ABSTRACT
This research aimed to explore the biological role of long non-coding RNA (lncRNA) ZFAS1 in bupivacaine-induced neurotoxicity. The levels of lncRNA ZFAS1, miR-421, and zinc finger protein 564 (ZNF564) were detected by RT-qPCR. MTT and TUNEL assays were utilized to evaluate cell viability and apoptosis, respectively. Caspase-3 activity was measured by the caspase-3 activity assay kit. The binding ability between miR-421 and ZFAS1 or ZNF564 was confirmed by Rip and dual-luciferase reporter assays. In this study, it was found that the levels of ZFAS1 and ZNF564 were gradually upregulated and miR-421 expression was downregulated with increasing concentrations of bupivacaine. Functional assays indicated that the silencing of ZFAS1 suppressed cell viability and facilitated cell apoptosis of SH-SY5Y cells, while overexpression of ZFAS1 had the opposite effects. Moreover, it was identified that miR-421 was a target of ZFAS1, and ZFAS1 regulated the bupivacaine-induced neurotoxicity via miR-421. In addition, we confirmed that ZNF564 was a downstream target of miR-421. The upregulation of miR-421 decreased the cell viability, and increased the cell apoptosis rate and caspase-3 activity, while the upregulation of ZND564 partially abolished these effects. Finally, it was demonstrated that ZFAS1 could upregulate the expression of ZNF564 by targeting miR-421. In conclusion, our results demonstrated that ZFAS1 alleviated bupivacaine-induced neurotoxicity through the miR-421/ZNF564 axis, suggesting a new strategy for the amelioration of bupivacaine-induced neurotoxicity.
Introduction
Bupivacaine, a sodium channel blocker, is a local anesthetic commonly used in surgical procedures, and alleviation of postoperative and chronic pains [Citation1,Citation2]. Although long-term exposure to bupivacaine has a good effect on analgesia, it also leads to neurotoxicity and irreversible neurological complications [Citation3,Citation4]. To prevent the toxic and side effects of bupivacaine, increasing studies were conducted to investigate the mechanisms of bupivacaine-induced neurotoxicity. Previous reports indicated bupivacaine could result in the activation of the apoptotic pathway and the inhibition of cell viability, which further caused neurotoxicity [Citation5,Citation6]. However, the precise mechanisms of bupivacaine-induced neurotoxicity are still unclear.
Long non-coding RNAs (lncRNAs) are a type of non-coding RNAs (ncRNA) more than 200 nucleotides in length, that are widely reported to participate in pathophysiological processes, including anesthesia-induced neurotoxicity [Citation7]. For instance, lncRNA BDNF-AS reduced bupivacaine-induced neurotoxicity through neurotrophin TrkB signaling [Citation8]. The overexpression of NR_030777 alleviated neurotoxicity by regulating the expression of Zfp326 and Cpne5 [Citation9]. The depletion of lncRNA PADNA facilitated bupivacaine-induced neurotoxicity and neuronal apoptosis via miR-194/FBXW7 axis [Citation10]. Recently, Zhang et al indicated that lncRNA ZFAS1 ameliorated neuronal injury and suppressed apoptosis, inflammation, and oxidative stress in cerebral ischemia/reperfusion injury [Citation11]. Nevertheless, the biological role and molecular mechanism of ZFAS1 in bupivacaine-induced neurotoxicity remain unknown.
MicroRNAs (miRNAs) are a group of highly conserved ncRNAs with 20–25 nucleotides in length [Citation12,Citation13]. With the increasing attention and research on miRNA, several miRNAs, such as miRNA-23a [Citation14], miR-21-5p [Citation15], and miR-330-3p [Citation16], have become the therapeutic targets and biomarkers for neurological disease [Citation17,Citation18]. miR-421 was reported to facilitate cell death and aggravate neurotoxicity in Parkinson’s disease by regulating MEF2D expression [Citation19]. Moreover, Yang et al indicated that the upregulation of miR-421 accelerated neuronal apoptosis in hippocampal neurons [Citation20]. Nevertheless, the involvement of miR-421 in bupivacaine-induced neurotoxicity has not been clarified.
