ABSTRACT
Tinnitus is deemed as the result of abnormal neural activities in the brain, and Homer proteins are expressed in the brain that convey nociception. The expression of Homer in tinnitus has not been studied. We hypothesized that expression of Homer in the auditory cortex was altered after tinnitus treatment. Mice were injected with sodium salicylate to induce tinnitus. Expression of Homer was detected by quantitative real-time polymerase chain reaction, western blotting, and immunohistochemistry assays. We found that Homer1 expression was upregulated in the auditory cortex of mice with tinnitus, while expression of Homer2 or Homer3 exhibited no significant alteration. Effects of two inhibitors of metabolic glutamate receptor 5 (mGluR5), noncompetitive 2-Methyl-6-(phenylethynyl)-pyridine (MPEP) and competitive α-methyl-4-carboxyphenylglycine (MCPG), on the tinnitus scores of the mice and on Homer1 expression were detected. MPEP significantly reduced tinnitus scores and suppressed Homer1 expression in a concentration dependent manner. MCPG had no significant effects on tinnitus scores or Homer1 expression. In conclusion, Homer1 expression was upregulated in the auditory cortex of mice after tinnitus, and was suppressed by noncompetitive mGluR5 inhibitor MPEP, but not competitive mGluR5 inhibitor MCPG.
Introduction
Tinnitus, with the prevalence of 10%–15% [Citation1], refers to the perception of sound without external sound stimulus and is usually accompanied by hearing loss. Tinnitus is distinguished from auditory hallucinations, the latter one involving the symptoms of hearing one or more talking voices. Around 1–2% of global population was interfered by severe tinnitus [Citation1]. In some cases, social isolation, depression, and suicide may be caused by tinnitus [Citation2]. The prevalence of sound-induced tinnitus is on the increase, especially in young people with the increased intake of white bread, carbonated beverages, and fast food [Citation3].
Tinnitus is perceived as originating from cochlea [Citation4], and the central nervous system [Citation5]. Some studies revealed that tinnitus is a conscious percept and is influenced by emotion, attention, memory, and learning [Citation1,Citation6,Citation7]. Functional neural imaging studies in patients with tinnitus showed alterations in the classical auditory pathway and the cortical regions associated with attention, emotion, memory, and perception functions [Citation8–11]. Therefore, tinnitus is deemed as the result of abnormal neural activities in some trigger zones of the brain, causing a series of changes in brain networks [Citation6].
The Homer proteins are products of three mammalian genes (Homer1-3), one Xenopus gene and one Drosophila gene [Citation12]. Homer proteins are similar in structure including a conserved amino-terminal EVH1 domain that binds to group1 metabotropic glutamate receptors (mGluRs) [Citation13], ryanodine receptors [Citation14], inositol-1,4,5-triphosphate receptors [Citation13], and transient receptor potential canonical-1 ion channels [Citation15]. Homer proteins are expressed in brain regions that convey nociception [Citation16,Citation17]. Homer1 is localized to chromosome 5 (5q14.2) and encodes many transcriptional variants in humans. Homer1 (but not Homer2) knockout leads to glutamate abnormalities in prefrontal cortex, providing evidence for the selective disadvantageous effects of Homer1 silencing on emotional, sensorimotor, cognitive, and motivational processing [Citation18]. Considering the close relationship between tinnitus and attention, emotion, memory, perception functions, we hypothesized that Homer1 is associated with tinnitus. Homer1 proteins bind to mGluR5 [Citation19]. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP; formula: C₁₄H₁₁N) is a potent, noncompetitive, orally active, and systemically active mGluR5 antagonist, while α-methyl-4-carboxyphenylglycine (MCPG; formula: C₁₀H₁₁NO₄) is a phenylglycine derivative and a competitive mGluR5 antagonist. Both MPEP and MCPG have been suggested to have antidepressant-like effects in rodents [Citation20,Citation21].
Salicylate can induce aberrant excitability of central and peripheral auditory neurons [Citation22]. Rodents injected with salicylate were commonly used for tinnitus research [Citation23–25]. We made a hypothesis that tinnitus is related to the abnormal expression of Homer genes in the auditory cortex. After establishing a salicylate-stimulated tinnitus mouse model, expression of Homer genes in the auditory cortex was detected and we identified upregulated expression of Homer1, but not of Homer2 or Homer3 in auditory cortex of mice after salicylate stimulation. The effects of two mGluR5 inhibitors, MPEP and MCPG, on tinnitus scores and on expression of Homer1 were subsequently investigated.
