ABSTRACT
Colon adenocarcinoma (COAD) is one of the most common types of malignancy and accounts for >3 million deaths worldwide each year. The present study aimed to evaluate the role of notum palmitoleoyl-protein carboxylesterase (NOTUM) in in vivo and in vitro, and to identify the relationship between NOTUM and the apoptosis of COAD. Moreover, the present study aimed to investigate whether NOTUM regulated Fas cell surface death receptor (FAS)-mediated apoptosis was affected by the Wnt signaling pathway. Gene expression profiling interactive analysis (GEPIA) was used to predict the potential function of NOTUM. Western blotting and reverse transcription-quantitative PCR were conducted to detect the protein and mRNA expression levels of NOTUM in different tissues or cell lines. The occurrence and development of COAD was detected after NOTUM knockdown lentivirus administration. The apoptosis of COAD was also observed. SKL2001 was applied to examine whether the role of NOTUM was regulated by Wnt. GEPIA analysis demonstrated that NOTUM expression in COAD tumor tissue was higher compared with in normal tissues. Pair-wise gene correlation analysis identified a potential relationship between NOTUM and Wnt. NOTUM protein and mRNA expression levels in colon carcinoma tissues and RKO cells were increased. NOTUM knockdown lentivirus serves a role in inhibiting COAD development by reducing tumor proliferation, reducing tumor size, and increasing the level of apoptosis in vitro and in vivo. Moreover, NOTUM could increase apoptosis in COAD, which was regulated by FAS, and SKL2001 blocked the progress of apoptosis after NOTUM regulation by NOTUM knockdown lentivirus in vitro and in vivo. Collectively, the present results suggested that NOTUM may be able to regulate the apoptosis of COAD, and that Wnt may be the down-stream target signaling of NOTUM in apoptosis.
KEYWORDS:
Introduction
The incidence of colon adenocarcinoma (COAD) demonstrates geographic variation; for instance, the age-adjusted incidence varies by up to 12-fold among different countries [Citation1], which suggests that the development of colonic cancer is difficult to track. COAD develops from specific types of neoplastic polyps in the colonic mucosa via a multistep process, in terms of molecular genetics [Citation2]. COAD has attracted global attention and there are numerous investigations into the development of novel therapies based on an understanding of the mechanisms that induce this disease, as well as to address key obstacles in the available treatments [Citation3].
Apoptosis is a type of programmed cell death that is regulated at the gene level and results in the orderly and efficient removal of damaged cells, including DNA damage or damage that occurs during development [Citation4]. Dysregulation of apoptosis is associated with unregulated cancer cell proliferation, development, progression and resistance to drug therapies [Citation5]. Therefore, therapeutic strategies targeting molecules involved in apoptotic resistance represent a viable approach and may help to overcome the challenges that have resulted in previous ineffective therapeutic methods [Citation6].
Fas cell surface death receptor (FAS) is a member of the tumor necrosis factor receptor superfamily, known as ‘death receptors’, and has been revealed to serve an important role in initiating cell apoptosis [Citation7]. However, the mechanism underlying FAS-mediated apoptosis requires further investigation.
Notum palmitoleoyl-protein carboxylesterase (NOTUM) was first discovered in fruit flies, and it is composed of four exons. When the expression of the NOTUM gene increases, fruit flies are born wingless [Citation8]. The NOTUM gene is involved in the production of vertebrate nerves and heads in the embryo, as well as in the regulation of brain growth in planaria [Citation9]. From fruit flies to humans, NOTUM genes have been identified to function mainly via an association with the Wnt signaling pathway [Citation10]. Recently, NOTUM has been reported to regulate cancer development in numerous tumor types, including invasive urothelial carcinoma of the bladder [Citation11] and colorectal cancer [Citation12]. NOTUM is also involved in abnormal development of the thorax and in apoptosis in Drosophila melanogaster [Citation13], and this has prompted researchers to examine whether NOTUM could be employed for the regulation of apoptosis in some diseases. However, the mechanism via which NOTUM regulates apoptosis in COAD is not yet fully understood.
The present study, we aimed to explore the role of NOTUM in COAD and used NOTUM knockdown lentivirus to enhance the apoptotic and development of tumor tissues in vivo and in vitro. Furthermore, Wnt agonists were used to induce Wnt level and to reverse the apoptosis by regulating NOTUM. The hypothesis of present findings indicated that NOTUM could possibly regulate Fas-mediated apoptosis via the Wnt signaling pathway in COAD. These results may provide novel strategies for COAD treatment by utilizing the mechanism of apoptosis.