The present study aimed to investigate the role and molecular mechanism of ZFAS1 in bupivacaine-induced neurotoxicity, and we hypothesized that ZFAS1 exhibited neuroprotective effects against neurotoxicity. Our results for the first demonstrated that ZFAS1 alleviated neurotoxicity by regulating the miR-421/zinc finger protein 564 (ZNF564) axis, which might provide a new strategy for preventing bupivacaine-induced neurotoxicity.
Materials and methods
Cell culture and treatment
Human neuroblastoma cell line (SH-SY5Y), obtained from the American Type Culture Collection (ATCC, USA), were cultured in Dulbecco’s modified Eagle’s medium (DMEM; catalog number: 11,995,065; Thermo Fisher Scientific) supplemented with 10% FBS (catalog number: F2442; Sigma-Aldrich) and 1% penicillin/streptomycin (catalog number: 15,070,063; Thermo Fisher Scientific) at 37°C. For drug treatment, the cells were incubated with 0.5, 1.0, 1.5, or 2.0 mM bupivacaine (catalog number: B5274; Sigma-Aldrich) for 24 h as previously described [Citation8].
Cell transfection
Short hairpin RNA (shRNA) targeting ZFAS1 (shZFAS1) with its control (shNC), miR-421 mimics with its control (NC mimics), miR-421 inhibitor with its control (NC inhibitor), ZFAS1 overexpression plasmid (pcDNA3.1/ZFAS1), and ZNF564 overexpression plasmid (pcDNA3.1/ZNF564) were synthesized by GenePharma (Shanghai, China). The transfection was performed using Lipofectamine 2000 (catalog number: 11,668,019; Invitrogen) [Citation21]. The sequences were as follows: shZFAS1: 5ʹ-CUGGCUGAACCAGUUCCACAA-3ʹ; shNC: 5ʹ-ACGUUGCUGAUGUACUACAGC-3ʹ; miR-421 mimics: 5ʹ-AGCUACUGCUACGACUUGAGU-3ʹ; NC mimics: 5ʹ-UCAUCAGUCAUGCAUGUAUGC-3ʹ; miR-421 inhibitor: 5ʹ-ACUGAUGAUCGUAGUCGGUAC-3ʹ; NC inhibitor (5ʹ-UAUGAUGCAUGCAUGCAUGCA-3ʹ.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted using TRIzol reagent (catalog number: 15,596,026; Invitrogen), and reverse-transcribed to cDNA using a PrimeScript RT Reagent Kit (catalog number RR047A; TaKaRa, China). RT-qPCR was then performed using the SYBR Green kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) on the ABI 7500 Real-time PCR system (Applied Biosystems). The relative gene expression was calculated using 2−ΔΔCt method [Citation22]. GAPDH or U6 was used as an internal control. The primer sequences were presented in .
Table 1. Primer sequences used for RT-qPCR
Cell viability assay
The transfected cells were seeded into 96-well plates and treated with different concentrations of bupivacaine for 24 h. Subsequently, 10 μL 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT; catalog number: M2128; Sigma-Aldrich) solution was added to each well and incubated for 4 h at 37°C. Cell viability was assessed by a microplate reader at 570 nm [Citation23].
Caspase-3 activity
The transfected SH-SY5Y cells were seeded in 6-well plates after different treatments, and the caspase-3 activity was detected using a Caspase-3 activity assay kit (Catalog number: C1115; Beyotime) following the manufacturer’s protocol [Citation24].
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
An in-situ Cell Death Detection Kit (catalog number: M2128; 11,684,817,910; Roche) was utilized to evaluate the apoptosis rate of SH-SY5Y cells [Citation25]. Briefly, SH-SY5Y cells were washed with PBS (catalog number: 10,010,023; Thermo Fisher Scientific) and fixed in paraformaldehyde (catalog number: P6148; Sigma-Aldrich). Next, the cells were treated with 0.1% Triton X-100 (catalog number: BP151-500; Thermo Fisher Scientific) for 2 min and cultured with TUNEL reaction mixture at 37°C for 1 h. Subsequently, the TUNEL-stained cells were counterstained with DAPI. Apoptotic cells were detected by a fluorescence microscope.
