ABSTRACT
As an oncogenic kinase in multiple cancers, LMTK3 was deeply implicated in cancer pathogenesis. Nevertheless, its biological function in colorectal cancer (CRC) is still unclear. In this study, LMTK3 mRNA expression was assessed by RT-qPCR. LMTK3, phospho-ERK1/2 (p-ERK1/2), ERK1/2, and cleaved caspase-3 protein levels were detected by western blotting. Cetuximab (CTX)-resistant CRC cell models were constructed to investigate the mechanism of LMTK3-regulated CTX resistance in CRC. CTX half-maximal inhibitory concentration (IC50), viability, apoptosis, cell cycle, migration, and invasion of CRC cells were analyzed via Cell Counting Kit-8 (CCK-8), flow cytometry, wound healing, and transwell assays. We found LMTK3 was distinctly upregulated in CRC tissues and cells, particularly in CTX-resistant CRC tissues and cells. LMTK3 inhibition lowered CTX half-maximal inhibitory concentration (IC50) value, inhibited cell viability, induced cell apoptosis, triggered cell-cycle arrest, and impaired cell metastatic capability in CTX-resistant CRC cells. Moreover, we also demonstrated that LMTK3 induced CTX resistance in CRC via the activation of ERK/MAPK signaling in vitro. These results suggested a novel molecular mechanism by which LMTK3 participates in the development of CTX resistance in CRC.
Introduction
Colorectal cancer (CRC), as a malignancy derived from the digestive system, is a major human cancer prevalent around the world, with approximately 1.8 million new cases diagnosed every year [Citation1,Citation2]. As the third most fatal malignancy, CRC has an estimated annual death toll exceeding 690,000 [Citation3,Citation4]. Owing to early detection, CRC incidence has decreased in the last few years [Citation5]. At present, surgical excision accompanied with chemotherapy and radiotherapy is still the most common therapeutic method for CRC patients [Citation6]. Nevertheless, almost 50% of CRC patients suffered from metastases [Citation7]. To prolong survival, slow down tumor progression, relieve cancer-related symptoms, and further improve the life quality of patients with metastatic CRC (mCRC), the development of therapeutic methods and application of chemotherapeutic drugs have made progress in the past two decades; however, the overall survival of mCRC patients is still only about 2 years [Citation8,Citation9].
Chemoresistance frequently arises during mCRC chemotherapy, which is a great challenge for mCRC treatment [Citation10]. As an EGF receptor (EGFR) monoclonal antibody, cetuximab (CTX) exerts its anti-cancer effect by inhibiting cancer cell growth via binding to the extracellular domain of EGFR [Citation11]. For mCRC cases, the application of CTX combined with conventional chemotherapy has been used as a typical therapeutic method to reduce progression risks and appears to be more active than cytotoxic chemotherapies alone [Citation12]. Therefore, it is of great importance to understand the specific mechanisms of CTX resistance in CRC.
As an oncoprotein, lemur tyrosine kinase-3 (LMTK3) serves as a tumor-promoting protein in various human malignancies [Citation13]. For example, Lu et al. reported that LMTK3 contributed to thyroid cancer progression [Citation14]. Xu et al. demonstrated that LMTK3 promoted breast cancer tumorigenesis by inhibiting tumor-suppressing genes via chromatin remodeling [Citation15]. In addition, it was found that the LMTK3 level was upregulated in CRC samples and high LMTK3 expression was closely related to the poorer overall survival of CRC patients, suggesting LMTK3 is a vital CRC prognostic marker [Citation16,Citation17]. Furthermore, Stebbing et al. reported that LMTK3 aggravated drug resistance in breast cancer [Citation18], indicating its promoting role in the development of drug resistance. Therefore, it was assumed that LMTK3 might facilitate tumor progression and chemoresistance in CRC. However, its detailed mechanism in regulating CRC tumorigenesis and chemoresistance to cetuximab is still unclear.
In this study, we intended to verify the regulatory effect and detailed mechanism of LMTK3 in CRC progression and CRC chemoresistance to CTX. We showed that LMTK3 promoted CTX resistance and aggravated malignant phenotypes in CRC cells via the ERK/MAPK pathway, thereby providing a new direction for improving chemotherapeutic efficacy in CRC.
Materials and methods
Bioinformatics analysis of TCGA data and GEO data
The TCGA data of LMTK3 levels in Colon adenocarcinoma (COAD) patients were obtained from the GEPIA database (http://gepia.cancer-pku.cn/). The LMTK3 expression data of CRC tumor and normal samples were obtained from the Gene Expression Omnibus (GEO) profile database (accession number: GSE10950 and GSE74602).