Materials and methods
Gene expression profiling interactive analysis (GEPIA)
COAD genomics datasets (Ensembl ID: ENSG00000185269.11) were analyzed via the GEPIA website (https://gepia.cancer-pku.cn/), as described in a previous study [Citation1]. The expression profile assay in different digestive system carcinomas, including cholangiocarcinoma, COAD, esophageal carcinoma (ESCA), rectum adenocarcinoma (READ) and stomach adenocarcinoma (STAD), and the isoform analysis in the boxplot for NOTUM expression of COAD were in the ‘profiles and box plot in expression DIY’ module. The pathological stage plot assay was in the ‘stage plot in expression DIY’ module. Survival analysis was in the module of ‘survival analysis’, and pair-wise gene correlation analysis was in ‘correlation analysis’ module.
Human tumor specimens
For this study, 20 patients (12 male and 8 females; age range, 40–69 years old) who were pathologically diagnosed with COAD were selected at the Digestive Surgery Department in Shidong Hospital (Shanghai, China) between March 2018 and March 2019, and 12 samples (6 male and 6 females; age range, 45–69 years old) of normal tissues collected from around the tumor (para-tumor) were used as the control group. The inclusion criteria were as follows: i) Complete clinical data; ii) diagnosed by the Pathology Department; and iii) medium to low level tubular adenocarcinoma pathologically. The exclusion criteria included: i) Incomplete clinical data; ii) combined with other intestinal tumors; and iii) tumor metastasis. All experimental procedures were approved by the Human Ethics Committee of Shidong Hospital (No.2018041201), and informed written consent was obtained from each subject prior to the study. lists the basic information of the patients.
Table 1. Clinicopathological features of patients with colon adenocarcinoma
Cell lines and cell cultures
The cell lines used were as follows: NCM460 cells (cat. no. CC-Y1550; EK-Bioscience GmbH; a human colon epithelial cell line), RKO cells (cat. no. CL- 196; Procell Life Science & Technology Co., Ltd.), HCT116 cells (cat. no. CL-0096, Procell Life Science & Technology Co., Ltd.) and SW620 cells (cat. no. CL-0225; Procell Life Science & Technology Co., Ltd.). RKO, HCT116 and SW620 are human COAD cell lines. Cells were cultured at 5% CO2 and 37°C with DMEM (Sigma-Aldrich; Merck KGaA), as described in a previous study [Citation14]. Cells were treated with 10 μg SKL2001 (Selleck Chemicals) for 7 days at 37°C for the last part of the experiments in vitro.
Animals
A total of 294 4-week-old healthy male Balb/c mice (weight, 30–40 g), which were supplied by the vivarium of the State Research Center of Virology and Biotechnology, were housed in an appropriate environment under specific pathogen-free (SPF) conditions with a humidity of 60 ± 5%, and were provided unlimited access to food and water. All animals used in this experiment were cared for in strict accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, revised 1996) [Citation15]. All experimental procedures were approved by the Animal Ethics Committee of the Shidong Hospital (No.2018041202).
Reagent and antibodies
The reagent used was as SKL2001 (cat. no. S8320; Selleck Chemicals). The primary antibodies used were as follows: Anti-NOTUM (cat. no. ab106448; Abcam), anti-FAS (cat. no. ab15285; Abcam), anti-BAX (cat. no. ab32503; Abcam), anti-Bcl-2 (cat. no. ab182858; Abcam), anti-cleaved caspase-3 (cat. no. ab49822; Abcam; 1:1,000), anti-cleaved poly (ADP-ribose) polymerase 1 (PARP1; cat. no. ab32064; Abcam; 1:1,000) and anti-β-actin (cat. no. M01263-2; Wuhan Boster Biological Technology, Ltd.; 1:5,000). The secondary antibodies used were conjugated to horseradish peroxidase anti-rabbit IgG (H + L) (cat. no. AS014; ABclonal Biotech Co., Ltd.; 1:5,000) and anti-mouse IgG (H + L) (cat. no. AS003; ABclonal Biotech Co., Ltd.; 1:5,000).
NOTUM knockdown lentivirus administration
NOTUM knockdown lentivirus (sh-NOTUM), sh-NC lentivirus and control lentivirus were designed and chemically synthesized by Shanghai GenePharma Co., Ltd, along with the transfection reagent. Lentivirus vectors were stored at −80°C and inoculated as described in a previous study [Citation1]. The cell protein or mRNA was extracted, centrifuged and precipitated using Phosphatase inhibitor cocktail B (cat. no. P1086; Beyotime Institute of Biotechnology) and Beyozol (cat. no. R0011; Beyotime Institute of Technology) from 6 well after 72 h. Cells transfected with lentivirus after passage were used to establish the COAD mouse model. The sequences of the lentivirus NOTUM are listed in .
Table 2. Sequence of NOTUM lentivirus
Subcutaneous xenograft mouse model
Male Balb/c mice (age, 4 weeks) were fed in an SPF environment, and 200 μl 1x105/ml RKO cells with PBS were injected subcutaneously in the back of the hind leg. After 7 days, vital signs and inoculation sites were observed. Mice were treated according to the ‘Laboratory Animal Welfare from the American College of Laboratory Animal Medicine, 2014, Pages 29–38, Chapter 4-Animal Welfare Assessment Considerations’ [Citation16]. The investigators observed the mice daily to ensure animal welfare and to determine the humane endpoints, as described in a previous study [Citation2]. In total, 10 mg/kg SKL2001 was administered to the mice for 7 days at 37°C for the last part of the experiments in vivo.