Bioinformatics analysis and dual-luciferase reporter assay
The starBase database (http://starbase.sysu.edu.cn/) was used to predict the potential binding sites between miR-421 and ZFAS1 or ZNF564. The wild-type (WT) and mutant (Mut) sequences of ZFAS1 and ZNF564 were sub-cloned into pmirGLO vectors (Promega). Then, these vectors were co-transfected with NC mimics or miR-421 mimics into SH-SY5Y cells using Lipofectamine 2000. The luciferase activity was detected by the Dual-luciferase Reporter System (catalog number: E1910, Promega) [Citation26].
RNA-binding protein immunoprecipitation (RIP) assay
RIP assay was utilized with Magna RIP RNA-Binding Protein Immunoprecipitation Kit (catalog number: 17–701; Merck Life Science, Shanghai, China) [Citation25]. The transfected cells were dissolved in RIP lysis buffer, and cell lysate was incubated with magnetic beads bound with the Ago2 antibody (catalog number: 2897; Cell Signaling Technology). IgG (catalog number: 14,708; Cell Signaling Technology) was used as a control group. Then, immunoprecipitated RNA was detected with RT-qPCR assay.
Statistical analysis
All experiments were conducted with three replicates. SPSS 17.0 (SPSS, Chicago, USA) was employed for statistical analysis and the data are presented as mean ± standard deviation. Student’s t-test and one-way ANOVA were performed to evaluate the difference between groups. P< 0.05 was regarded as significant statistically.
Results
In this study, we aimed to explore the role and molecular mechanism of ZFAS1 in bupivacaine-induced neurotoxicity. A series of in vitro assays were conducted, and the results indicated that ZFAS1 exhibited neuroprotective effects against neurotoxicity. Mechanistic investigations revealed ZFAS1 alleviated bupivacaine-induced neurotoxicity by regulating the miR-421/ZNF564 axis.
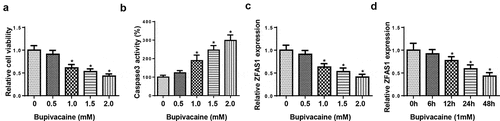
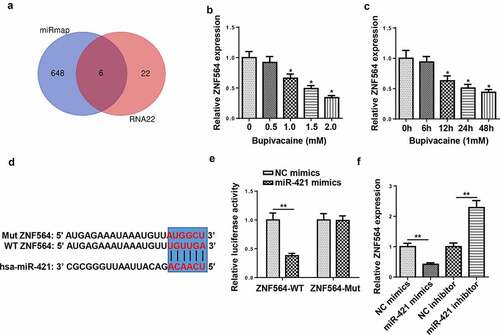
LncRNA ZFAS1 expression was downregulated with the increasing concentrations of bupivacaine
SH-SY5Y human neuroblastoma cells are commonly used as neuronal models [Citation27]. To induce neurotoxicity, SH-SY5Y cells were treated with different bupivacaine concentrations (0, 0.5, 1.0, 1.5, or 2.0 mM). As presented in , with the increasing concentrations of bupivacaine, the cell viability was decreased and the activity of caspase-3 was increased. Subsequently, RT-qPCR indicated that ZFAS1 expression was gradually downregulated with the increase of bupivacaine concentration ()). Moreover, we also detected lncRNA ZFAS1 expression in SH-SY5Y cells at 0 h, 6 h, 12 h, 24 h, and 48 h after exposure to 1.0 mM bupivacaine. These results demonstrated that ZFAS1 expression was downregulated with the increase of time ()).
