ABSTRACT
Recurrent spontaneous abortion (RSA) is a threat to human reproductive health worldwide. CircPUM1 has been reported to participate in the pathogenesis of various diseases. However, there has been no report on its association with RSA yet. In this study, gene expressions were examined by RT-qPCR. Protein levels of JUNB and cleaved caspases-3 were detected by Western blotting. ELISA was used to detect TNF-α, IL-6, and IL-8 levels. Cell viability, migration, invasion, and apoapsis were analyzed using CCK-8, transwell, and flow cytometry assays. The association between miR-30a-5p and circPUM1 or JUNB was identified by bioinformatics analysis, dual-luciferase reporter assay, and RIP assay. Herein, we found circPUM1 was significantly downregulated in RSA placental samples. CircPUM1 knockdown induced decreased proliferation, migration, and invasion, but increased apoptosis, pro-apoptotic protein (cleaved caspases-3) level, and proinflammatory factor (TNF-α, IL-6, and IL-8) secretion in trophoblast cells. Furthermore, we confirmed that circPUM1 was a sponge for miR-30a-5p, and JUNB was directly targeted by miR-30a-5p. It was demonstrated that miR-30a-5p inhibition could reverse trophoblast cell dysfunction and inflammation induced by circPUM1 knockdown. In addition, we found that JUNB expression was negatively modulated by miR-30a-5p and positively regulated by circPUM1. Moreover, circPUM1 inhibition exacerbated dysfunction and inflammation in trophoblast cells via targeting JUNB. To sum up, our study indicated that circPUM1 could impair RSA occurrence and development by facilitating trophoblast cellular processes and protecting against inflammation via the miR-30a-5p/JUNB axis, providing a new target for the improvement of RSA diagnosis and treatment.
Introduction
As a kind of common pregnancy complication causing female infertility, recurrent spontaneous abortion (RSA) is characterized as two or more continuous spontaneous miscarriages before 24 weeks of pregnancy [Citation1,Citation2]. Statistically, almost 1% to 5% of pregnant women experience RSA at childbearing age, which poses a threat to human reproductive health [Citation1]. However, the detailed molecular mechanisms of RSA pathogenesis remain unknown. In early pregnancy, trophoblast cells exert crucial biological effects in placenta anchorage, maternal spiral artery modeling, decidual angiogenesis regulation, hormone, and cytokine secretion, as well as crosstalk with maternal immune cells [Citation3]. Trophoblast cell dysfunction and inflammation could lead to pregnancy-related complications including RSA [Citation4,Citation5]. Previous research also demonstrated that pro-inflammatory cytokines repressed the migrative and invasive abilities of trophoblast cells, thus resulting in RSA [Citation6]. Therefore, mechanisms associated with trophoblast cell dysfunction and inflammation may help to enhance understanding of RSA pathogenesis.
Circular RNAs (CircRNAs) are a category of covalent ring-like RNAs [Citation7]. As demonstrated by previous studies, a number of genes regulating cellular activities are associated with RSA susceptibility, while there is limited knowledge on the role of circRNAs in RSA [Citation8,Citation9]. A report from Li et al. revealed that circ_ZUFSP improves RSA by facilitating trophoblast cell migration and invasion via miR-203/STOX1 axis [Citation10]. Recently, multiple studies have demonstrated that circRNAs are deeply implicated in preeclampsia (PE), another obstetrical complication that manifests trophoblast cell dysfunction and inflammation and may also cause pregnancy loss [Citation11]. For example, Shen et al. reported circTRNC18 restrained trophoblast cell migration and epithelial–mesenchymal transition (EMT) in preeclampsia via the miR-762/Grhl2 axis [Citation12]. Zheng et al. also revealed that downregulated circ_HIPK3 contributed to PE development by inhibiting trophoblast cell proliferation, migration, invasion, and tube formation [Citation13]. A previous work identified circRNA PUM1 (circPUM1) as a down-regulated circRNA in preeclampsia [Citation14]. Therefore, it was assumed that circPUM1 might be also downregulated in RSA and implicated in RSA occurrence and development.
In this work, we intended to explore the potential functions of circPUM1 in RSA and investigate the detailed mechanism by which circPUM1 regulates biological activities and inflammatory response of trophoblast cells. Our findings might provide a promising biomarker for RSA diagnosis and treatment.
Materials and Methods
Patients and tissue samples
30 RSA patients and 30 age-matched healthy pregnant women (with at least one successful pregnancy and without spontaneous abortion, preterm labor, or PE history) were recruited at the Third Affiliated Hospital of Soochow University. Each participant signed the informed consent. RSA placental tissue samples were collected in surgical abortion immediately (within 24 hours) after determination of fetal death. Normal placental tissue samples were collected from healthy pregnant women in artificial abortion performed to terminate unplanned pregnancy within 6 to 12 gestation weeks. All placental tissue samples were immediately frozen and kept in liquid nitrogen for subsequent experiments. The Ethic Committee of the Third Affiliated Hospital of Soochow University permitted this study.
Cell culture and transfection
Human placenta trophoblast cells (HTR-8/SVneo) acquired from BeNa Culture Collection (Beijing, China) were cultured in DMEM (10% FBS, 100 IU/ml penicillin as well as 10 mg/mL streptomycin) under a humid environment at 37°C with 5% CO2.