Mice were sacrifice via heart perfusion with 0.9% saline solution. The mice were anesthetized chloral hydrate (400 mg/kg; intraperitoneal), an upper abdominal incision was made, and the heart was exposed. Then, 0.9% saline solution was injected into the apex of heart before cutting open the right auricle and pumped into the systemic circulation.
The experimental groups (n = 6 mice/group) were as follows: Con group (untransfected COAD cells), sh-NOTUM group and control sh-NOTUM group. In total, 96 mice were involved in this experiment. The skin was cut directly, and tumor tissue was collected subcutaneously after modeling for 28 days. Tumor volume was calculated as 0.5 x length x width 2 (mm3).
Western blot analysis
Radio-Immunoprecipitation Assay (RIPA) lysis buffer (Beyotime Institute of Biotechnology) containing the protease inhibitor was used to extract total protein from the cells and the tissues, and the supernatant was collected after centrifugation (12,000 x g for 20 min at 4°C). In brief, ~40 μg protein from each tissue sample were subjected to 12% SDS-PAGE and transferred to a PVDF membrane as described in a previous study [Citation3]. All the samples were detected using a ChemiDoc™ Touch Imaging system (Bio-Rad Laboratories, Inc.) and were analyzed using Image Lab 3.0 software (Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative (RT-q)PCR
Total RNA was extracted from frozen tissue using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) and reverse transcribed into cDNA using a PrimeScript™ RT Reagent kit with gDNA Eraser (Takara Biotechnology Co., Ltd.) according to the manufacturer’s instructionsas, as described in a previous study [Citation4]. The cDNA was checked using a cocktail of random primers and reverse-transcriptase enzyme. 50 nmol primer sequences for amplification were as follows: NOTUM forward, 5ʹ-CTCCATTTTACAAGCAGCAG-3ʹ and reverse, 5ʹ-GCTCTTTCCTATCCTGTTCA-3ʹ; and β-actin forward, 5ʹ-TGTTTGAGACCTTCAACACC-3ʹand reverse, 5ʹ-CGCTCATTGCCGATAGTGAT-3ʹ (Invitrogen; Thermo Fisher Scientific, Inc.). A total of 40 μl RNA was reverse transcribed into cDNA in 42°C, 55 min and 98°C, 5 min. The PCR reactions were performed with Takara SYBR Premix Ex Taq II (TliRnaseH Plus; Takara Biotechnology Co., Ltd.) on a PCR amplifier in 95°C, 10s, 95°C, 5s, 65°C, 15s and 72°C, 15s in 40 thermocyclings. NOTUM mRNA expression was calculated according to its ratio to β-actin, and the expression levels were calculated using the 2−∆∆Cq method [Citation17].
Propidium Iodide (PI) staining
Cell viability was assessed using the PI-Hoechst assay (Beijing Solarbio Science & Technology Co., Ltd), according to the manufacturer’s instruction. Cells were observed using fluorescence microscopy at x400 magnification. Images were acquired with a Nikon Eclipse Ni inverted microscope (TE2000; Nikon Corporation).
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling assay (TUNEL) staining
Apoptotic cells were detected using a TUNEL assay (Beijing Solarbio Science & Technology Co., Ltd.). The TUNEL assay was performed according to the manufacturer’s protocol. Briefly, 5 μl Tdt enzyme (10%) and 45 μl fluorescence-labeled solution (90%) was incubated with the sections for 1 h at 37°C. The section was washed withphosphate belanced solution (PBS) three times and mounted with mounting medium (Sigma-Aldrich; Merck KGaA). The nuclei of the cells were stained with DAPI for 1 min at 37°C. Images were captured using fluorescence microscopy at x400 magnification in 10 randomly selected fields of view [Citation18].
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. After stirring the solution for 30 min, cells in the logarithmic growth stage were inoculated into 96-well plates in groups. Cells with a concentration of 200 ml of 4x104/ml were inoculated into each well as described in a previous study [Citation2]. The absorbance values of the wells were determined using an enzyme-linked immunoassay reader at 490 nm.
Statistical analysis
Data are presented as the mean ± standard deviation (SD). Data statistical analysis and correlation analysis were performed using GraphPad Prism 6.0 (GraphPad Software, Inc.). Survival analysis was performed using SPSS 19.0 (IBM Corp.). The homogeneity variance and analysis of variance were compared in every group. Differences were analyzed using one-way ANOVA, and multiple comparisons were analyzed using the Sidak test. The survival rates were analyzed using log-rank test and pair-wise gene correlation was analyzed using correlation test. P < 0.05 was considered to indicate a statistically significant difference.