Materials and methods
Animals and grouping
Because tinnitus is most prevalent in older humans, we used the senescence-accelerated mouse-prone 8 (SAMP8) mice in the present study, which is consistent with the previous studies [Citation26,Citation27]. Male SAMP8 mice (three-month-old; 22–33 g) were randomly divided into 8 groups: saline (n = 10), 300 mg/kg salicylate (n = 20) [Citation26], salicylate + 100 nmol/L of MPEP (n = 20), salicylate + 250 nmol/L of MPEP (n = 20), salicylate + 300 nmol/L of MPEP (n = 20), salicylate + 100 nmol/L of MCPG (n = 20), salicylate + 250 nmol/L of MCPG (n = 20), salicylate + 300 nmol/L of MCPG (n = 20). Concentrations of MPEP and MCPG were determined according to our preliminary assays. Animal experiments were approved by the Institutional Animal Care and Use Committee of Hubei Provincial Hospital of Traditional Chinese Medicine (Hubei, China).
Conditioning paradigm
The conditioning to the task is composed of 6 sessions that were performed daily for 5 days (days 1–5) [Citation26]. There were 10 trials for each session that lasts 15–20 min with the intervals of at least 1 min. The conditioned stimulus was a 50 dB pure tone (frequency: 10 kHz; duration: 3 seconds), while the unconditioned stimulus was an electric foot-shock (3.7 mA; duration: no more than 30 seconds) according to Guitton’s protocols [Citation28]. The interval between the conditioned and the unconditioned stimuli was 1 second. After the coupled conditioned and unconditioned stimulus, mice climbed the pole to a safe area. When the animal climbed correctly, the electrical shocks would be stopped. The ‘truepositive’ score was evaluated according to times the mice climbed correctly in response to sound. When the score reached at least 80% in 3 continuous sessions, mice were considered as conditioned, and were used for the tinnitus experiments.
Induction and testing of tinnitus
Upon conditioned, the mice had a rest for 1 day (day 6). On the next 4 consecutive days (days 7–10), an active avoidance task was conducted 2 h after intraperitoneal injections of saline alone or that containing sodium salicylate (300 mg/kg; Sigma, St. Louis, MO, USA) [Citation26]. The active avoidance task included 10 trials. To compensate for hearing loss induced by salicylate, the intensity of sound that produced the behavioral responses was increased to 70 dB for mice in the salicylate-treated group to make sure that mice in both groups had similar sound sensation levels. During testing, 3-second sound was given to the mice. After that, mice were observed to see whether they would climb to the safe area as conditioned in 5 seconds (true-positive). If the mice stayed in the safe area for more than 10 seconds, they would be returned to the floor for following observation, otherwise, mice will be given with electrical shocks until they climbed to the safe area. Finally, the total number of times that the mice climbed during the inter-trial silent period of 1 minute for 10 trials were calculated as the tinnitus scores.
Tissue preparation and combined in situ hybridization and immunohistochemistry
Auditory cortices were identified according to a previous study [Citation29]. Tissues within the anterior and posterior ectosylvian sulci (roughly delineating A1) were dissected. RNA was isolated from tissues as a previous study described [Citation30]. Combined in situ hybridization and immunohistochemistry method was used for detection of existence of Homer1 mRNA and protein in auditory cortex according to a previous study [Citation31]. The primary antibody against Homer1 protein (1:500, ab184955) and the secondary antibody (1:100, ab7090) used in the immunohistochemistry was purchased from Abcam.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from auditory cortex tissue using a TRIzol™ Plus RNA Purification Kit (Invitrogen, CA, USA). Concentration and purity of RNA were evaluated using a photometer (NanoDrop 8000, Thermo, USA). Extracted RNAs were reverse transcribed to cDNA using a PrimeScript RT reagent Kit (Takara, Dalian, China) at 25°C for 5 min, 50°C for 45 min. Inactivation of the reverse transcriptase was performed by heating to 85°C for 5 min. The mRNA expression levels were calculated by the 2−ΔΔCt method [Citation32] and were normalized to Gapdh gene expression. Relative primer sequences used for PCR were listed as follows: Homer1, forward (F), 5ʹ-CAGGAATCAGCAGGAGGAG-3ʹ, reverse (R), 5ʹ-GATTGCTGAACTAGCATGAGAG-3ʹ; Homer2, F, 5ʹ-AGGAGATGGAGTTGAAAGATCTC-3ʹ, R, 5ʹ-AGGAGATGGAGTTGAAAGATCTC-3ʹ; Homer3, F, 5ʹ-TCAGGAGGTGAAAGAAGCTG-3ʹ, R, 5ʹ-CTTGGAGGAACCTGATGGG-3ʹ; Gapdh, F, 5ʹ-CATCTTCTTGTGCAGTGCC-3ʹ, R, 5ʹ-CAAATCCGTTCACACCGAC-3ʹ.