Small interfering RNA (si-RNA) against circPUM1 (si-circPUM1: 5′-ACCUGAUCAUCUACAACAUTT-3′) and relevant negative control (si-NC: 5′-GUAUUGUAGAUGAUGGUCATT-3′), pcDNA3.1 empty vector (Vector), circPUM1 overexpression vector (oe-circPUM1), JUNB overexpression vector (oe-JUNB), miR-30a-5p mimics (5ʹ-UGUAAACAUCCUCGACUGGAAG-3ʹ), miR-30a-5p inhibitor (5ʹ-CTTCCAGTCGAGGATGTTTACA-3ʹ) and their negative controls (NC mimics: 5ʹ-ACAUAGGGCCCAUGCUAACUGC-3ʹ; NC inhibitor:5ʹ-UCACAACCUCCUAGAAAGAGUA-3ʹ) obtained from GenePharma (Shanghai, China) were transfected into HTR-8/SVneo cells via LipofectamineTM 2000 (Invitrogen). The HTR-8/SVneo cells were cultivated for 24 h after transfection and then collected for following tests.
RT-qPCR
Trizol (Invitrogen) was applied for total RNA extraction. In short, RNAs were transcribed into cDNA in reverse via PrimeScript™ RT reagent Kit (Takara, China). Then, qPCR was performed with Bestar®SybrGreen qPCR mastermix (DBI). Relative gene expressions were determined by 2−ΔΔCt method, with circPUM1, JUNB and miR-30a-5p expression respectively normalized to GAPDH or U6 [Citation15–18]. The primer sequences applied were as listed below: circPUM1 Forward: 5′-GATTATTCAGGCACGCAGGT-3′, Reverse: 5′-CCCTCCTCCTTCAAATCTCC-3′; JUNB Forward: 5′-GACACAGGCGCATCTCTGAAG-3ʹ,Reverse: 5′-GATCACGCCGTTGCTGTTG-3ʹ; GAPDH Forward: 5′-GCACCGTCAAGGCTGAGAAC-3′, Reverse: 5′-ATGGTGGTGAAGACGCCAGT-3ʹ; miR-30a-5p Forward: CGCGATGTTGAAACATCCTCGAC-3ʹ, Reverse: 5ʹ-ATCCAGTGCAGGGTCCGAGG-3ʹ; U6 Forward: 5′-CCTGCGCAAGGATGAC-3′, Reverse: 5′- -GCTTCGGCAGCACATATACTAAAAT-3′.
Western blotting
Total protein obtained from each sample was equally separated by 10% SDS-PAGE before the transfer to PVDF membranes (Millipore, USA). Then, PVDF membranes were blocked with 5% skimmed milk and cultivated with primary antibodies against cleaved caspases-3, JUNB and GAPDH at 4°C overnight. Afterward, the membranes were cultivated with the secondary antibody. The protein bands were visualized with an enhanced chemiluminescent substrate (ECL, USA).
CCK-8
CCK-8 was adopted for cell viability assessment. In brief, transfected HTR-8/SVneo cells were seeded to 96-well plates (5 × 103 cells/well) and then cultivated at 37°C for 0, 24, 48, or 72 h. After 10 μL CCK-8 reagent (Dojindo Laboratories, Japan) was supplemented at the time mentioned above, the cells were cultured at 37°C for another 2 h. The absorbance was analyzed via a microplate reader at 450 nm [Citation19].
Transwell
Transwell inserts with or without Matrigel coating on the membrane of upper wells were used to assess cell invasion or migration via Transwell assays. Briefly, HTR-8/SVneo cells (5 × 104 cells/well) were seeded to upper wells filled with 100 μL serum-free DMEM. The lower wells were filled with 800 μL DMEM containing 10% FBS. HTR-8/SVneo cells below the membrane were fixed and stained with 10% methanol and 0.5% crystal violet for 20 min after incubation for 48 h at 37°C with 5% CO2. Ultimately, the migrated or invaded HTR-8/SVneo cells were counted with an optical microscope (Olympus) [Citation20].
Flow cytometry
Flow cytometry assay was carried out for cell apoptotic rate analysis. Briefly, transfected HTR-8/SVneo cells were rinsed with PBS and digested in 0.25% trypsin. Then, the collected HTR-8/SVneo cells were subject to centrifugation for 5 min at 2000 rpm to remove supernatant and resuspended in binding buffer. Next, the re-suspended HTR-8/SVneo cells were cultured with 5 μl Annexin V-FITC and 5 μl PI for 20 min in darkness at 4°C for staining. Finally, cell apoptosis was analyzed by flow cytometry (FACSCalibur, USA) [Citation21].
Enzyme-linked immunosorbent assay (ELISA)
The levels of the inflammatory factors-tumor necrosis factor-α (TNF-α), interleukin (IL) −6, and IL-8 were measured using TNF-α ELISA kit (R&D Systems), IL-6 ELISA kit (Sangon Biotech), and IL-8 ELISA kit (R&D Systems) according to the manufacture’s protocols, respectively [Citation22].