Patients and sample collection
CRC samples and adjacent non-tumor tissue specimens were obtained from 56 patients diagnosed with CRC at Changzhou No.3 People’s Hospital. The inclusion criteria were as follows: i) patients were diagnosed with CRC for the first time; ii) patients with complete medical records; and iii) patients had not been treated before admission. The exclusion criteria were as follows: i) patients with previous history of malignancy; ii) patients who were transferred from other hospitals. Written informed consent was offered by each participant. The diagnoses were pathologically confirmed by two pathologists independently. The patients were grouped into CTX-sensitive group (patients free of recurrence or suffering recurrence beyond 6 months as of CTX chemotherapy completion, n = 32) and CTX-resistant group (patients suffering recurrence within 6 months as of CTX chemotherapy completion, n = 24). All the specimens were kept at −80°C before use. This study was permitted by the Ethics Committee of Changzhou No.3 People’s Hospital.
Cell culture and establishment of CTX-resistant CRC cell models
Normal colonic epithelial cell line (NCM460) and CRC cell lines (LS174T, SW837, HT-29, RKO, and HR8348) obtained from BeNa Culture Collection (Beijing, China) were cultured in RPMI 1640 containing 10% FBS at 37°C with 5% CO2.
Parental RKO and SW837 cells were exposed to gradient CTX concentrations () for 6 continuous months to construct CTX-resistant CRC cells (RKO/CTX and SW837/CTX). Thereafter, the CTX-resistant CRC cell lines (RKO/CTX and SW837/CTX) established were cultured in containing 200 μg/mL CTX to maintain drug resistance.
Plasmids and transfection
Lentiviral vectors containing siRNA targeting LMTK3 (si-LMTK3: 5ʹ-GCTGCCGTTTCTGCTGATTAT-3ʹ) and scramble (si-NC: 5ʹ‐UUCUCCGAACGUGUCACGUTT-3ʹ), as well as lentivirus vectors cloned with PCR product covering the LMTK3 open reading frame (oe-LMTK3) and scramble (Vector) were constructed by GeneChem (Shanghai, China). RKO/CTX and SW837/CTX cells were stably transfected with these vectors via Lipofectamine 2000 and selected by puromycin.
RT-qPCR
Total RNA was extracted from tissues and cells via TRIzol. Then, cDNA synthesis was performed with PrimerScript RT Master kit (Takara, China). Thereafter, qPCR was performed to detect LMTK3 mRNA level via SYBR Green PCR Master Mix (Roche, Germany) on an ABI 7900 Real-Time PCR System (Applied Biosystems, USA). The relative LMTK3 expression was normalized to GAPDH and calculated by 2−ΔΔCt method [Citation19]. The specific primers were listed below: LMTK3: Forward Primer 5′-CAAGTGCTGTGGTTGTGTAATG-3′ and Reverse Primer 5′-CAGGCATCTTGTCGAGGATGG-3′; GAPDH: Forward Primer 5′-GAAGGTGAAGGTCGGAGTC- 3′ and Reverse Primer 5′-GAAGATGGTGATGGGATTTC- 3′.
Western blotting
Total protein exacts were obtained from tissues and cells with RIPA buffer. Subsequently, protein concentrations were quantified with BCA Protein System Kit (Thermo, USA). The protein samples were resolved on SDS-PAGE and transferred onto a PVDF membrane. The membrane was cultured with primary antibodies against LMTK3, phospho-ERK1/2 (p-ERK1/2), ERK1/2, cleaved caspase-3, or GAPDH after blocked in 5% nonfat milk for 4 h. Next, the membrane was cultivated with the secondary antibody for another 2 h at room temperature. Finally, the protein blots were visualized by ECL kit (Pierce Chemical, USA).
CCK-8 assay
For cytotoxicity assay, cells (1 × 104 cells/well) were put into a 96-well plate and exposed to serial CTX dilutions for 48 h. After 10 μL CCK-8 (BestBio, China) was added into each well, the cells were cultured for another 2 h. Then, absorbance was detected with Microplate Reader (Thermo Scientific) at 450 nm. Finally, the half-maximal inhibitory concentration (IC50) was calculated based on dose-response curves by nonlinear regression analysis with GraphPad Prism 6.0 [Citation20].
To assess cell viability, CCK-8 solution was added to each well at 0, 24, 48, and 72 h after transfection. Then, the cell viability was also examined as above.
Wound healing assay
Transfected cells were seeded into 6-well plates (5 × 105 cells/well) and cultivated till 90% cell confluence. Thereafter, a 10 μl pipette tip was applied to create wounds. Then, cells were washed with PBS (Sigma-Aldrich) to remove cell debris. The wounds were photographed and examined and at 0 and 24 h under an inverted microscope (Nikon) [Citation2].