Results
We aimed to explore the role of NOTUM in COAD and used NOTUM knockdown lentivirus to enhance the apoptotic and development of tumor tissues in vivo and in vitro. Furthermore, SKL2001 was used to induce Wnt level and to reverse the apoptosis. The hypothesis was that NOTUM could regulate Fas-mediated apoptosis via the Wnt signaling pathway in COAD.
Bioinformatic analysis of the role of NOTUM in COAD and other digestive system carcinomas using the GEPIA server
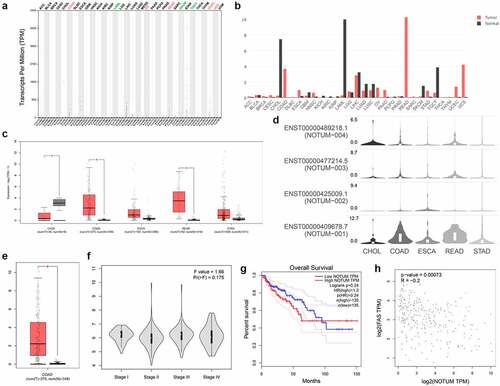
The predicted function of NOTUM in COAD and other digestive system carcinomas from GEPIA and TCGA were presented in . As demonstrated in –d, the expression profile and transcripts per million was quantified in different cancer types. NOTUM expression in COAD, ESCA, READ and STAD tumor tissues was significantly higher compared with that in normal tissues, especially in COAD and READ. From the isoform analysis shown in a boxplot (), the NOTUM isoform was over-expressed in COAD, compared with that in normal tissues. The expression of NOTUM in different pathological stages is illustrated in , and the difference was not statistically significant among various stages. Patients with COAD with a high expression of NOTUM isoform had a poor prognostic outcome (). Pair-wise gene correlation analysis of NOTUM with FAS demonstrated a potential relationship between NOTUM and FAS ().
Figure 1. Bioinformatic analysis of the role of NOTUM in COAD and other digestive system carcinomas using the GEPIA serve. (a) Specific quantified value. (b) Expression profile in different cancer types from the GEPIA databases. (c and d) Protein expression profile assay in different digestive system carcinomas. (e) Isoform analysis for NOTUM in COAD, presented as a boxplot. (f) Pathological stage plot assay for NOTUM in COAD. (g) Survival analyses in COAD cancer type. (h) Pair-wise gene correlation analysis of NOTUM and FAS. NOTUM, notum palmitoleoyl-protein carboxylesterase; COAD, colon adenocarcinoma; FAS, Fas cell surface death receptor; T, tumor; N, normal; TPM, transcripts per million
Varying levels of NOTUM expression in different tissues or cell lines
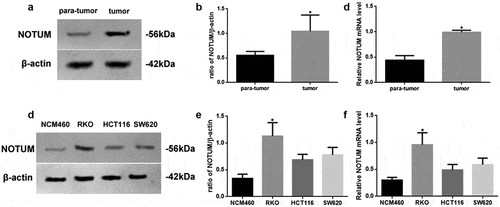
The protein and mRNA levels of NOTUM in different tissues or cell lines were examined using western blot analysis and RT-qPCR (). NOTUM protein and mRNA expression levels in colon carcinoma tissues were increased (P < 0.05), compared with those in para-tumor tissue (–c). In addition, NOTUM protein and mRNA expression levels in RKO cells were significantly increased (P < 0.05), compared with other cells (–f). Thus, RKO cells were used as the colon carcinoma model for the follow-up experiments.
Figure 2. Expression levels of NOTUM in different tissues or cell lines. (a) Western blot analysis of NOTUM expression in tumor and para-tumor tissues, and (b) semi-quantification of results. (c) RT-qPCR assay for mRNA expression of NOTUM in tumor and para-tumor tissues. (d) Western blot analysis of NOTUM expression in NCM460, RKO, HCT116 and SW620 cells, and (e) semi-quantification of results. (f) RT-qPCR assay for mRNA expression of NOTUM in NCM460, RKO, HCT116 and SW620 cells. Protein and mRNA expression levels were normalized to β-actin. n = 6 per group. Data are presented as the mean ± SD. *P < 0.05, vs. para-tumor or NCM460 cells. NOTUM, notum palmitoleoyl-protein carboxylesterase; RT-qPCR, reverse transcription-quantitative PCR
Knockdown of NOTUM could regulate colon carcinoma development and apoptosis in vitro
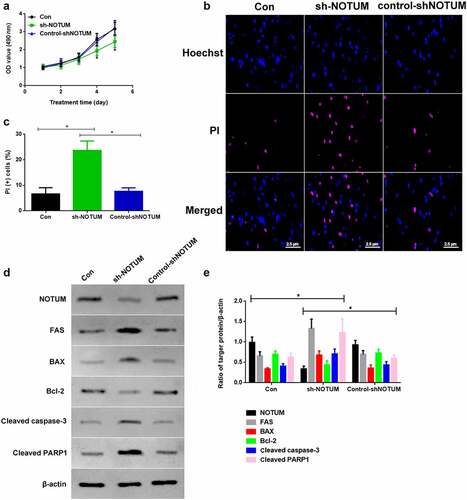
As NOTUM expression is up-regulated in tumors and may be correlated with tumor progression, NOTUM expression was blocked using a NOTUM knockdown lentivirus in vitro to examine the effects (). The cell proliferation of Con, sh-NOTUM and control-shNOTUM RKO cells was investigated. The absorbance values of the MTT assay on 4th and 5th days in the sh-NOTUM group was significantly lower compared with those in the other two groups (). To investigate the apoptotic function of NOTUM, PI staining was used to observe the changes of the number of the apoptotic cells (). The apoptotic level of cells in the sh-NOTUM group was increased compared with the Con and control-shNOTUM groups (P < 0.05; ). In addition, proteins associated with apoptosis were detected via western blotting (). The expression levels of FAS, BAX, cleaved caspase-3 and cleaved PARP1 were increased in the sh-NOTUM group (P < 0.05) compared with the Con and control-shNOTUM groups, while NOTUM and Bcl-2 expression levels demonstrated the opposite trends ().