Dual-Luciferase Reporter Assay
Binding sequences between miR-30a-5p and circPUM1 (circPUM1-WT) or 3′UTR of JUNB (JUNB-WT) and respective mutated sequences (circPUM1-MUT and JUNB-MUT) were cloned into pmirGLO vectors (Promega, USA). Thereafter, HTR-8/SVneo cells were transfected with the above-mentioned plasmids, together with miR-30a-5p mimics or NC mimics, and then collected and lysed. Finally, Dual-Glo® Luciferase Assay System (Promega) was employed for analysis of Firefly and Renilla luciferase activities [Citation23].
RNA immunoprecipitation (RIP) assay
The RIP Kit (Millipore, USA) was applied for RIP assay. Briefly, HTR-8/SVneo cells were lysed with RIP lysis buffer. Then, the supernatant was cultivated with protein A/G magnetic beads in combination with anti-Argonaute-2 (Anti-Ago2) or anti-Immunoglobulin G (Anti-IgG) [Citation24]. Then, circPUM1 and miR-30a-5p levels in the precipitates were determined by RT-qPCR.
Statistical analysis
All data were acquired from results of at least three independent tests and shown as mean ± SD. GraphPad Prism 6.0 was applied for data analysis. To acquire statistical differences, Student’s t-test or one-way ANOVA was utilized for two-group or multiple-group comparisons. Statistical differences with P value <0.05 were believed significant.
Results
Herein, we intended to investigate the role of circPUM1 in regulating the functions of trophoblast cells in RSA. Through a series of in vitro assays, we found that circPUM1 attenuates trophoblast cell dysfunction and inflammation in RSA through the miR-30a-5p/JUNB axis. Therefore, our findings, for the first time, explored the functional effects of circPUM1 on the regulation of trophoblast cellular processes, providing a novel target for RSA diagnosis and treatment.
CircPUM1 knockdown induces dysfunction and inflammation in HTR-8/SVneo cells
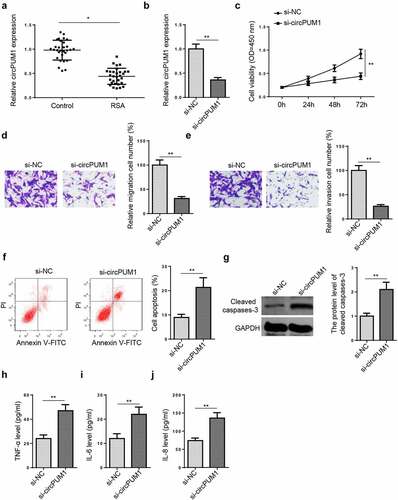
Firstly, circPUM1 expression was measured in 30 RSA patients and 30 normal pregnant women via RT-qPCR. The RSA group showed significantly lower circPUM1 levels in placental tissues (), relative to the control group. To investigate the biological role of circPUM1 in RSA, circPUM1 was knocked down in HTR-8/SVneo cells. According to RT-qPCR, HTR-8/SVneo cells transfected with si-circPUM1 transfection showed a significant reduction in circPUM1 expression, compared with that in si-NC group (). CCK-8 assay suggested circPUM1 inhibition reduced cell viability (). Transwell assays showed that circPUM1 depletion inhibited the migrative and invasive abilities of HTR-8/SVneo cells ( and e). Flow cytometry assay showed that circPUM1 deficiency accelerated HTR-8/SVneo cell apoptosis (). Similarly, the cleaved caspases-3 level was also increased significantly after circPUM1 knockdown (). As indicated by ELISA assay, the levels of pro-inflammatory factors (TNF-α, IL-6, and IL-8) were substantially increased by circPUM1 knockdown (). To sum up, circPUM1 improved trophoblast cell dysfunction and inflammation.