Figure 1. LncRNA ZFAS1 expression was downregulated with the increasing concentrations of bupivacaine
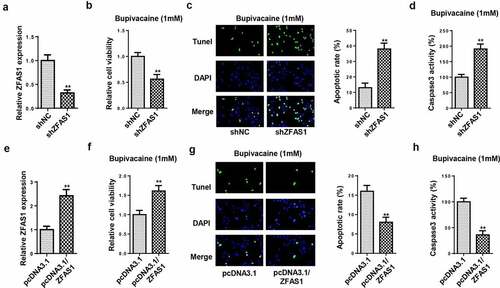
LncRNA ZFAS1 regulated cell viability and apoptosis in SH-SY5Y cells
To examine the role of ZFAS1 in bupivacaine-induced neurotoxicity, loss- or gain-of-function experiments were performed. RT-qPCR indicated that ZFAS1 expression was markedly decreased in cells transfected with shZFAS1 ()). Next, MTT and TUNEL assays showed that the ZFAS1 knockdown significantly decreased cell viability and accelerated cell apoptosis in SH-SY5Y cells treated with 1.0 mM bupivacaine (). Meanwhile, the knockdown of ZFAS1 remarkably promoted caspase-3 activity ()). Subsequently, SH-SY5Y cells were transfected with ZFAS1 overexpression plasmid, and the transfection efficiency was validated by RT-qPCR ()). Functional assays revealed that the upregulation of ZFAS1 markedly increased the cell viability and decreased the apoptosis rate of bupivacaine-treated SH-SY5Y cells (–h)). The above results demonstrated that ZFAS1 alleviated bupivacaine-induced neurotoxicity.
Figure 2. LncRNA ZFAS1 regulated cell viability and apoptosis in SH-SY5Y cells
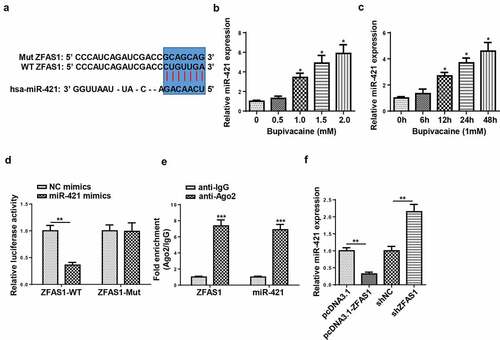
ZFAS1 directly interacted with miR-421
Previous studies revealed that ZFAS1 acted as a sponge and negatively regulated the expression of miR-421 [Citation28,Citation29], which prompted us to hypothesize that miR-421 might be a downstream target of ZFAS1 in SH-SY5Y cells. Through using starbase website (http://starbase.sysu.edu.cn/), miR-421 was predicted to bind with ZFAS1. Then, RT-qPCR indicated that miR-421 expression was gradually upregulated with increasing bupivacaine concentration or exposure duration (). Moreover, miR-421 mimics significantly reduced the luciferase activity of ZFAS1-WT instead of ZFAS1-Mut in SH-SY5Y cells ()). RIP assay revealed that miR-421 and ZFAS1 were markedly enriched in the Ago2 group compared with the IgG group (). Moreover, the overexpression of ZFAS1 restrained miR-421 expression, while the knockdown of ZFAS1 upregulated miR-421 expression in SH-SY5Y cells ()). Overall, our data revealed that ZFAS1 could negatively regulate miR-421 expression.
Figure 3. ZFAS1 directly interacted with miR-421
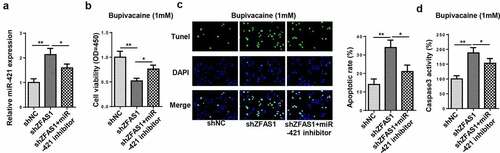
ZFAS1 mitigated bupivacaine-induced neurotoxicity via miR-421
To investigate whether ZFAS1 regulated bupivacaine-induced neurotoxicity through miR-421, SH-SY5Y cells were transfected with shNC, shZFAS1, and shZFAS1+ miR-421 inhibitor. RT-qPCR revealed that the downregulation of miR-421 partially abolished the promoting effect of ZFAS1 knockdown on miR-421 expression ()). In addition, the depletion of ZFAS1 inhibited the viability of SH-SY5Y cells, which was neutralized following miR-421 inhibitor transfection (). Furthermore, the interference of ZFAS1 increased cell apoptosis and caspase-3 activity, whereas miR-421 inhibition abrogated the effects caused by ZFAS1 silencing (). In sum, these findings revealed that ZFAS1 regulated bupivacaine-induced neurotoxicity by targeting miR-421.