Figure 3. Knockdown of NOTUM regulates colon adenocarcinoma development and apoptosis in vitro. (a) Proliferation assay was conducted over 5 days. (b) PI staining assay was performed to examine apoptosis. (c) PI (+) cells assay was performed in Con, sh-NOTUM and control-shNOTUM groups in vitro. (d) Western blot analysis of NOTUM, FAS, BAX, Bcl-2, cleaved caspase-3 and cleaved PARP1 expression levels in vitro. (e) Semi-quantification of NOTUM, FAS, BAX, Bcl-2, cleaved caspase-3 and cleaved PARP1 expression levels. Protein levels were normalized to β-actin. n = 6 per group. *P < 0.05, Con and control-shNOTUM groups vs. sh-NOTUM group. NOTUM, notum palmitoleoyl-protein carboxylesterase; FAS, Fas cell surface death receptor; PARP1, poly (ADP-ribose) polymerase 1; Con, control; shRNA, short hairpin RNA; OD, optical density
Knockdown of NOTUM could regulate colon carcinoma development and apoptosis in vivo
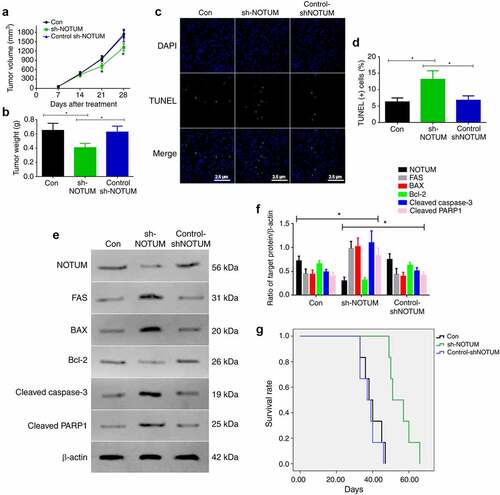
As the knockdown of NOTUM could regulate colon carcinoma development and apoptosis in vitro, this process was also examined in vivo (). Firstly, tumor volume and tumor weights were measured in the Con, sh-NOTUM and control-sh-NOTUM mice. The tumor volume on 21 and 28 days in sh-NOTUM mice was significantly lower compared with that in the other two groups (P < 0.05; ). The tumor weight in sh-NOTUM mice was also decreased (P < 0.05; ). Next, in order to investigate the apoptotic function of NOTUM, TUNEL staining was conducted to observe the changes of the apoptotic cells (), and the level of apoptotic cells in sh-NOTUM mice was increased, compared with the Con and control-shNOTUM groups (P < 0.05; ). In addition, proteins associated with apoptosis were detected using western blot analysis (). The expression levels of FAS, BAX, cleaved caspase-3 and cleaved PARP1 were increased in the sh-NOTUM group (P < 0.05), compared with their expression in the other groups, and NOTUM and Bcl-2 demonstrated contrary results (). Moreover, the survival rate was significantly increased in sh-NOTUM mice compared with the Con and control-shNOTUM groups (P < 0.05; ).