Western blotting analysis
Tissue samples were lysed using RIPA buffer (Beyotime, Shanghai, China) with protease inhibitor cocktail (Sigma). A Bicinchoninic Acid Protein Assay Kit (Thermo Fisher) was used for protein quantification. The same amount (50 μg/lane) of protein was separated from SDS-PAGE and blotted onto PVDF membranes followed by incubation with the primary antibodies against Homer1 (1/1000; ab184955; Abcam), Homer2 (1/1000; PA5-70,541; Invitrogen), Homer3 (1/2000; PA5-79,384; Invitrogen), GAPDH (1/2500; ab9485; Abcam) overnight at 4°C. Next, samples were incubated with the secondary antibodies at room temperature for 2 h. GAPDH served as an internal control. Protein visualization was performed with an ECL chemiluminescence kit (Thermo Fisher). The ImageJ version 1.8.0 software (National Institutes of Health) was used to quantify each protein band.
Statistical analysis
Statistical analysis was conducted using SPSS 20 software. GraphPad Prism 7 was used for graph drawing. Student’s t test was performed to analyze the difference between 2 groups, while one-way or two-way analysis of variance was used for difference analysis among 3 or more groups. Correlation between tinnitus scores and expression level of Homer1 was assessed by linear regression analysis. P values less than 0.05 were defined significant.
Results
Effects of salicylate on tinnitus scores, body weight, and midbrain weight in mice
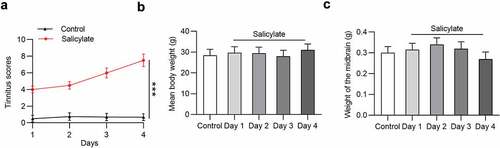
Effects of salicylate on tinnitus scores, body weight, and midbrain weight in mice were detected. The tinnitus scores were not elevated after injection of normal saline into mice in the following 4 days, but were significantly increased in salicylate-treated mice in a day-dependent manner (). The mean tinnitus score of mice after salicylate stimulation for 4 days was increased by 10.87 folds compared to that for 1 day. No significant difference was found in the mean body weight () and midbrain weight () of mice between the control group and the salicylate group.
Figure 1. The daily tinnitus scores, weight of body and midbrain of mice in the control group and the salicylate group. (a) Tinnitus scores of mice after saline or salicylate injection for 4 days. (b) Body weight of mice in the control group and the salicylate group after injections of saline or salicylate for 4 days. (c) Weight of midbrain of mice after injections of saline or salicylate for 4 days. ***p < 0.001
Changes of homer expression in the auditory cortex of mice after salicylate stimulation
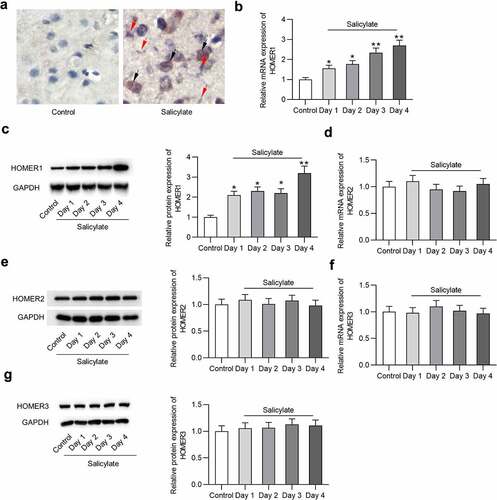
Effects of salicylate on Homer expression in the auditory cortex of mice after salicylate stimulation were detected. reveals the existence of Homer1 mRNA and protein in the auditory cortex of mice ex vivo. reveals that salicylate induced the upregulation of Homer1 mRNA (by 2.7 folds) and protein (by 3.2 folds) expression in the auditory cortex of mice in a day-dependent manner. We also found a positive correlation between tinnitus scores and expression level of Homer1 (coefficient: 5.22; standard error: 0.3; 95% confident interval: 4.75–5.15; P < 0.001). Interestingly, expression of Homer2 () or Homer3 () in the auditory cortex was not significantly affected by salicylate.