Figure 4. ZFAS1 mitigated bupivacaine-induced neurotoxicity via miR-421
ZNF564 was directly targeted by miR-421
To investigate the downstream regulatory mechanism of miR-421, miRmap and RNA22 database were employed and 6 candidate genes (AC010422.6, TRAPPC6B, DCAF5, ERO1A, KCNB1, and ZNF564) were screened ()). RT-qPCR showed that the levels of ZNF564 were downregulated with the increase of bupivacaine concentration and exposure duration (). Subsequently, miR-421 overexpression decreased the luciferase activity of the wild-type ZNF564, but not the mutant ZNF564 (). In addition, miR-421 overexpression inhibited ZNF564 expression, and miR-421 silence increased ZNF564 expression ()). These findings indicated that miR-421 negatively regulated ZNF564 expression by direct interaction.
Figure 5. ZNF564 was the direct target of miR-421
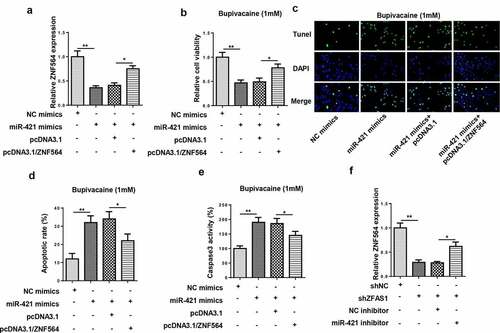
ZFAS1 regulated bupivacaine-induced neurotoxicity via the miR-421/ZNF564 axis
To further investigate whether ZNF564 was a downstream regulator of ZFAS1/miR-421 axis in bupivacaine-induced neurotoxicity, SH-SY5Y cells were transfected with NC mimics, miR-421 mimics, miR-421 mimics+pcDNA3.1, and miR-421 mimics+ pcDNA3.1/ZNF564. RT-qPCR showed that overexpression of miR-421 decreased ZNF564 expression, which was counteracted by ZNF564 overexpression ()). Moreover, functional assays revealed that the upregulation of miR-421 decreased the cell viability, and increased the cell apoptosis rate and caspase-3 activity, while the upregulation of ZND564 partially abolished these effects (–e)). In addition, the downregulation of miR-421 reversed the inhibitory effect of ZFAS1 knockdown on the expression of ZNF564 ()). Thus, these data indicated that ZFAS1 ameliorated bupivacaine-induced neurotoxicity via the miR-421/ZNF564 axis.
Figure 6. ZFAS1 regulated bupivacaine-induced neurotoxicity via the miR-421/ZNF564 axis
Discussion
Increasing evidence indicates that lncRNA plays a vital role in anesthetic-induced neurotoxicity. For example, Song et al reported that lncRNA IGF2AS expression was upregulated in ketamine-treated neural stem cells, and knockdown of IGF2S alleviated the apoptosis in ketamine-injured neural stem cells [Citation30]. Zhao et al indicated that lncRNA Gm15621 prevented neurotoxicity through the inhibition of apoptosis and inflammation response in sevoflurane-induced primary hippocampal neurons [Citation31]. A previous study revealed that the ZFAS1 level was significantly decreased in patients with cerebral ischemia/reperfusion injury, and overexpression of ZFAS1 mitigated neuronal damage and reduced cerebral ischemia/reperfusion-induced apoptosis [Citation11]. In addition, Zhang et al indicated that the depletion of ZFAS1 suppressed the migration, invasion, and proliferation, but facilitated apoptosis in glioma cells [Citation32]. In this study, we observed that bupivacaine decreased the level of ZFAS1 in SH-SY5Y cells, especially in the high concentration bupivacaine group. Moreover, the upregulation of ZFAS1 prominently facilitated cell viability and inhibited cell apoptosis in bupivacaine-treated SH-SY5Y cells. Our results revealed that ZFAS1 could mitigate bupivacaine-induced neurotoxicity.