Figure 1. CircPUM1 knockdown induces dysfunction and inflammation in HTR-8/SVneo cells. (a) CircPUM1 expression was determined by RT-qPCR in placental tissues of RSA patients and normal controls. (b) CircPUM1 expression was determined in HTR-8/SVneo cells after transfection with si-circPUM1 and si-NC. (c) CCK-8 analysis revealed a significant reduction in cell viability following circPUM1 knockdown. (d and e) Transwell assays showed that circPUM1 depletion inhibited cell migration and invasion. (f) Flow cytometry revealed an increase in apoptosis following circPUM1 knockdown. (g) The cleaved caspase-3 level was detected by Western blotting. (h-j) CircPUM1 knockdown promoted TNF-α (h), IL-6 (i), and IL-8 (j) expressions, as determined by ELISA. All data were obtained from three independent experiments (n = 3). *P < 0.05 and **P < 0.01
CircPUM1 sponges miR-30a-5p in HTR-8/SVneo cells
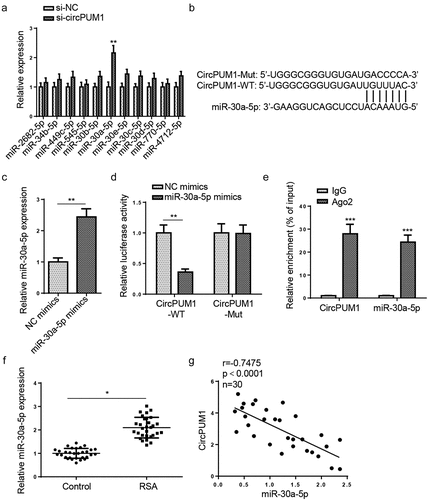
To further probe into the latent circPUM1-mediated mechanism in RSA, we used StarBase website to find downstream miRNAs of circPUM1 under certain conditions (Degradome Data: high stringency (≥3); CLIP Data: strict stringency (≥5)). Among the 11 miRNAs predicted (miR-2682-5p, miR-34b-5p, miR-449 c-5p, miR-545-5p, miR-30b-5p, miR-30a-5p, miR-30e-5p, miR-30 c-5p, miR-30d-5p, miR-770-5p, and miR-4712-5p), miR-30a-5p expression was remarkably higher in HTR-8/SVneo cells transfected with si-circPUM1 (). It has been demonstrated by previous reports that miR-30a-5p represses cell viability, migration, and invasion, accelerates cell apoptosis, and exacerbates inflammatory response [Citation25–27]. Therefore, miR-30a-5p was selected as the target miRNA for circPUM1 in this work. The binding site between circPUM1 and miR-30a-5p was shown in . To further confirm the binding condition between miR-30a-5p and circPUM1, dual-luciferase reporter assay was conducted in HTR-8/SVneo cells. Firstly, the transfection efficiency of miR-30a-5p mimics was assessed in HTR-8/SVneo cells by RT-qPCR (). It was observed that miR-30a-5p overexpression diminished the luciferase activity of circPUM1-WT, rather than circPUM1-MUT (). RIP assay confirmed that the expression levels of both circPUM1 and miR-30a-5p were enriched in Ago2 precipitates, relative to control IgG precipitates (). In addition, miR-30a-5p expression was also markedly elevated in RSA placental tissue samples, relative to the control group (). Pearson’s correlation analysis showed that miR-30a-5p expression was negatively correlative to circPUM1 expression in RSA placental tissues (). Collectively, these results indicated that circPUM1 directly targeted and adversely regulated miR-30a-5p in RSA.
Figure 2. CircPUM1 sponges miR-30a-5p in HTR-8/SVneo cells. (a) The expression of miRNAs was appraised in cells transfected with si-circPUM1 and si-NC. (b) The binding site between miR-30a-5p and circPUM1 was predicted by StarBase website. (c) The expression of miR-30a-5p was examined in cells transfected with miR-30a-5p mimics and NC mimics. (d and e) Luciferase reporter and RIP assays were conducted to assess the binding of miR-30a-5p and circPUM1 in HTR-8/SVneo cells. (f) miR-30a-5p expression was tested in placental tissues of RSA patients and normal controls. (g) Pearson’s correlation analysis was used to analyze the association between miR-30a-5p and circPUM1 expressions in RSA placental tissues. All data were obtained from three independent experiments (n = 3). *P < 0.05, **P < 0.01 and ***P < 0.001
CircPUM1 knockdown aggravates HTR-8/SVneo cell dysfunction and inflammation via interaction with miR-30a-5p
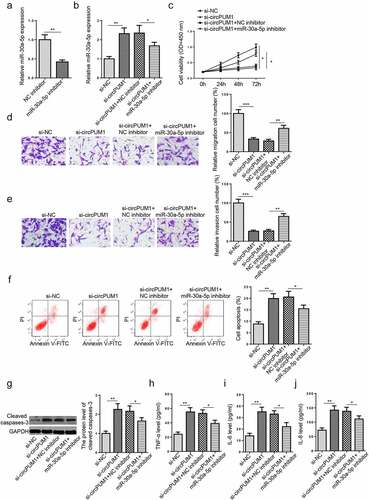
To determine the functions of miR-30a-5p in the biological activities of HTR-8/SVneo cells mediated by circPUM1, rescue assays were performed. As revealed by RT-qPCR assay, miR-30a-5p inhibitor visibly restrained miR-30a-5p enrichment in HTR-8/SVneo cells (). Subsequently, si-NC, si-circPUM1, si-circPUM1+ NC inhibitor, or miR-30a-5p inhibitor were severally transfected into HTR-8/SVneo cells. RT-qPCR results exhibited that circPUM1 silencing significantly increased miR-30a-5p level in HTR-8/SVneo cells; whereas such an increase was abolished by miR-30a-5p suppression to a certain extent (). Data acquired from rescue assays also showed that miR-30a-5p inhibition partly eliminated the impacts of circPUM1 blocking on HTR-8/SVneo cell viability, migration, invasion, apoptosis, cleaved caspases-3 level, and secretion of proinflammatory cytokines (). In sum, circPUM1 ameliorated trophoblast cell dysfunction and inflammation via miR-30a-5p.