Figure 2. Expression of Homer1/2/3 in the auditory cortex of mice. (a) Homer1 mRNA and protein is expressed in the auditory cortex (temporal cortex area 3) of mice in the salicylate group. Scale bar: 500 μm. Red arrow points to Homer1 protein (bluish violet) and black arrow points to Homer1 mRNA (claybank). (b) Relative Homer1 mRNA expression in the auditory cortex of mice after injections of saline or salicylate on Day 1, Day 2, Day 3, Day 4 was detected by PCR analysis. (c) Relative Homer1 protein expression in the auditory cortex of mice after injections of saline or salicylate on Day 1, Day 2, Day 3, Day 4 was detected by western blotting analysis. (d-e) Relative Homer2 expression at the mRNA and protein levels in the auditory cortex of mice in the control group and the salicylate group on Day 1, Day 2, Day 3, Day 4 was detected by PCR and western blotting analysis. (f-g) Relative Homer3 expression at the mRNA and protein levels in the auditory cortex of mice in the control group and the salicylate group on Day 1, Day 2, Day 3, Day 4 was detected by PCR and western blotting analysis. *p < 0.05, **p < 0.01
Effects of MPEP and MCPG on tinnitus scores, body weight, and midbrain weight of salicylate-treated mice
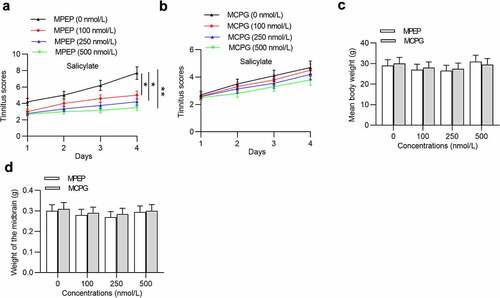
Next, we investigated the influence of MPEP and MCPG on tinnitus scores, body weight, and midbrain weight of mice after injection with salicylate. Injection of MPEP significantly reduced tinnitus scores in a concentration-dependent manner (), while injection of MCPG had no significant effects on tinnitus scores (). Moreover, neither MPEP nor MCPG can induce significant changes in the mean body weight () and midbrain weight ().
Figure 3. Effects of MPEP and MCPG on the tinnitus scores, weight of body and midbrain of mice in the salicylate group. (a) Daily tinnitus scores of mice injected with salicylate combined with different concentrations of MPEP (0, 100, 250, 500 nmol/L). (b) Daily tinnitus scores of mice injected with salicylate combined with different concentrations of MCPG (0, 100, 250, 500 nmol/L). (c) Body weight of mice injected with salicylate combined with different concentrations of MPEP (0, 100, 250, 500 nmol/L) was evaluated on the 4th day after injections. (d) Body weight of mice injected with salicylate combined with different concentrations of MCPG (0, 100, 250, 500 nmol/L) was evaluated on the 4th day after injections. *p < 0.05, **p < 0.01
Effects of MPEP and MCPG on Homer1 expression in the auditory cortex of mice
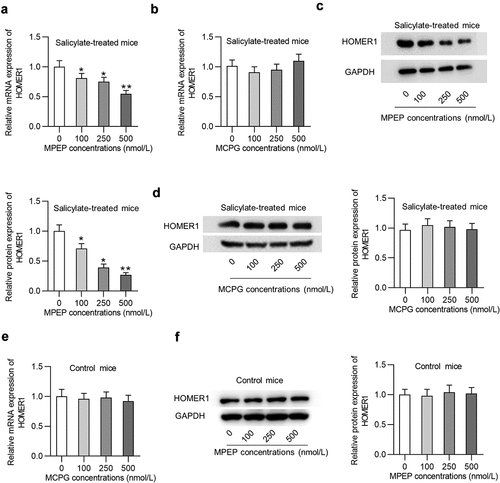
Whether MPEP and MCPG regulate Homer1 expression in the auditory cortex of mice was explored. Injection of MPEP significantly reduced the mRNA expression of Homer1 () in the auditory cortex of mice in the salicylate group in a concentration-dependent manner (decreased by 45% by 500 nmol/L of MPEP), while injection of MCPG had no significant effects on the mRNA expression of Homer1 (). Similarly, Homer1 protein levels were concentration-dependently decreased by MPEP in the auditory cortex of mice in the salicylate group (, decreased by 73% by 500 nmol/L of MPEP) and were not impacted by MCPG (). MPEP suppresses Homer1 expression at the mRNA and protein levels in the auditory cortex of control mice, indicating that the MPEP-medaited degradation of Homer1 is independent of salicylate ().