Transwell assay
Cell invasion was testified by Transwell assay. The upper chamber was equipped with an 8-μm-pore polycarbonate filter pre-coated with Matrigel (BD Biosciences). Briefly, 5 × 104 cells were suspended in 200 μL serum-free DMEM and transferred to the upper chamber. Thereafter, the culture medium containing 10% FBS was supplemented to the lower chamber. Then, the cells were incubated for 24 h. Next, the invaded cells were fixed with paraformaldehyde, followed by staining with crystal violet. The cells invaded were counted under a light microscope [Citation21].
Flow cytometry
For cell apoptosis detection, BD Pharmingen FITC annexin V Apoptosis Detection Kit I (BD Biosciences). Briefly, after CTX treatment for 48 h, the cells were harvested by centrifugation and then resuspended in binding buffer (1x105 cells/ml). Thereafter, the cells were cultivated with 5 μl annexin V-FITC and 5 μl PI in darkness for 15 min. Eventually, the apoptotic rate was analyzed by flow cytometry (Beckman Coulter) [Citation22].
For cell cycle detection, cells were digested with trypsin, washed with ice-cold PBS, and fixed in 70% ethanol for at least 12 h at 4°C. Then, fixed cells were washed 3 times with PBS, suspended in PBS containing 10 µg/ml propidium iodide and 100 µg/ml RNase A, and then incubated at 37°C for at least 30 min in darkness. Finally, cell cycle was detected by flow cytometry [Citation23].
Statistical analysis
Every experiment in this study was repeated three times. All the data were presented as mean ± standard deviation (SD). All the analyses were conducted with GraphPad Prism 6.0. Any comparison between two groups or more groups was severally assessed by Student’s t-test or one-way ANOVA. Receiver operating characteristic curve (ROC) analysis was applied for the assessment of the diagnostic value of LMTK3 expression in CRC. A difference with P value < 0.05 was regarded as significant in statistics.
Results
Here, we intended to investigate the role of LMTK3 in regulating chemoresistance of CRC to CTX. We performed a series of in vitro assays and found that LMTK3 promoted CTX resistance in CRC via activating the ERK/MAPK signaling pathway. Therefore, our findings demonstrated the functional effects of LMTK3 in regulating CTX chemoresistance within CRC, providing a novel target for CRC chemotherapy.
LMTK3 is highly expressed in CRC
Based on the GEPIA colon adenocarcinoma (COAD) database, LMTK3 level was distinctly elevated in tumor tissues ()). Furthermore, GEO datasets revealed that LMTK3 expression was significantly increased in CRC tumors compared with that in normal tissues ()). For the sake of confirming ectopic LMTK3 expression in CRC, we evaluated LMTK3 expression in CRC tissues, corresponding non-tumor tissue, representative CRC cell lines, and normal colonic epithelial cells. RT-qPCR and western blotting revealed that LMTK3 mRNA and protein levels were upregulated in 56 CRC tissues, relative to normal tissues ()). Moreover, it was uncovered by RT-qPCR that LMTK3 expression was markedly enhanced in CRC cell lines (LS174T, SW837, HT-29, RKO, and HR8348) than normal colonic epithelial cell line (NCM460) ()). Since LMTK3 level was higher in RKO and SW837 cell lines, they were applied for sequential experiments. As revealed by statistical results, high LMTK3 expression was correlated with high TNM stage and lymph node metastasis (). Finally, ROC curve analysis indicated that LMTK3 was of great diagnostic value in CRC, with an area under the curve (AUC) of 0.908 ()). The above data exhibited that LMTK3 was overexpressed in CRC tissues and cells, indicating LMTK3 might be implicated in CRC progression.