Figure 3. CircPUM1 knockdown aggravates HTR-8/SVneo cell dysfunction and inflammation via interaction with miR-30a-5p. (a) MiR-30a-5p expression was tested in HTR-8/SVneo cells after transfection with NC inhibitor or miR-30a-5p inhibitor. (b) HTR-8/SVneo cells were transfected with si-NC, si-circPUM1, si-circPUM1+ NC inhibitor, or miR-30a-5p inhibitor, respectively. (c) Cell proliferation was analyzed by CCK-8 assay. (d and e) Transwell assay was conducted to detect cell migration and invasion abilities. (f) Cell apoptosis was analyzed using Flow cytometry analysis. (g) The cleaved caspase-3 level was detected by Western blotting. (h-j) TNF-α (h), IL-6 (i), and IL-8 (j) expressions were determined by ELISA. All data were obtained from three independent experiments (n = 3). *P < 0.05 and **P < 0.01
CircPUM1 positively regulates JUNB expression via interaction with miR-30a-5p
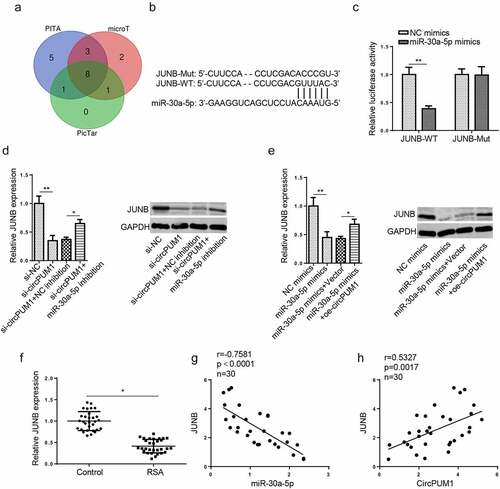
To explore the downstream regulatory pathway of miR-30a-5p, bioinformatics analysis (PicTar, PITA, and microT) was conducted to predict target genes. 8 candidate mRNAs (ZNF644, ARID3A, JUNB, SRSF7, AMOTL1, GNAI2, ELOVL5, MARCKS) were found under the following conditions: Degradome Data: high stringency (≥3); CLIP Data: strict stringency (≥5) (). Among them, JUNB has been demonstrated to facilitate cell proliferation, migration, and invasion, and inhibit cell apoptosis [Citation28–30]. As revealed by Zhang et al., JUNB negatively regulates TNF-α expression and exerts an inhibitory effect in NF-κB-dependent inflammation [Citation31]. Therefore, JUNB was chosen as a downstream target of miR-30a-5p in our study. The potential binding site between 3′ UTR of JUNB and miR-30a-5p was presented in . A dual-luciferase reporter assay showed that miR-30a-5p overexpression decreased the luciferase activity of JUNB-WT reporter but exhibited nearly no effects on JUNB-MUT reporter (), confirming miR-30a-5p directly targets JUNB in HTR-8/SVneo cells. Besides, it was discovered that JUNB mRNA and protein levels were decreased by circPUM1 knockdown or miR-30a-5p overexpression, while such a reduction could be partially reversed by miR-30a-5p inhibition or circPUM1 upregulation ( and e). In addition, we found that JUNB expression was decreased in RSA placental tissues (). It was also confirmed by Pearson’s correlation analysis that JUNB expression was negatively associated with miR-30a-5p expression but positively related to circPUM1 expression in RSA placental tissues ( and h). Therefore, circPUM1 positively regulates JUNB expression in RSA as a sponge for miR-30a-5p.
Figure 4. CircPUM1 positively regulates JUNB expression via interaction with miR-30a-5p. (a) Venn diagram showed the downstream target genes which could bind with miR-30a-5p in PicTar, PITA, and microT databases. (b) StarBase website predicted the putative binding site between miR-30a-5p and JUNB. (c) A luciferase reporter assay was conducted to verify the combination between miR-30a-5p and JUNB. (d) JUNB mRNA and protein levels were detected by RT-qPCR and western blotting in HTR-8/SVneo cells transfected with si-NC, si-circPUM1, si-circPUM1+ NC inhibitor, or si-circPUM1+ miR-30a-5p inhibitor, respectively. (e) JUNB mRNA and protein levels were detected by RT-qPCR and western blotting in HTR-8/SVneo cells transfected with NC mimics, miR-30a-5p mimics, miR-30a-5p mimics+Vector, or miR-30a-5p mimics+oe-circPUM1, respectively. (f) JUNB mRNA expression was tested in placental tissues of RSA patients and normal controls. (g and h) Pearson’s correlation analysis was used to analyze the association between JUNB mRNA expression and miR-30a-5p or circPUM1 enrichment in RSA placental tissues. All data were obtained from three independent experiments (n = 3). *P < 0.05 and **P < 0.01
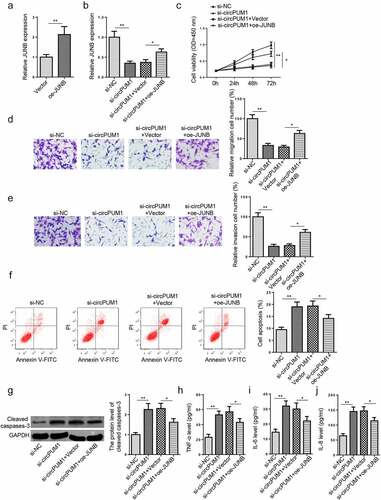
CircPUM1 depletion causes HTR-8/SVneo cell dysfunction and inflammation via JUNB
Considering JUNB exhibited lower expression in RSA placental tissues than normal tissues and circPUM1 regulated JUNB expression via miR-30a-5p, we speculated that JUNB could be involved in the circPUM1-mediated biological activities and inflammatory response of HTR-8/SVneo cells. To validate this possibility, si-NC, si-circPUM1, si-circPUM1+ Vector, and si-circPUM1+ oe-JUNB were respectively transfected into HTR-8/SVneo cells. JUNB overexpression efficiency in HTR-8/SVneo cells was confirmed by RT-qPCR (). RT-qPCR results exhibited that circPUM1 blocking significantly reduced JUNB level in HTR-8/SVneo cells, while such a reduction was partly eliminated by JUNB overexpression (). Rescue assays also uncovered that JUNB addition partially abrogated the effects of circPUM1 silencing on HTR-8/SVneo cell viability, migration, invasion, apoptosis, cleaved caspases-3, and production of proinflammatory cytokines (). Altogether, the results illustrated that circPUM1 inhibition exacerbated dysfunction and inflammation in trophoblast cells via targeting JUNB.