Figure 4. Homer1 expression was inhibited by MPEP but not MCPG in the auditory cortex of mice in the salicylate group. (a) Relative mRNA expression of HOMER1 in the auditory cortex of mice injected with salicylate combined with different concentrations of MPEP (0, 100, 250, 500 nmol/L). (b) Relative mRNA expression of HOMER1 in the auditory cortex of mice injected with salicylate combined with different concentrations of MCPG (0, 100, 250, 500 nmol/L). (c) Relative protein expression of HOMER1 in the auditory cortex of mice injected with salicylate combined with different concentrations of MPEP (0, 100, 250, 500 nmol/L). (d) Relative protein expression of HOMER1 in the auditory cortex of mice injected with salicylate combined with different concentrations of MCPG (0, 100, 250, 500 nmol/L). (e-f) Relative mRNA and protein expression of HOMER1 in the auditory cortex of mice injected with saline combined with different concentrations of MPEP (0, 100, 250, 500 nmol/L) were detected by PCR and western blotting analysis. *p < 0.05, **p < 0.01
Discussion
We confirmed tinnitus induction in mice after injection of salicylate. The present study revealed that Homer1 expression at the mRNA and protein levels was day-dependently upregulated in the auditory cortex of salicylate-treated mice, while Homer2 or Homer3 expression showed no significant alteration in response to salicylate, indicating that salicylate-induced tinnitus may result from downregulation of the Homer1 gene in the autitory cortex. Tinnitus can be caused by noise, and Homer1 was reported to be significantly downregulated in the ventral cochlear nucleus of mice 2 days after exposure to noise [Citation33]. According to previous studies, abnormal expression of Homer1 can be induced by many external stimuluses, for example, exposure to novel environments induces the upregulation of Homer1 in cortical structures including the frontal cortex [Citation34]. Psychotomimetic drugs induce Homer1 upregulation in several limbo-corticostriatal structures [Citation35–37] and antipsychotic medications [Citation38,Citation39]. Moreover, a previous study revealed that Homer1 has the same regulatory elements with miR-96 and miR-1271, and the mutation of miR-96 and miR-1271 leads to deafness in humans and mice [Citation40], which indicates the close association between Homer1 and hearing-related disease.
mGluRs can mediate ionotropic glutamate receptors, neurotransmitter release and various calcium and potassium channels [Citation41] that are involved in the pathophysiology of central nervous system injury. mGluRs are categorized into group I, group II, and group III. A previous study revealed that mice mimicking human syndromic deafness exhibited impaired glutamate uptake in the cochlear nuclei [Citation42]. mGluR5 belongs to group I and consists of heptahelical domain, cysteine-rich region, and binding domain [Citation43]. The role of mGluR5 is controversial due to lacking authentic selective antagonists. Selective mGluR5 inhibitors including noncompetitive MPEP [Citation44] and competitive MCPG [Citation45] have been identified. We identified that injection of MPEP significantly reduced tinnitus scores in mice in a concentration-dependent manner, while injection of MCPG had no significant effects on tinnitus scores. Importantly, MPEP reduced Homer1 mRNA expression and protein levels in the auditory cortex of tinnitus mice, while MCPG had no significant effects on Homer1 expression. These findings indicated that MPEP, as a mGluR5 inhibitor, has an inhibitory effect on tinnitus by suppressing Homer1. Tinnitus has been reported to be highly associated with gene expression of NMDA receptor [Citation27], and mGluR5 positive modulators enhance NMDA receptor activation [Citation46]. MPEP can function as a weak NMDA antagonist [Citation47]. There is also evidence for a functional interaction between mGluR5 and NMDA receptors [Citation48]. We hypothesized that MPEP suppresses tinnitus also via inhibiting NMDA receptor expression and/or its functions.