Table 1. The correlation between LMTK3 expression and clinicopathological features in CRC patients
Figure 1. LMTK3 is highly expressed in CRC. (a) LMTK3 level in colon adenocarcinoma (COAD) and tumor tissues was provided by GEPIA database (P = 0.0342). (b) LMTK3 level in CRC tumors and normal tissues in GSE10950 (P = 0.00647) and GSE74602 (P = 0.00875) datasets from GEO database. (c and d) The mRNA and protein expressions of LMTK3 in 56-paired CRC tissues and adjacent normal tissues was analyzed by RT-qPCR (P = 0.00895) and western blotting (P = 0.00762). (e) LMTK3 mRNA expression in CRC cell lines (LS174T, SW837, HT-29, RKO, and HR8348) and normal colonic epithelial cell line (NCM460) were analyzed by RT-qPCR. (f) ROC curve for prognostic value of LMTK3 in CRC, with AUC = 0.908. *P < 0.05, **P < 0.01
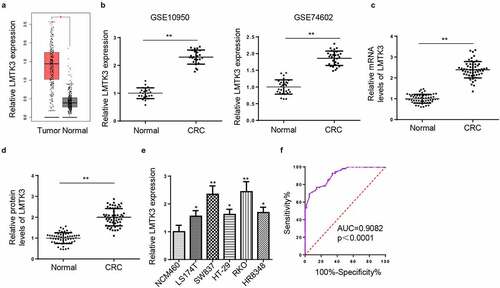
LMTK3 knockdown decreases CTX resistance and cell proliferation and induces cell apoptosis and cell-cycle arrest in CTX-resistant CRC cells
As shown in ), LMTK3 level was distinctly higher in CTX-resistant CRC tissues, relative to CTX-sensitive CRC tissues. To explore the molecular mechanism of CTX resistance in CRC, CTX-resistant CRC cell models (RKO/CTX and SW837/CTX cells) were established. As indicated by CCK-8, RKO/CTX and SW837/CTX manifested higher CTX IC50 values than parental RKO and SW837 cells ()), indicating the successful establishment of CTX-resistant CRC cells. Similarly, LMTK3 expression was also manifestly elevated in RKO/CTX and SW837/CTX cells, compared with parental RKO and SW837 cells ()). To evaluate the biological role of LMTK3 in CTX-resistant CRC, CCK-8 and flow cytometry analysis were performed. Firstly, the efficiency of LMTK3 knockdown was confirmed in RKO/CTX and SW837/CTX cells ()). It was exhibited that LMTK3 silencing greatly reduced CTX IC50 values compared with the control group ()). Besides, LMTK3 knockdown impaired cell proliferation in RKO/CTX and SW837/CTX cells ()). In addition, LMTK3 silencing significantly accelerated apoptosis in RKO/CTX and SW837/CTX cells ()). Consistently, the cleaved caspase-3 level was considerably increased after LMTK3 knockdown ()). Furthermore, LMTK3 depletion initiated cell cycle arrest in the G0/G1 phase in RKO/CTX and SW837/CTX cells ()). In short, LMTK3 expression is closely related to CTX chemoresistance, cell proliferation, cell apoptosis, and cell-cycle arrest of CTX-resistant CRC cells.
Figure 2. LMTK3 knockdown decreases CTX resistance and cell proliferation and induces cell apoptosis and cell-cycle arrest in CTX-resistant CRC cells. (a) LMTK3 mRNA expression in CTX-sensitive CRC tissues (n = 32) and CTX-resistant CRC tissues (n = 24) was analyzed by RT-qPCR. (b) CTX IC50 values of CTX-resistant CRC cells (RKO/CTX and SW837/CTX) and parental CRC cells (RKO and SW837) were determined by the CCK-8 assay. (c and d) LMTK3 mRNA and protein expressions in CTX-resistant CRC cells (RKO/CTX and SW837/CTX) and parental CRC cells (RKO and SW837) were analyzed by RT-qPCR and western blotting. (e and f) RKO/CTX and SW837/CTX cells were respectively transfected with si-LMTK3 or si-NC, and the transfection efficiency was evaluated by RT-qPCR and western blotting. (g) CTX IC50 values of RKO/CTX and SW837/CTX cells respectively transfected with si-LMTK3 and si-NC were determined by CCK-8 assay. (h) CCK-8 assay was performed to determine cell vitality. (i) Flow cytometry was performed to detect cell apoptosis. (j) Cleaved caspase-3 level was detected by Western blotting. (k) Flow cytometry was performed to detect cell-cycle. *P < 0.05 and **P < 0.01
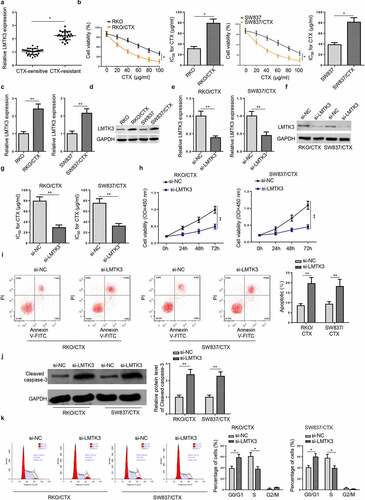
LMTK3 deficiency attenuates CTX-resistant CRC cell migration and invasion
To further evaluate the role of LMTK3 in CRC migration and invasion, wound healing and transwell assays were performed. The results exhibited a remarkable decline in migration ability of CTX-resistant CRC cells after LMTK3 knockdown, compared with si-NC group ()). Similarly, the number of invaded RKO/CTX and SW837/CTX cells were decreased in si-LMTK3 group than controls ()). Therefore, LMTK3 might facilitate cell metastatic capabilities of CTX-resistant CRC cells.