Figure 5. CircPUM1 depletion causes HTR-8/SVneo cell dysfunction and inflammation via JUNB. (a) JUNB mRNA expression in HTR-8/SVneo cells transfected with Vector and oe-JUNB was measured by RT-qPCR. (b) JUNB mRNA expression in HTR-8/SVneo cells transfected with si-NC, si-circPUM1, si-circPUM1+ Vector, si-circPUM1+ oe-JUNB was measured by RT-qPCR. (c) Cell proliferation was analyzed by CCK-8 assay. (d and e) Transwell assay was conducted to detect cell migration and invasion abilities. (f) Cell apoptosis was analyzed using Flow cytometry analysis. (g) The cleaved caspase-3 level was detected by Western blotting. (h-j) TNF-α (h), IL-6 (i), and IL-8 (j) expressions were determined by ELISA. All data were obtained from three independent experiments (n = 3). *P < 0.05 and **P < 0.01
Discussion
Due to indefinite etiology and lack of effective diagnostic and therapeutic methods, RSA is a serious reproductive disease threatening women at childbearing age [Citation32]. Earlier studies have elucidated that circRNAs exert important effects in the detection and diagnosis of diseases by serving as biomarkers [Citation33]. CircRNAs are also deeply implicated in trophoblast cell proliferation, migration, invasion, apoptosis, and inflammatory response, and abnormal circRNA expression may also lead to placental dysfunction, suggesting their significant clinical value in obstetrical complications, including RSA [Citation10].
In this study, we observed that circPUM1 exhibited lower abundance in RSA placental tissue samples, relative to the control group. CircPUM1 was recognized as a promotor for cell proliferation, migration, and invasion by preceding works. To cite an instance, Guan et al. discovered that circPUM1 interacted with miR-615-5p and miR-6753-5p to accelerate ovarian cancer cell proliferation, migration, and invasion and reduce apoptosis [Citation34]. Chen et al. found that circPUM1 facilitated proliferation, migration, and invasion but restrained apoptosis of lung adenocarcinoma cells as a sponge of miR-326 [Citation35]. In consonance with the above studies, circPUM1 knockdown impaired the proliferative, migrative, and invasive abilities but accelerated apoptosis of HTR-8/SVneo cells. Inflammatory response activated by pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-8, could impair trophoblast cell functions and even cause RSA [Citation36,Citation37]. For example, TNF-α contributes significantly to pregnancy maintenance; however, abnormal TNF-α expression may result in RSA [Citation37,Citation38]. Moreover, IL-6 and IL-8 expressions were also significantly upregulated in RSA [Citation36,Citation39]. In our study, it was found that circPUM1 inhibition promoted the production of TNF-α, IL-6, and IL-8. These results suggested that circPUM1 promoted proliferation, migration, and invasion, while inhibited apoptosis and inflammatory response in trophoblast cells.
A considerable number of works have identified the roles of circRNAs in regulating cell proliferation, metastasis, apoptosis, and inflammatory response by regulating mRNA levels as competitive endogenous RNAs (ceRNAs) for miRNAs [Citation40,Citation41]. In our study, miR-30a-5p was upregulated in RSA. In addition, miR-30a-5p was directly targeted and negatively regulated by circPUM1 in RSA. Zhang et al. disclosed that miR-30a-5p conferred inflammation in myocarditis-induced cardiac injury via regulating SOCS1 [Citation42]. Moreover, Guo et al. uncovered that miR-30a-5p impaired proliferation and metastasis of trophoblast cells through the ERK/AKT pathway via targeting B3GNT5 [Citation25]. Herein, rescue assays also demonstrated that circPUM1 facilitated proliferation, migration, and invasion, but suppressed apoptosis and proinflammatory secretion in trophoblast cells as a sponge for miR-30a-5p.