However, some limitations of our study must be addressed. Despite that sodium salicylate at the dose of 350 mg/kg used in the present study is widely applied in rodent experiments on tinnitus, there are some side reactions such as the systemic toxicity and peripheral deafness [Citation49]. Our findings only provided the primary evidence for the expression of Homer1 in sodium salicylate-induced tinnitus in mice, while experiments using neurons are needed to confirm the results.
Conclusion
Our findings innovatively demonstrated that Homer1 is upregulated in the auditory cortex of mice with salicylate-induced tinnitus. A noncompetitive mGluR5 antagonist, MPEP, can reduce tinnitus scores of mice and suppress the expression of Homer1 in the auditory cortex in a concentration-dependent manner, while a competitive mGluR5 antagonist, MCPG, exerted no significant effects. Our findings could contribute to further understanding of pathophysiology and therapy of tinnitus, while more studies are needed to reveal the detailed mechanisms linking Homer1 and tinnitus in the future.
Additional information
Funding
References
- Langguth B, Kreuzer PM, Kleinjung T, et al. Tinnitus: causes and clinical management. Lancet Neurol. 2013 Sep;12(9):920–930.
- Gollnast D, Tziridis K, Krauss P, et al. Analysis of audiometric differences of patients with and without tinnitus in a large clinical database. Front Neurol. 2017;8:31.
- Tomanic M, Belojevic G, Jovanovic A, et al. Dietary factors and tinnitus among adolescents. Nutrients. 2020 Oct 27;12(11):11.
- Evans EF, Borerwe TA. Ototoxic effects of salicylates on the responses of single cochlear nerve fibres and on cochlear potentials. Br J Audiol. 1982 May;16(2):101–108.
- Paul AK, Lobarinas E, Simmons R, et al. Metabolic imaging of rat brain during pharmacologically-induced tinnitus. Neuroimage. 2009 Jan 15;44(2):312–318.
- De Ridder D, Elgoyhen AB, Romo R, et al. Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci U S A. 2011 May 17;108(20):8075–8080.
- Searchfield GD, Morrison-Low J, Wise K. Object identification and attention training for treating tinnitus. Prog Brain Res. 2007;166:441–460.
- Lanting CP, de Kleine E, van Dijk P. Neural activity underlying tinnitus generation: results from PET and fMRI. Hear Res. 2009 Sep;255(1–2):1–13.
- Schlee W, Mueller N, Hartmann T, et al. Mapping cortical hubs in tinnitus. BMC Biol. 2009 Nov;23(7):80.
- Shulman A, Goldstein B, Strashun AM. Final common pathway for tinnitus: theoretical and clinical implications of neuroanatomical substrates. Int Tinnitus J. 2009;15(1):5–50.
- Zhang J, Chen YC, Feng X, et al. Impairments of thalamic resting-state functional connectivity in patients with chronic tinnitus. Eur J Radiol. 2015 Jul;84(7):1277–1284.
- Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol. 2007;8(2):206.
- Tu JC, Xiao B, Yuan JP, et al. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998 Oct;21(4):717–726.
- Hwang SY, Wei J, Westhoff JH, et al. Differential functional interaction of two Vesl/Homer protein isoforms with ryanodine receptor type 1: a novel mechanism for control of intracellular calcium signaling. Cell Calcium. 2003 Aug;34(2):177–184.
- Yuan JP, Kiselyov K, Shin DM, et al. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003 Sep 19;114(6):777–789.
- Lominac KD, Oleson EB, Pava M, et al. Distinct roles for different Homer1 isoforms in behaviors and associated prefrontal cortex function. J Neurosci. 2005 Dec 14;25(50):11586–11594.
- Szumlinski KK, Lominac KD, Oleson EB, et al. Homer2 is necessary for EtOH-induced neuroplasticity. J Neurosci. 2005 Jul 27;25(30):7054–7061.
- Szumlinski KK, Dehoff MH, Kang SH, et al. Homer proteins regulate sensitivity to cocaine. Neuron. 2004 Aug 5;43(3):401–413.
- Bridi M, Schoch H, Florian C, et al. Transcriptional corepressor SIN3A regulates hippocampal synaptic plasticity via Homer1/mGluR5 signaling. JCI Insight. 2020 Mar 12;5(5):5.
- Wang Y, He W, Zhang H, et al. mGluR5 mediates ketamine antidepressant response in susceptible rats exposed to prenatal stress. J Affect Disord. 2020 Jul 1;272:398–408.