Figure 3. LMTK3 deficiency attenuates CTX-resistant CRC cell migration and invasion. RKO/CTX and SW837/CTX cells were respectively transfected with si-LMTK3 or si-NC. (a) Wound healing was applied to detect cell migration. (b) Transwell assay was applied to assess cell invasion. **P < 0.01 and ***P < 0.001
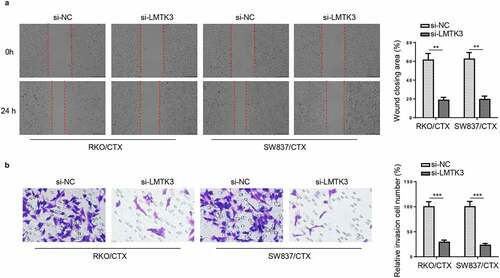
LMTK3 activates ERK/MAPK pathway in CTX-resistant CRC cells
Previous reports revealed that ERK/MAPK actively participated in the development of chemoresistance to multiple drugs during chemotherapy for human cancers [Citation24–26]. Besides, Raghav et al. elucidated the ERK/MAPK pathway activated by HGF/MET axis could animate oncogenic signaling in CTX-resistant tumors to further aggravate chemoresistance [Citation27], indicating that ERK/MAPK signaling contributed to CTX resistance in tumors. In addition, Sun et al. revealed that ERK/MAPK pathway was deeply involved in CRC tumorigenesis [Citation28]. Moreover, Ye et al. also uncovered that ERK/MAPK signaling inhibition could eliminate the chemoresistance of CRC cells [Citation29]. Therefore, we assumed that LMTK3 might activate the ERK/MAPK signaling pathway to exacerbate CTX resistance in CRC. To verify such an assumption, we detected the levels of proteins associated with ERK/MAPK signaling in RKO/CTX and SW837/CTX cells expressing oe-LMTK3 or si-LMTK3. Firstly, the efficiency of LMTK3 overexpression was confirmed in RKO/CTX and SW837/CTX cells ()). Then, it was confirmed that phospho-ERK1/2 (p-ERK1/2) level in RKO/CTX and SW837/CTX cells was obviously declined due to LMTK3 inhibition, while there were no significant changes in ERK1/2 level ()). On the contrary, LMTK3 overexpression visibly increased p-ERK1/2 level in RKO/CTX and SW837/CTX cells ()). These results implied that LMTK3 might activate the ERK/MAPK signaling in CTX-resistant CRC cells.
Figure 4. LMTK3 activates ERK/MAPK pathway in CTX-resistant CRC cells. (a) RKO/CTX and SW837/CTX cells were respectively transfected with Vector or oe-LMTK3, and the transfection efficiency was evaluated by western blotting. (b) ERK/MAPK pathway-related proteins (p-ERK1/2 and ERK1/2) in RKO/CTX and SW837/CTX cells transfected with si-LMTK3 or si-NC were analyzed by western blotting. (c) Protein levels of p-ERK1/2 and ERK1/2 in RKO/CTX and SW837/CTX cells transfected with Vector or oe-LMTK3 were analyzed by western blotting. **P < 0.01
Inactivation of ERK/MAPK signaling reverses the enhanced CTX resistance induced by LMTK3 overexpression in vitro
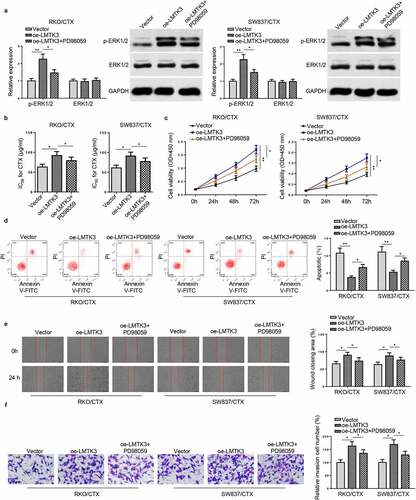
To further validate the functions of the ERK/MAPK signaling in LMTK3-regulated CRC resistance to CTX. PD98059, an ERK inhibitor, was applied to treat RKO/CTX and SW837/CTX cells. As shown in ), PD98059 treatment reversed the increased p-ERK1/2 protein level in RKO/CTX and SW837/CTX cells caused by LMTK3 overexpression. Rescue assays exhibited that LMTK3 upregulation increased CTX IC50, boosted cell viability, reduced cell apoptosis, and expedited cell migration and invasion in OXA-resistant CRC cells, while PD98059 could reverse these impacts caused by LMTK3 overexpression ()). To sum up, LMTK3 exacerbated CTX resistance in CRC cells via activation of the ERK/MAPK signaling.