As a subunit of AP-1 transcription factor family, JUNB is an inflammation suppressor [Citation43]. JUNB has also been proven as a promotor of cell proliferation, migration, and invasion [Citation29,Citation44]. In this study, JUNB was verified as a downstream target for miR-30a-5p. It was also proven that circPUM1 functioned as a ceRNA for miR-30a-5p to regulate the JUNB level in HTR-8/SVneo cells. Besides, the suppressive effect on cell viability, migration, and invasion, as well as the promoting impact on cell apoptosis and secretion of proinflammatory factors in HTR-8/SVneo cells induced by circPUM1 inhibition could also be reversed by JUNB overexpression.
Conclusion
To sum up, our findings revealed that circPUM1 attenuates trophoblast cell dysfunction and inflammation via the miR-30a-5p/JUNB axis in RSA. This work significantly increases the understanding of RSA occurrence and development in vitro and provides a molecular target for RSA diagnosis and treatment. However, our study is still limited due to small sample volume. Future research needs to base upon a larger sample size or apply extra in vitro and in vivo assays.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Stirrat GM. Recurrent miscarriage. Lancet. 1990;336:673–675.
- Baek KH, Lee EJ, Kim YS. Recurrent pregnancy loss: the key potential mechanisms. Trends Mol Med. 2007;13:310–317.
- Zhang Y, Zhou J, Li MQ, et al. MicroRNA-184 promotes apoptosis of trophoblast cells via targeting WIG1 and induces early spontaneous abortion. Cell Death Dis. 2019;10:223.
- Wu L, Cheng B, Liu Q, et al. CRY2 suppresses trophoblast migration and invasion in recurrent spontaneous abortion. J Biochem. 2020;167:79–87.
- Giannubilo SR, Landi B, Pozzi V, et al. The involvement of inflammatory cytokines in the pathogenesis of recurrent miscarriage. Cytokine. 2012;58:50–56.
- Tian FJ, Qin CM, Li XC, et al. Decreased stathmin-1 expression inhibits trophoblast proliferation and invasion and is associated with recurrent miscarriage. Am J Pathol. 2015;185:2709–2721.
- Ashwal-Fluss R, Meyer M, Pamudurti NR, et al. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56:55–66.
- Chen X, Guo DY, Yin TL, et al. Non-Coding RNAs Regulate Placental Trophoblast Function and Participate in Recurrent Abortion. Front Pharmacol. 2021;12:646521.
- Fang Z, Yang Y, Xu Y, et al. LncRNA HULC Polymorphism Is Associated With Recurrent Spontaneous Abortion Susceptibility in the Southern Chinese Population. Front Genet. 2019;10:918.
- Li Z, Zhou G, Tao F, et al. circ-ZUFSP regulates trophoblasts migration and invasion through sponging miR-203 to regulate STOX1 expression. Biochem Biophys Res Commun. 2020;531:472–479.
- Shafabakhsh R, Mirhosseini N, Chaichian S, et al. Could circRNA be a new biomarker for pre-eclampsia? Mol Reprod Dev. 2019;86:1773–1780.
- Shen XY, Zheng LL, Huang J, et al. CircTRNC18 inhibits trophoblast cell migration and epithelial-mesenchymal transition by regulating miR-762/Grhl2 pathway in pre-eclampsia. RNA Biol. 2019;16:1565–1573.
- Zhang Y, Cao L, Jia J, et al. CircHIPK3 is decreased in preeclampsia and affects migration, invasion, proliferation, and tube formation of human trophoblast cells. Placenta. 2019;85:1–8.
- Zhou W, Wang H, Wu X, et al. The profile analysis of circular RNAs in human placenta of preeclampsia. Exp Biol Med (Maywood). 2018;243:1109–1117.
- Jiao H, Chen R, Jiang Z, et al. miR-22 protect PC12 from ischemia/reperfusion-induced injury by targeting p53 upregulated modulator of apoptosis (PUMA). Bioengineered. 2020;11:209–218.
- Hui G, Ying Y. Quantitative Rapid Analysis Method for Ofloxacin in Raw Milk Based on Molecule-Specific Recognition and Electrochemical Impedance Spectrum. Trans ASABE. 2017;60:1439–1443.
- Hui G, Zhang J, Li J, et al. Sucrose quantitative and qualitative analysis from tastant mixtures based on Cu foam electrode and stochastic resonance. Food Chem. 2016;197(Pt B):1168–1176.
- Fang X, Mei Z, Chen J, et al. Sensor Based on Ni Foam Material Modified with Graphene Oxidated and Non-linear Analysis Model. Food Analytical Methods. 2021. DOI:10.1007/s12161-021-02028-x
- Yao Y, Li X, Cheng L, et al. Circular RNA FAT atypical cadherin 1 (circFAT1)/microRNA-525-5p/spindle and kinetochore-associated complex subunit 1 (SKA1) axis regulates oxaliplatin resistance in breast cancer by activating the notch and Wnt signaling pathway. Bioengineered. 2021;12:4032–4043.
- Wang X, Li T. Ropivacaine inhibits the proliferation and migration of colorectal cancer cells through ITGB1. Bioengineered. 2021;12:44–53.
- Guohua H, Hongyang L, Zhiming J, et al. Study of small-cell lung cancer cell-based sensor and its applications in chemotherapy effects rapid evaluation for anticancer drugs. Biosens Bioelectron. 2017;97:184–195.