Figure 4. Knockdown of NOTUM regulates colon adenocarcinoma development and apoptosis in vivo. (a) Tumor volume over 28 days period and (b) tumor weight at 28 day in the Con, sh-NOTUM and control-shNOTUM mice. (c and d) Immunofluorescence assay of TUNEL staining (magnification, x400) and (d) TUNEL(+) cells. (e) Western blot analysis of NOTUM, FAS, BAX, Bcl-2, cleaved caspase-3 and cleaved PARP1 expression levels in vivo. (f) Semi-quantification of NOTUM, FAS, BAX, Bcl-2, cleaved caspase-3 and cleaved PARP1 expression levels. Protein levels were normalized to β-actin. *P < 0.05, Con and control-shNOTUM groups vs. sh-NOTUM group. (g) Survival analysis of Con, sh-NOTUM and control-shNOTUM mice. n = 6 per group. NOTUM, notum palmitoleoyl-protein carboxylesterase; FAS, Fas cell surface death receptor; PARP1, poly(ADP-ribose) polymerase 1; Con, control; shRNA, short hairpin RNA
NOTUM regulates FAS-mediated apoptosis via the Wnt signaling pathway
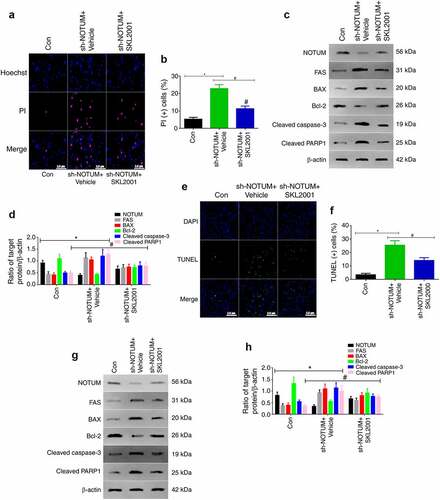
To investigate whether NOTUM is able to regulate Fas-mediated apoptosis via the Wnt signaling, Wnt expression was increased by using SKL2001, which is a Wnt agonist (18). In the experiment, SKL2001 was used to enhance Wnt expression, and apoptosis in cells was examined via western blot analysis and immunofluorescence in vitro and in vivo (). As presented in and b, compared with the sh-NOTUM + Vehicle group, the level of apoptotic cells was significantly decreased in the sh-NOTUM + SKL2001 group in vitro (P < 0.05). The expression levels of FAS, BAX, cleaved caspase-3 and cleaved PARP1 in the sh-NOTUM + SKL2001 group were also decreased (P < 0.05), compared with the sh-NOTUM + Vehicle group. Moreover, the opposite results were obtained for NOTUM and Bcl-2 expression levels ( and d). In vivo, as presented in and f, compared with the sh-NOTUM + Vehicle group, the level of apoptotic cells was significantly decreased in the sh-NOTUM + SKL2001 group (P < 0.05). The expression levels of FAS, Bax, cleaved caspase-3 and cleaved PARP1 in the sh-NOTUM + SKL2001 group were also decreased (P < 0.05), compared with the sh-NOTUM + Vehicle group. Moreover, the opposite results were obtained for NOTUM and Bcl-2 expression levels ( and h).
Figure 5. NOTUM regulates FAS-mediated apoptosis via the Wnt signaling pathway (a) PI staining assay of apoptosis and (b) quantification of PI (+) cells in Con, sh-NOTUM + Vehicle and sh-NOTUM + SKL2001 mice. (c) Western blot analysis of NOTUM, FAS, BAX, Bcl-2, cleaved caspase-3 and cleaved PARP1 expression levels in vitro. (d) Semi-quantification of NOTUM, FAS, BAX, Bcl-2, cleaved caspase-3 and cleaved PARP1 expression levels. (e) Immunofluorescence assay of TUNEL staining (magnification, x400) and (f) number of TUNEL(+) cells in Con, sh-NOTUM + Vehicle and sh-NOTUM + SKL2001 mice. (g) Western blot analysis of NOTUM, FAS, BAX, Bcl-2, cleaved caspase-3 and cleaved PARP1 expression levels in vivo, and (h) semi-quantification of results. Protein levels were normalized to β-actin. n = 6 per group. *P < 0.05, sh-NOTUM + vehicle group vs. Con group; #P < 0.05, sh-NOTUM + SKL2001 group vs. sh-NOTUM + vehicle group. NOTUM, notum palmitoleoyl-protein carboxylesterase; FAS, Fas cell surface death receptor; PARP1, poly(ADP-ribose) polymerase 1; Con, control; shRNA, short hairpin RNA
Discussion
Colorectal cancer is the third most common cancer type and the fourth most common cause of mortality globally, accounting for ~12 million new cases and 600,000 mortalities per year [Citation19]. Previous studies have provided evidence for the ongoing discussion of the requirement to performed molecular tumor analysis in all patients with COAD who received adjuvant chemotherapy [Citation20,Citation21].
Although blood-based protein biomarkers are already utilized in clinical settings, including P53, isocitrate dehydrogenase 1 and Bcl-2, they cannot be used alone to screen or diagnose cancer as the levels of these markers can be abnormal for reasons other than cancer [Citation22]. Thus, there is an urgent requirement for novel biomarkers and molecular targets with relatively high sensitivity and specificity.