Figure 5. Inactivation of ERK/MAPK signaling reverses the enhanced CTX resistance induced by LMTK3 overexpression in vitro. (a) Protein levels of p-ERK1/2 and ERK1/2 in RKO/CTX and SW837/CTX cells transfected with Vector, oe-LMTK3, or oe-LMTK3+ PD98059 were analyzed by western blotting. (b) CTX IC50 values of RKO/CTX and SW837/CTX cells respectively transfected with Vector, oe-LMTK3, or oe-LMTK3+ PD98059 were determined by CCK-8 assay. (c) CCK-8 assay was performed to determine cell vitality. (d) Flow cytometry was performed to detect cell apoptosis. (e and f) Cell migration and invasion were assessed by wound healing and transwell assays. *P < 0.05 and ** P < 0.01
Discussion
Since intensive studies have been conducted in the field of diagnostic, surgical, and chemotherapeutic strategies for CRC patients, the mortality rate of CRC patients has been relatively reduced [Citation6]. However, drug resistance is a major challenge for CRC chemotherapy [Citation30]. As the first anti-EGFR antibody for CRC chemotherapy permitted by FDA, CTX has been applied in adjuvant therapy for CRC, whereas chemoresistance to CTX is still a major challenge in clinical practice [Citation31].
Multiple genes were proven to regulate CTX chemoresistance in CRC. For example, Hou et al. revealed that SETDB1 facilitated tumorigenesis and enhanced CTX resistance in CRC [Citation32]. Tan et al. demonstrated that PRSS aggravated chemoresistance to CTX in CRC [Citation33]. Besides, Rosa et al. also reported that SphK1 conferred CTX resistance in CRC [Citation34]. In this study, data from GEPIA and GEO databases identified that LMTK3 was upregulated in CRC. Consistently, we confirmed LMTK3 level was elevated in CRC tissues and cell lines, particularly in CTX-resistant CRC tissues and cell lines. These results indicated LMTK3 might be a regulator for regulating CTX chemoresistance in CRC.
LMTK3 has been recognized as an oncogenic kinase in various types of human tumors. To cite an instance, a report from Xu et al. demonstrated that LMTK3 contributed to breast cancer invasion via integrin beta1 induced by GRB2 [Citation35]. LMTK3 facilitated the progression of KIT-dependent gastrointestinal stromal tumor and melanoma [Citation36]. In addition, Zhang et al. also uncovered that serum LMTK3 served as a biomarker for the detection of primary non-small cell lung cancer [Citation37]. Herein, we found that LMTK3 deficiency distinctly restrained malignant phenotypes of CTX-resistant CRC cells, as indicated by declined CTX IC50 value, inhibited cell proliferation, increased cell apoptosis, arrested cell cycle, as well as impaired cell migration and invasion, suggesting that LMTK3 induced CTX resistance in CRC cells.
The ERK/MAPK pathway has been proven to play vital roles in regulating cell growth, differentiation, proliferation, apoptosis, cell cycle, as well as migration functions [Citation38,Citation39]. ERK/MAPK signaling is also closely associated with chemoresistance regulation in human cancers, including CRC. For instance, Yang et al. uncovered that KRAS aggravated chemoresistance in pancreatic cancer by activating the ERK/MAPK signaling via RKIP inhibition [Citation40]. Wang et al. disclosed that UCH-L1 upregulation exacerbated multidrug resistance and metastasis in breast cancer via activation of the ERK/MAPK pathway [Citation41]. Furthermore, Zhang et al. revealed that FOXC2 activated ERK/MAPK pathway to enhance oxaliplatin resistance in CRC via EMT [Citation42]. A previous study from Jiang et al. also indicated that the activation of ERK/MAPK signaling was highly related to high LMTK3 expression [Citation43]. Similarly, our study demonstrated that LMTK3 depletion could inhibit the expression of proteins associated with the ERK/MAPK pathway, thereby inactivating the ERK/MAPK signaling, while LMTK3 overexpression exhibited opposite effects. Moreover, the ERK inhibitor, PD98059, reversed the effect of LMTK3 overexpression on p-ERK1/2 protein level in CTX-resistant CRC cells. Further rescue assays manifested that PD98059 treatment eliminated the promotive effect of LMTK3 upregulation on CTX resistance and malignant phenotypes of CTX-resistant CRC cells. Taken together, LMTK3 promoted CTX resistance in CTX-resistant CRC cells by targeting the ERK/MAPK signaling.