- Li J, Deng Q, Fan W, et al. Melatonin-induced suppression of DNA methylation promotes odontogenic differentiation in human dental pulp cells. Bioengineered. 2020;11:829–840.
- Shang J, Sun S, Zhang L, et al. miR-211 alleviates ischaemia/reperfusion-induced kidney injury by targeting TGFbetaR2/TGF-beta/SMAD3 pathway. Bioengineered. 2020;11:547–557.
- Long H, Li Q, Xiao Z, et al. LncRNA MIR22HG promotes osteoarthritis progression via regulating miR-9-3p/ADAMTS5 pathway. Bioengineered. 2021;12:3148–3158.
- Guo Z, Sun Q, Liao Y, et al. MiR-30a-5p inhibits proliferation and metastasis of hydatidiform mole by regulating B3GNT5 through ERK/AKT pathways. J Cell Mol Med. 2020;24:8350–8362.
- Lv XB, Niu QH, Zhang M, et al. Critical functions of microRNA-30a-5p-E2F3 in cardiomyocyte apoptosis induced by hypoxia/reoxygenation. Kaohsiung J Med Sci. 2021;37:92–100.
- Shangxun Z, Junjie L, Wei Z, et al. ADAR1 Alleviates Inflammation in a Murine Sepsis Model via the ADAR1-miR-30a-SOCS3 Axis. Mediators Inflamm. 2020;(2020):9607535.
- Nakamura M, Yoshida H, Takahashi E, et al. transcription factor JunB functions in Xenopus tail regeneration by positively regulating cell proliferation. Biochem Biophys Res Commun. 2020;522:990–995.
- Hyakusoku H, Sano D, Takahashi H, et al. JunB promotes cell invasion, migration and distant metastasis of head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2016;35:6.
- Gurzov EN, Ortis F, Bakiri L, et al. JunB Inhibits ER Stress and Apoptosis in Pancreatic Beta Cells. PLoS One. 2008;3:e3030.
- Zhang X, Jin JY, Wu J, et al. RNA-Seq and ChIP-Seq reveal SQSTM1/p62 as a key mediator of JunB suppression of NF-kappaB-dependent inflammation. J Invest Dermatol. 2015;135:1016–1024.
- Feng X, Jiang S, Leung W, et al. BuShen HuoXue Decoction Promotes Decidual Stromal Cell Proliferation via the PI3K/AKT Pathway in Unexplained Recurrent Spontaneous Abortion. Evid Based Complement Alternat Med. 2020;2020:6868470.
- Zhang Z, Yang T, Xiao J. Circular RNAs: promising Biomarkers for Human Diseases. EBioMedicine. 2018;34:267–274.
- Guan X, Zong ZH, Liu Y, et al. circPUM1 Promotes Tumorigenesis and Progression of Ovarian Cancer by Sponging miR-615-5p and miR-6753-5p. Mol Ther Nucleic Acids. 2019;18:882–892.
- Chen J, Xu S, Chen S, et al. CircPUM1 promotes the malignant behavior of lung adenocarcinoma by regulating miR-326. Biochem Biophys Res Commun. 2019;508:844–849.
- Huang Z, Du G, Huang X, et al. The enhancer RNA lnc-SLC4A1-1 epigenetically regulates unexplained recurrent pregnancy loss (URPL) by activating CXCL8 and NF-kB pathway. EBioMedicine. 2018;38:162–170.
- Hua F, Li CH, Wang H, et al. Relationship between expression of COX-2, TNF-alpha, IL-6 and autoimmune-type recurrent miscarriage. Asian Pac J Trop Med. 2013;6:990–994.
- Zhang C, Deng X, Zhang X, et al. Association between Serum TNF-alpha Levels and Recurrent Spontaneous Miscarriage: a Meta-analysis. Am J Reprod Immunol. 2016;75:86–93.
- Zhu L, Chen H, Liu M, et al. Treg/Th17 Cell Imbalance and IL-6 Profile in Patients With Unexplained Recurrent Spontaneous Abortion. Reprod Sci. 2017;24:882–890.
- Yang J, Cheng M, Gu B, et al. CircRNA_09505 aggravates inflammation and joint damage in collagen-induced arthritis mice via miR-6089/AKT1/NF-kappaB axis. Cell Death Dis. 2020;11:833.
- Su Y, Lv X, Yin W, et al. CircRNA Cdr1as functions as a competitive endogenous RNA to promote hepatocellular carcinoma progression. Aging (Albany NY). 2019;11:8183–8203.
- Zhang Y, Cai S, Ding X, et al. MicroRNA-30a-5p silencing polarizes macrophages toward M2 phenotype to alleviate cardiac injury following viral myocarditis by targeting SOCS1. Am J Physiol Heart Circ Physiol. 2021;320:H1348–H1360.
- Kiesow K, Bennewitz K, Miranda LG, et al. Junb controls lymphatic vascular development in zebrafish via miR-182. Sci Rep. 2015;5:15007.
- Wang H, Xu T, Wu L, et al. Molecular mechanisms of MCM3AP-AS1 targeted the regulation of miR-708-5p on cell proliferation and apoptosis in gastric cancer cells. Eur Rev Med Pharmacol Sci. 2020;24:2452–2461.