NOTUM is a 56-kDa evolutionarily conserved secreted deacylase that is associated the Wnt signaling pathway [Citation23]. NOTUM is overexpressed in hepatocellular carcinoma [Citation24], gastric cancer [Citation25], invasive urothelial carcinoma of the bladder [Citation11] and colorectal cancer [Citation12]. These findings suggest that NOTUM serves a role in multiple cancer types. In order to investigate the potential role of NOTUM in COAD, GEPIA databases were used to predict the potential function of NOTUM in the present study. The current results suggested that NOTUM expression in COAD, ESCA, READ and STAD tumor tissues was higher compared with that in normal tissues, especially in COAD and READ. Moreover, patients with COAD with high expression of the NOTUM isoform had a poor prognostic outcome. Pair-wise gene correlation analysis identified a potential relationship between NOTUM and FAS. Thus, these results indicated that NOTUM may serve a role in apoptosis in COAD.
In the present study, western blot analysis and RT-qPCR were used to analyze the protein and mRNA expression levels of NOTUM in different tissue or cell lines, and it was found that NOTUM expression was increased. This suggests that NOTUM may be involved in the development and progression of colon cancer.
FAS stimulates the initiation of apoptosis, which leads to the recruitment of FAS-associated death domain proteins and self-activates caspase-8, ultimately leading to apoptosis [Citation26]. To investigate the relationship between NOTUM and FAS, PI and TUNEL staining were conducted, and apoptotic-related protein expression levels were detected via western blotting after NOTUM knockdown lentivirus administration in vitro and in vivo. The apoptotic cell levels were increased in the sh-NOTUM group. In addition, NOTUM knockdown lentivirus administration improved proliferation, tumor volume, tumor weight and survival rate. These results demonstrated that low levels of NOTUM can significantly inhibit the proliferation and metastasis of colon cancer cells by regulating FAS-mediated apoptosis, as well as reduce the tumor volume and ultimately improve the prognosis.
The Wnt signaling pathway is associated with cell differentiation, polarization and migration during development [Citation27]. Previous studies have reported that the Wnt signaling pathway is a key pathway involved in various processes of colon cancer [Citation28]. To date, studies have examined the roles and mechanisms of Wnt signaling in regulating cell apoptosis, stimulating angiogenesis and maintaining highly resistant CSCs [Citation29,Citation30]. The Wnt signaling pathway has recently been revealed to be associated with NOTUM [Citation31]. However, the mechanism via which NOTUM regulates apoptosis via the Wnt signaling pathway in COAD is not fully understood. To address this issue, the present study used Wnt agonists to increase Wnt signaling activity. The present results demonstrated that the Wnt agonists reversed the progress of apoptosis by NOTUM regulation. Thus, it was suggested that NOTUM regulated Fas-mediated apoptosis via Wnt signaling.
However, the present study also has several limitations; firstly, the direct relationship between NOTUM and Fas was not analyzed using co-immunoprecipitation, thus it was not fully proved that NOTUM and Fas bind. Secondly, for technical reasons, flow cytometric analysis of apoptosis was not performed. Lastly, the mechanism of the signaling pathway should be further investigated.
Conclusion
The present findings indicated that NOTUM knockdown significantly activated apoptotic function and inhibited the development of COAD by modulating FAS and prolonging the survival time of mice. Collectively, these results may provide novel strategies for COAD treatment by utilizing the mechanism of apoptosis.
Highlights
NOTUM expression is up-regulated in COAD and may be correlated with tumor progression.
NOTUM regulates FAS-mediated apoptosis in COAD.
NOTUM regulates apoptosis via the Wnt signaling pathway in COAD.
Ethics approval and consent to participate
All experimental procedures were approved by the Human Ethics Committee of Shidong Hospital, and the informed consent were obtained from each subject (No.2018041201).
All animals used in this experiment were cared for in strict accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). All experimental procedures were approved by Animal Ethics Committee of Shidong Hospital (No.2018041202).
Authors’ contribution
HG & QN designed, performed the study and wrote the first draft. YZ & KH collected, analyzed and interpreted the data. YW, XX, & KH analyzed the data, as well as wrote and revised the paper. All authors read and approved the final manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Cappell MS. Pathophysiology, clinical presentation, and management of colon cancer. Gastroenterol Clin North Am. 2008 Mar;37(1):1–24.
- Ji X, Peng Q, Wang M. Anti-colon-cancer effects of polysaccharides: a mini-review of the mechanisms. Int J Biol Macromol. 2018 Jul 15;114:1127–1133.
- Nikodinovic-Runic J, Mojic M, Kang Y, et al. Undecylprodigiosin conjugated monodisperse gold nanoparticles efficiently cause apoptosis in colon cancer cells in vitro. J Mater Chem B. 2014 Jun 7;2(21):3271–3281.
- Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011 Nov 11;147(4):742–758.
- Fulda S. Tumor resistance to apoptosis. Int J Cancer. 2009 Feb 1;124(3):511–515.
- Fulda S. Targeting apoptosis for anticancer therapy. Semin Cancer Biol. 2015 Apr;31:84–88.
- Nagata S. Fas-mediated apoptosis. Adv Exp Med Biol. 1996;406:119–124.