Conclusion
Our study demonstrated that LMTK3 promoted CTX resistance in CRC via activation of the ERK/MAPK signaling pathway in vitro. Therefore, targeting LMTK3 could be a promising therapeutic strategy for the effective treatment of CRC. However, our study is still lack of network analysis and in vivo studies, which is a limitation of this work. In the future, network analysis and in vivo studies will be performed to further verify our findings.
Research highlights
LMTK3 is highly expressed in CRC.
LMTK3 knockdown decreases CTX resistance in CTX-resistant CRC cells.
LMTK3 activates ERK/MAPK pathway in CTX-resistant CRC cells.
LMTK3 aggravates CTX resistance by activating the ERK/MAPK signaling.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Shi Z, Shen C, Yu C, et al. Long non-coding RNA LINC00997 silencing inhibits the progression and metastasis of colorectal cancer by sponging miR-512-3p. Bioengineered. 2021;12(1):627–639.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29.
- Liao R, Ma QZ, Zhou CY, et al. Identification of biomarkers related to Tumor-Infiltrating Lymphocytes (TILs) infiltration with gene co-expression network in colorectal cancer. Bioengineered. 2021;12(1):1676–1688.
- Li YP, Du XR, Zhang R, et al. Interleukin-18 promotes the antitumor ability of natural killer cells in colorectal cancer via the miR-574-3p/TGF-beta1 axis. Bioengineered. 2021;12(1):763–778.
- Liu B, Yan X, Hou Z, et al. Impact of Bupivacaine on malignant proliferation, apoptosis and autophagy of human colorectal cancer SW480 cells through regulating NF-kappaB signaling path. Bioengineered. 2021;12(1):2723–2733.
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193.
- Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet. 2010;375(9719):1030–1047.
- Van Cutsem E, Cervantes A, Nordlinger B, et al. Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii1–9.
- Lee GY, Lee JS, Son CG, et al. Combating drug resistance in colorectal cancer using herbal medicines. Chin J Integr Med. 2020;27(7):551-560.
- Jing C, Ma R, Cao H, et al. Long noncoding RNA and mRNA profiling in cetuximab-resistant colorectal cancer cells by RNA sequencing analysis. Cancer Med. 2019;8(4):1641–1651.
- Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer. JAMA. 2017;317(23):2392–2401.
- Ditsiou A, Cilibrasi C, Simigdala N, et al. The structure-function relationship of oncogenic LMTK3. Sci Adv. 2020;6(46):eabc3099.
- Lu L, Yuan X, Zhang Q, et al. LMTK3 knockdown retards cell growth and invasion and promotes apoptosis in thyroid cancer. Mol Med Rep. 2017;15(4):2015–2022.
- Xu Y, Zhang H, Nguyen VT, et al. LMTK3 represses tumor suppressor-like genes through chromatin remodeling in breast cancer. Cell Rep. 2015;12(5):837–849.
- Shi H, Wu J, Ji M, et al. Serum lemur tyrosine kinase 3 expression in colorectal cancer patients predicts cancer progression and prognosis. Med Oncol. 2013;30(4):754.
- Shi H, Li Q, Ji M, et al. Lemur tyrosine kinase-3 is a significant prognostic marker for patients with colorectal cancer. Int J Clin Exp Pathol. 2014;7:1101–1107.
- Stebbing J, Shah K, Lit LC, et al. LMTK3 confers chemo-resistance in breast cancer. Oncogene. 2018;37(23):3113–3130.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408.
- Yao Y, Li X, Cheng L, et al. Circular RNA FAT atypical cadherin 1 (circFAT1)/microRNA-525-5p/spindle and kinetochore-associated complex subunit 1 (SKA1) axis regulates oxaliplatin resistance in breast cancer by activating the notch and Wnt signaling pathway. Bioengineered. 2021;12(1):4032–4043.
- Wang X, Li T. Ropivacaine inhibits the proliferation and migration of colorectal cancer cells through ITGB1. Bioengineered. 2021;12(1):44–53.
- Liu W, Miao Y, Zhang L, et al. MiR-211 protects cerebral ischemia/reperfusion injury by inhibiting cell apoptosis. Bioengineered. 2020;11(1):189–200.
- Ge C, Zeng B, Li R, et al. Knockdown of STIM1 expression inhibits non-small-cell lung cancer cell proliferation in vitro and in nude mouse xenografts. Bioengineered. 2019;10(1):425–436.