LncRNAs are widely reported to act as competing endogenous RNAs (ceRNAs) by specifically adsorbing miRNAs, and then modulate the expression of target genes [Citation33,Citation34]. For example, Ke et al indicated that NEAT1 enhanced neuronal injury in Alzheimer’s disease by regulating miR-107 [Citation35]. Lin et al showed that HOTAIR silencing decreased the number of α-synuclein-positive cells and TH-positive cells by sponging miR-126-5p to inhibit the progression of Parkinson’s disease [Citation36]. In anesthetic-induced neurotoxicity, Wang et al demonstrated that knockdown of lncRNA ATB protected neuronal cells against Aβ25-35-induced neurotoxicity through the miR-200/ZNF217 axis [Citation37]. Zhang et al showed that lncRNA Rik-203 promoted sevoflurane-induced neurotoxicity by regulating miR-101a-3p [Citation38]. miR-421 has been reported to facilitate neuronal apoptosis in Parkinson’s Disease [Citation19] and epilepsy [Citation20], and act as a downstream target of ZFAS1 in various diseases, such as oral squamous cell carcinoma [Citation28] and epilepsy [Citation29]. Herein, the miR-421 level was increased by bupivacaine in the time- and dose-dependent manner in SH-SY5Y cells. Moreover, it was found that ZFAS1 regulated the cell viability and apoptosis of SH-SY5Y cells by directly binding to miR-421. These results suggested ZFAS1 modulated bupivacaine-induced neurotoxicity by regulating miR-421.
Zinc finger protein (ZNF) is one of the most abundant and versatile proteins in eukaryotic genomes [Citation39], which have been previously reported to be involved in biological processes, such as proliferation and apoptosis [Citation40,Citation41]. In this study, we confirmed that ZNF564 was the downstream gene of miR-421. Moreover, the upregulation of miR-421 inhibited cell viability, and promoted the cell apoptosis of bupivacaine-treated SH-SY5Y cells, while ZND564 overexpression partially abolished these effects. Finally, we demonstrated that ZFAS1 upregulated ZNF564 expression by targeting miR-421.
Conclusion
Our research indicated that the ZFAS1/miR-421/ZNF564 axis played a crucial role in protecting bupivacaine-induced neurotoxicity. These findings suggested that ZFAS1 could be considered as a new therapeutic target for bupivacaine-induced neurotoxicity treatment. However, this study was not without its limitations. For example, only SH-SY5Y cell line was used for the verification experiment. In future research, more cell lines will be used to verify the biological role of the ZFAS1/miR-421/ZNF564 axis in bupivacaine-induced neurotoxicity.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Kendall MC, Castro Alves LJ, De Oliveira G Jr. Liposome bupivacaine compared to plain local anesthetics to reduce postsurgical pain: an updated meta-analysis of randomized controlled trials. Pain Res Treat. 2018;2018:5710169.
- Chalkiadis GA, Sommerfield D, Low J, et al. Comparison of lumbar epidural bupivacaine with fentanyl or clonidine for postoperative analgesia in children with cerebral palsy after single-event multilevel surgery. Dev Med Child Neurol. 2016;58:402–408.
- Sakura S. Research on local anesthetic neurotoxicity using intrathecal and epidural rat models. J Anesth. 2007;21:533–534.
- Verlinde M, Hollmann MW, Stevens MF, et al. Local Anesthetic-Induced Neurotoxicity. Int J Mol Sci. 2016;17:339.
- Werdehausen R, Fazeli S, Braun S, et al. Apoptosis induction by different local anaesthetics in a neuroblastoma cell line. Br J Anaesth. 2009;103:711–718.
- Radwan IA, Saito S, Goto F. The neurotoxicity of local anesthetics on growing neurons: a comparative study of lidocaine, bupivacaine, mepivacaine, and ropivacaine. Anesth Analg. 2002;94: 319–324. table of contents.