- Liang Q, Peng T, Sun B, et al. Gene expression patterns determine the differential numbers of dorsocentral macrochaetes between Musca domestica and Drosophila melanogaster. Genesis. 2018 Dec;56(11–12):e23258.
- Roberts-Galbraith RH, Newmark PA. Follistatin antagonizes activin signaling and acts with notum to direct planarian head regeneration. Proc Natl Acad Sci USA. 2013 Jan 22;110(4):1363–1368.
- Petersen CP, Reddien PW. Polarized notum activation at wounds inhibits Wnt function to promote planarian head regeneration. Science. 2011 May 13;332(6031):852–855.
- Kuang S, Li H, Feng J, et al. Correlation of BRCA2 gene mutation and prognosis as well as variant genes in invasive urothelial carcinoma of the bladder. Cancer Biomark. 2019;25(2):203–212.
- Yoon JH, Kim D, Kim J, et al. NOTUM is involved in the progression of colorectal cancer. Cancer Genomics Proteomics. 2018 Nov-Dec;15(6):485–497.
- Binh TD, Pham TLA, Men TT, et al. Dysfunction of LSD-1 induces JNK signaling pathway-dependent abnormal development of thorax and apoptosis cell death in Drosophila melanogaster. Biochem Biophys Res Commun. 2019 Aug 20;516(2):451–456.
- Zheng LZ, Chen SZ. shRNAinduced knockdown of the SPERT gene inhibits proliferation and promotes apoptosis of human colorectal cancer RKO cells. Oncol Rep. 2018 Aug;40(2):813–822.
- Pitts M. Applied research ethics national association/ office of laboratory animal welfare. Institutional Animal C, Use C. A guide to the new ARENA/OLAW IACUC guidebook. Lab Anim (NY). 2002 Oct;31(9):40–42.
- MacArthur Clark JA, Sun D. Guidelines for the ethical review of laboratory animal welfare People’s Republic of China National Standard GB/T 35892-2018 [Issued 6 February 2018 Effective from 1 September 2018]. Anim Model Exp Med. 2020 Mar 1;3:103–113.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001 Dec;25(4):402–408.
- Liao S, Chen H, Liu M, et al. Aquaporin 9 inhibits growth and metastasis of hepatocellular carcinoma cells via Wnt/beta-catenin pathway. Aging (Albany NY). 2020 Jan 22;12(2):1527–1544.
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–1502.
- Glynne-Jones R, Harrison M. FOLFOXIRI reintroduction in metastatic colorectal cancer. Lancet Oncol. 2020;21(4):468–469.
- Chadi SA, Malcomson L, Ensor J, et al. Factors affecting local regrowth after watch and wait for patients with a clinical complete response following chemoradiotherapy in rectal cancer (InterCoRe consortium): an individual participant data meta-analysis. Lancet Gastroenterol Hepatol. 2018;3(12):825–836.
- He L, Khanal P, Morse CI, et al. Differentially methylated gene patterns between age-matched sarcopenic and non-sarcopenic women. J Cachexia Sarcopenia Muscle. 2019 Dec;10(6):1295–1306.
- Zhang X, Cheong SM, Amado NG, et al. Notum is required for neural and head induction via Wnt deacylation, oxidation, and inactivation. Dev Cell. 2015 Mar 23;32(6):719–730.
- Torisu Y, Watanabe A, Nonaka A, et al. Human homolog of NOTUM, overexpressed in hepatocellular carcinoma, is regulated transcriptionally by beta-catenin/TCF. Cancer Sci. 2008 Jun;99(6):1139–1146.
- Madan B, Ke Z, Lei ZD, et al. NOTUM is a potential pharmacodynamic biomarker of Wnt pathway inhibition. Oncotarget. 2016 Mar 15;7(11):12386–12392.
- Xu J, Liu F, Xiong Z, et al. The cleft palate candidate gene BAG6 supports FoxO1 acetylation topromote FasL-mediated apoptosis during palate fusion. Exp Cell Res. 2020 Nov 15;396(2):112310.
- Taciak B, Pruszynska I, Kiraga L, et al. Wnt signaling pathway in development and cancer. J Physiol Pharmacol. 2018 Apr;69(2). DOI:10.26402/jpp.2018.2.07.
- Rodriguez-Salas N, Dominguez G, Barderas R, et al. Clinical relevance of colorectal cancer molecular subtypes. Crit Rev Oncol Hematol. 2017 Jan;109:9–19.
- Reyes-Farias M, Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int J Mol Sci. 28 Jun 2019;20(13). DOI:10.3390/ijms20133177.
- Guo YZ, Xie XL, Fu J, et al. SOX9 regulated proliferation and apoptosis of human lung carcinoma cells by the Wnt/beta-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2018 Aug;22(15):4898–4907.
- Zhao Y, Ren J, Hillier J, et al. Structural characterization of melatonin as an inhibitor of the Wnt deacylase Notum. J Pineal Res. 2020 Mar;68(2):e12630.
