus floor elevation: A pilot study. J Oral Implantol 2012; 38:345-59; PMID:22913307; https://doi.org/10.1563/AAID-JOI-D-12-00083
- Li Y. A shape-memory alloy kit for lifting of mucous membrane of a maxillary sinus. Int Patent App 2015; Application number: PCT/CN2015/089842
- Schwarz L, Unger E, Watzek G, Pommer B. Novel devices to prevent membrane perforation in transcrestal sinus floor augmentation surgery Recent Patents on Biomedical Engineering 2013; 6:179-87.
- Petruzzi M, Ceccarelli R, Testori T, Grassi FR. Sinus floor augmentation with a hydropneumatic technique: A retrospective study in 40 patients. Int J Periodontics Restorative Dentistry 2012; 32:205-10; PMID:22292150
- Trombelli L, Minenna P, Franceschetti G, Minenna L, Farina R. Transcrestal sinus floor elevation with a minimally invasive technique. J Periodontol 2010; 81:158-66; PMID:20059428; https://doi.org/10.1902/jop.2009.090275
- Pommer B, Watzek G. Gel-pressure technique for flapless transcrestal maxillary sinus floor elevation: A preliminary cadaveric study of a new surgical technique. Int J Oral Maxillofacial Implants 2009; 24:817-22; PMID:19865621
- Sohn DS, Lee JS, An KM, Choi BJ. Piezoelectric internal sinus elevation (pise) technique: A new method for internal sinus elevation. Implant Dentistry 2009; 18:458-63; PMID:20009598; https://doi.org/10.1097/ID.0b013e3181b8e17f
- Ucer C. Nasal suction technique for maxillary sinus floor elevation: A report of 24 consecutive patients. Int J Oral Maxillofacial Implants 2009; 24:1138-43; PMID:20162120
- Stelzle F, Rohde M. Elevation forces and resilience of the sinus membrane during sinus floor elevation: Preliminary measurements using a balloon method on ex vivo pig heads. Int J Oral Maxillofacial Implants 2014; 29:550-7; PMID:24818193; https://doi.org/10.11607/jomi.3069
- Hu X, Lin Y, Metzmacher AR, Zhang Y. Sinus membrane lift using a water balloon followed by bone grafting and implant placement: A 28-case report. Int J Prosthodontics 2009; 22:243-247; PMID:19548405
- Tilotta F, Lazaroo B, Gaudy JF. Gradual and safe technique for sinus floor elevation using trephines and osteotomes with stops: A cadaveric anatomic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 2008; 106:210-6; https://doi.org/10.1016/j.tripleo.2007.12.030
- Lopez MA, Bassi MA, Confalone L, Carinci F. Maxillary sinus floor elevation via crestal approach: The evolution of the hydraulic pressure technique. J Craniofacial Surg 2014; 25:e127-32; PMID:24448532; https://doi.org/10.1097/SCS.0000000000000457
- Kim JM, Sohn DS, Heo JU, Park JS, Jung HS, Moon JW, Lee JH, Park IS. Minimally invasive sinus augmentation using ultrasonic piezoelectric vibration and hydraulic pressure: A multicenter retrospective study. Implant Dentistry 2012; 21:536-42; PMID:23149505; https://doi.org/10.1097/ID.0b013e31826a56c3
- Mazor Z, Kfir E, Lorean A, Mijiritsky E, Horowitz RA. Flapless approach to maxillary sinus augmentation using minimally invasive antral membrane balloon elevation. Implant Dentistry 2011; 20:434-8; PMID:22067602; https://doi.org/10.1097/ID.0b013e3182391fe3
- Chen L, Cha J. An 8-year retrospective study: 1,100 patients receiving 1,557 implants using the minimally invasive hydraulic sinus condensing technique. J Periodontol 2005; 76:482-491; PMID:15857085; https://doi.org/10.1902/jop.2005.76.3.482
- Ahn SH, Park EJ, Kim ES. Reamer-mediated transalveolar sinus floor elevation without osteotome and simultaneous implant placement in the maxillary molar area: Clinical outcomes of 391 implants in 380 patients. Clin Oral Implants Res 2012; 23:866-72; PMID:21722189; https://doi.org/10.1111/j.1600-0501.2011.02216.x
- Al-Dajani M. Recent trends in sinus lift surgery and their clinical implications. Clin Implant Dentistry Related Res 2016; 18:204-12; PMID:25274014; https://doi.org/10.1111/cid.12275
- Călin C, Petre A, Drafta S. Osteotome-mediated sinus floor elevation: A systematic review and meta-analysis. Int J Oral Maxillofacial Implants 2014; 29:558-76; PMID:24818194; https://doi.org/10.11607/jomi.3206
- Zitzmann NU, Schärer P. Sinus elevation procedures in the resorbed posterior maxilla: Comparison of the crestal and lateral approaches. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 1998; 85:8-17; https://doi.org/10.1016/S1079-2104(98)90391-2
- Hwang JH, Jung BY, Lim CS, Cha IH, Park W. Posterior maxillary segmental osteotomy concomitant with sinus lift using a piezoelectric device. J Oral Maxillofacial Surg 2011; 69:2339-44; PMID:21802189; https://doi.org/10.1016/j.joms.2011.04.023