- Glen H. Lenvatinib therapy for the treatment of patients with advanced renal cell carcinoma. Future Oncol. 2016;12:2195–2204.
- Zhang Y, Yan L, Cao Y, et al. Long noncoding RNA BDNF-AS protects local anesthetic induced neurotoxicity in dorsal root ganglion neurons. Biomed Pharmacothe. 2016;80:207–212.
- Yang H, Lin Q, Chen N, et al. LncRNA NR_030777 alleviates paraquat-induced neurotoxicity by regulating Zfp326 and Cpne5. Toxicol Sci. 2020;178(1):173–188.
- Yuning F, Liang C, Tenghuan W, et al. Knockdown of lincRNA PADNA promotes bupivacaine-induced neurotoxicity by miR-194/FBXW7 axis. Mol Med. 2020;26:79.
- Zhang Y, Zhang Y. lncRNA ZFAS1 improves neuronal injury and inhibits inflammation, oxidative stress, and apoptosis by sponging miR-582 and upregulating NOS3 expression in cerebral Ischemia/Reperfusion Injury. Inflammation. 2020;43:1337–1350.
- Ge Y, Zhang L, Nikolova M, et al. Strand-specific in vivo screen of cancer-associated miRNAs unveils a role for miR-21(*) in SCC progression. Nat Cell Biol. 2016;18:111–121.
- Yoon JH, Abdelmohsen K, Gorospe M. Functional interactions among microRNAs and long noncoding RNAs. Semin Cell Dev Biol. 2014;34:9–14.
- Pan Z, Shan Q, Gu P, et al. miRNA-23a/CXCR4 regulates neuropathic pain via directly targeting TXNIP/NLRP3 inflammasome axis. J Neuroinflammation. 2018;15:29.
- Li X, Giri V, Cui Y, et al. LncRNA FTX inhibits hippocampal neuron apoptosis by regulating miR-21-5p/SOX7 axis in a rat model of temporal lobe epilepsy. Biochem Biophys Res Commun. 2019;512:79–86.
- Peng C, Zhang C, Su Z, et al. DGCR5 attenuates neuropathic pain through sponging miR-330-3p and regulating PDCD4 in CCI rat models. J Cell Physiol. 2019;234:7292–7300.
- Peng T, Liu X, Wang J, et al. Long noncoding RNA HAGLROS regulates apoptosis and autophagy in Parkinson’s disease via regulating miR-100/ATG10 axis and PI3K/Akt/mTOR pathway activation. Artif Cells Nanomed Biotechnol. 2019;47:2764–2774.
- Li LH, Tu QY, Deng XH, et al. Mutant presenilin2 promotes apoptosis through the p53/miR-34a axis in neuronal cells. Brain Res. 2017;1662:57–64.
- Dong Y, Xiong J, Ji L, et al. MiR-421 aggravates neurotoxicity and promotes cell death in Parkinson’s disease models by directly targeting MEF2D. Neurochem Res. 2020;46(2):299–308.
- Yang B, Liang RS, Wu XY, et al. LncRNA TUG1 inhibits neuronal apoptosis in status epilepticus rats via targeting the miR-421/mTOR axis. Cell Signal. 2020;76:109787.
- Yang Z, OuYang X, Zheng L, et al. Long intergenic noncoding RNA00265 promotes proliferation of gastric cancer via the microRNA-144-3p/Chromobox 4 axis. Bioengineered. 2021;12:1012–1025.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408.
- Feng J, Li J, Qie P, et al. Long non-coding RNA (lncRNA) PGM5P4-AS1 inhibits lung cancer progression by up-regulating leucine zipper tumor suppressor (LZTS3) through sponging microRNA miR-1275. Bioengineered. 2021;12:196–207.
- Zhang X, Liu Z, Shu Q, et al. LncRNA SNHG6 functions as a ceRNA to regulate neuronal cell apoptosis by modulating miR-181c-5p/BIM signalling in ischaemic stroke. J Cell Mol Med. 2019;23:6120–6130.