- Heckler MM, Thakor H, Schafer CC, et al. ERK/MAPK regulates ERRgamma expression, transcriptional activity and receptor-mediated tamoxifen resistance in ER+ breast cancer. FEBS J. 2014;281(10):2431–2442.
- Liu J, Liu Y, Liu Y, et al. Anticancer action of Psilostachyin-A in 5-fluorouracil-resistant human liver carcinoma are mediated through autophagy induction, G2/M phase cell cycle arrest and inhibiting extracellular-signal-regulated kinase/mitogen activated protein kinase (ERK/MAPK) signaling pathway. Med Sci Monit. 2019;25:6711–6718.
- Nie Y, Ding Y, Yang M. GRHL2 upregulation predicts a poor prognosis and promotes the resistance of serous ovarian cancer to cisplatin. Onco Targets Ther. 2020;13:6303–6314.
- Raghav KP, Gonzalez-Angulo AM, Blumenschein GR Jr. Role of HGF/MET axis in resistance of lung cancer to contemporary management. Transl Lung Cancer Res. 2012;1:179–193.
- Sun H, Ou B, Zhao S, et al. USP11 promotes growth and metastasis of colorectal cancer via PPP1CA-mediated activation of ERK/MAPK signaling pathway. EBioMedicine. 2019;48:236–247.
- Ye DM, Ye SC, Yu SQ, et al. Drug-resistance reversal in colorectal cancer cells by destruction of flotillins, the key lipid rafts proteins. Neoplasma. 2019;66(4):576–583.
- Shen W, Xu T, Chen D, et al. Targeting SREBP1 chemosensitizes colorectal cancer cells to gemcitabine by caspase-7 upregulation. Bioengineered. 2019;10(1):459–468.
- Han Y, Peng Y, Fu Y, et al. MLH1 deficiency induces cetuximab resistance in colon cancer via Her-2/PI3K/AKT signaling. Adv Sci (Weinh). 2020;7(13):2000112.
- Hou Z, Sun L, Xu F, et al. Blocking histone methyltransferase SETDB1 inhibits tumorigenesis and enhances cetuximab sensitivity in colorectal cancer. Cancer Lett. 2020;487:63–73.
- Tan Z, Gao L, Wang Y, et al. PRSS contributes to cetuximab resistance in colorectal cancer. Sci Adv. 2020;6(1):eaax5576.
- Rosa R, Marciano R, Malapelle U, et al. Sphingosine kinase 1 overexpression contributes to cetuximab resistance in human colorectal cancer models. Clin Cancer Res. 2013;19(1):138–147.
- Xu Y, Zhang H, Lit LC, et al. The kinase LMTK3 promotes invasion in breast cancer through GRB2-mediated induction of integrin beta(1). Sci Signal. 2014;7(330):ra58.
- Klug LR, Bannon AE, Javidi-Sharifi N, et al. LMTK3 is essential for oncogenic KIT expression in KIT-mutant GIST and melanoma. Oncogene. 2019;38(8):1200–1210.
- Zhang K, Chen L, Deng H, et al. Serum lemur tyrosine kinase-3: a novel biomarker for screening primary non-small cell lung cancer and predicting cancer progression. Int J Clin Exp Pathol. 2015;8:629–635.
- Santarpia L, Lippman SM, El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):103–119.
- Yang CY, Wang J, Zhang JQ, et al. Human circular RNA hsa_circRNA_101705 (circTXNDC11) regulates renal cancer progression by regulating MAPK/ERK pathway. Bioengineered. 2021;12(1):4432–4441.
- Yang K, Li Y, Lian G, et al. KRAS promotes tumor metastasis and chemoresistance by repressing RKIP via the MAPK-ERK pathway in pancreatic cancer. Int J Cancer. 2018;142(11):2323–2334.
- Wang W, Zou L, Zhou D, et al. Overexpression of ubiquitin carboxyl terminal hydrolase-L1 enhances multidrug resistance and invasion/metastasis in breast cancer by activating the MAPK/Erk signaling pathway. Mol Carcinog. 2016;55(9):1329–1342.
- Chen Y, Deng G, Fu Y, et al. FOXC2 promotes oxaliplatin resistance by inducing epithelial-mesenchymal transition via MAPK/ERK signaling in colorectal cancer. Onco Targets Ther. 2020;13:1625–1635.
- Xu T, Wu K, Zhang L, et al. Long non-coding RNA LINC00858 exerts a tumor-promoting role in colon cancer via HNF4alpha and WNK2 regulation. Cell Oncol (Dordr). 2020;43(2):297–310.