- Zhang CC, Li Y, Feng XZ, et al. Circular RNA circ_0001287 inhibits the proliferation, metastasis, and radiosensitivity of non-small cell lung cancer cells by sponging microRNA miR-21 and up-regulating phosphatase and tensin homolog expression. Bioengineered. 2021;12:414–425.
- Zhang H, Liu S, Tang L, et al. Long non-coding RNA (LncRNA) MRPL23-AS1 promotes tumor progression and carcinogenesis in osteosarcoma by activating Wnt/β-catenin signaling via inhibiting microRNA miR-30b and upregulating myosin heavy chain 9 (MYH9). Bioengineered. 2021;12:162–171.
- Şahin M, Öncü G, Yılmaz MA, et al. Transformation of SH-SY5Y cell line into neuron-like cells: investigation of electrophysiological and biomechanical changes. Neurosci Lett. 2021;745:135628.
- Wang X, Hao R, Wang F, et al. ZFAS1 promotes cisplatin resistance via suppressing miR-421 expression in oral squamous cell carcinoma. Cancer Manag Res. 2020;12:7251–7262.
- Hu F, Shao L, Zhang J, et al. Knockdown of ZFAS1 inhibits hippocampal neurons apoptosis and autophagy by activating the PI3K/AKT pathway via Up-regulating miR-421 in epilepsy. Neurochem Res. 2020;45:2433–2441.
- Song C, Song C, Chen K, et al. Inhibition of long non-coding RNA IGF2AS protects apoptosis and neuronal loss in anesthetic-damaged mouse neural stem cell derived neurons. Biomed Pharmacothe. 2017;85:218–224.
- Zhao Y, Ai Y. Overexpression of lncRNA Gm15621 alleviates apoptosis and inflammation response resulting from sevoflurane treatment through inhibiting miR-133a/Sox4. J Cell Physiol. 2020;235:957–965.
- Zhang B, Chen J, Cui M, et al. LncRNA ZFAS1/miR-1271-5p/HK2 promotes glioma development through regulating proliferation, migration, invasion and apoptosis. Neurochem Res. 2020;45:2828–2839.
- Yang J, Qiu Q, Qian X, et al. Long noncoding RNA LCAT1 functions as a ceRNA to regulate RAC1 function by sponging miR-4715-5p in lung cancer. Mol Cancer. 2019;18:171.
- Thomson DW, Dinger ME. Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet. 2016;17:272–283.
- Ke S, Yang Z, Yang F, et al. Long Noncoding RNA NEAT1 Aggravates Abeta-Induced Neuronal Damage by Targeting miR-107 in Alzheimer’s Disease. Yonsei Med J. 2019;60:640–650.
- Lin Q, Hou S, Dai Y, et al. LncRNA HOTAIR targets miR-126-5p to promote the progression of Parkinson’s disease through RAB3IP. Biol Chem. 2019;400:1217–1228.
- Wang J, Zhou T, Wang T, et al. Suppression of lncRNA-ATB prevents amyloid-beta-induced neurotoxicity in PC12 cells via regulating miR-200/ZNF217 axis. Biomed Pharmacothe. 2018;108:707–715.
- Zhang L, Yan J, Liu Q, et al. LncRNA Rik-203 contributes to anesthesia neurotoxicity via microRNA-101a-3p and GSK-3beta-mediated neural differentiation. Sci Rep. 2019;9:6822.
- Xie W, Qiao X, Shang L, et al. Knockdown of ZNF233 suppresses hepatocellular carcinoma cell proliferation and tumorigenesis. Gene. 2018;679:179–185.
- Huan C, Xiaoxu C, Xifang R. Zinc finger protein 521, negatively regulated by MicroRNA-204-5p, promotes proliferation, motility and invasion of gastric cancer cells. Technol Cancer Res Treat. 2019;18:1533033819874783.
- Schnabl B, Valletta D, Kirovski G, et al. Zinc finger protein 267 is up-regulated in hepatocellular carcinoma and promotes tumor cell proliferation and migration. Exp Mol Pathol. 2011;91:695–701.