- Wang W, Zhang Z, Shang J, et al. Activation of group I metabotropic glutamate receptors induces long-term depression in the hippocampal CA1 region of adult rats in vitro. Neurosci Res. 2008 Sep;62(1):43–50.
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004 Nov;27(11):676–682.
- Jastreboff PJ, Brennan JF, Coleman JK, et al. Phantom auditory sensation in rats: an animal model for tinnitus. Behav Neurosci. 1988 Dec;102(6):811–822.
- Du Y, Liu J, Jiang Q, et al. Paraflocculus plays a role in salicylate-induced tinnitus. Hear Res. 2017 Sep;353:176–184.
- Puel JL, Guitton MJ. Salicylate-induced tinnitus: molecular mechanisms and modulation by anxiety. Prog Brain Res. 2007;166:141–146.
- Hwang JH, Chen JC, Yang SY, et al. Expression of tumor necrosis factor-α and interleukin-1β genes in the cochlea and inferior colliculus in salicylate-induced tinnitus. J Neuroinflammation. 2011 Apr 9;8(1):30.
- Hwang JH, Chen JC, Yang SY, et al. Expression of COX-2 and NMDA receptor genes at the cochlea and midbrain in salicylate-induced tinnitus. Laryngoscope. 2011 Feb;121(2):361–364.
- Guitton MJ, Caston J, Ruel J, et al. Salicylate induces tinnitus through activation of cochlear NMDA receptors. J Neurosci. 2003 May 1;23(9):3944–3952.
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Compact sixth edition ed. 2009;3(2):6.
- Tan J, Rüttiger L, Panford-Walsh R, et al. Tinnitus behavior and hearing function correlate with the reciprocal expression patterns of BDNF and Arg3.1/arc in auditory neurons following acoustic trauma. Neuroscience. 2007 Mar 16;145(2):715–726.
- Panford-Walsh R, Singer W, Rüttiger L, et al. Midazolam reverses salicylate-induced changes in brain-derived neurotrophic factor and arg3.1 expression: implications for tinnitus perception and auditory plasticity. Mol Pharmacol. 2008 Sep;74(3):595–604.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001 Dec;25(4):402–408.
- Manohar S, Ramchander PV, Salvi R, et al. Synaptic Reorganization Response in the Cochlear Nucleus Following Intense Noise Exposure. Neuroscience. 2019 Feb;10(399):184–198.
- Vazdarjanova A, McNaughton BL, Barnes CA, et al. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. J Neurosci. 2002 Dec 1;22(23):10067–10071.
- Cochran SM, Fujimura M, Morris BJ, et al. Acute and delayed effects of phencyclidine upon mRNA levels of markers of glutamatergic and GABAergic neurotransmitter function in the rat brain. Synapse. 2002 Dec 1;46(3):206–214.
- Nichols CD, Sanders-Bush E. A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology. 2002 May;26(5):634–642.
- Fujiyama K, Kajii Y, Hiraoka S, et al. Differential regulation by stimulants of neocortical expression of mrt1, arc, and homer1a mRNA in the rats treated with repeated methamphetamine. Synapse. 2003 Sep 1;49(3):143–149.
- de Bartolomeis A, Aloj L, Ambesi-Impiombato A, et al. Acute administration of antipsychotics modulates Homer striatal gene expression differentially. Brain Res Mol Brain Res. 2002 Jan 31;98(1–2):124–129.
- Polese D, de Serpis AA, Ambesi-Impiombato A, et al. Homer 1a gene expression modulation by antipsychotic drugs: involvement of the glutamate metabotropic system and effects of D-cycloserine. Neuropsychopharmacology. 2002 Dec;27(6):906–913.
- Jensen KP, Covault J. Human miR-1271 is a miR-96 paralog with distinct non-conserved brain expression pattern. Nucleic Acids Res. 2011 Jan;39(2):701–711.
- Mahato PK, Ramsakha N, Ojha P, et al. Group I metabotropic glutamate receptors (mGluRs): ins and outs. Adv Exp Med Biol. 2018;1112:163–175.
- Fuentes-Santamaría V, Alvarado JC, Rodríguez-de la Rosa L, et al. Neuroglial involvement in abnormal glutamate transport in the cochlear nuclei of the Igf1 (-/-) mouse. Front Cell Neurosci. 2019;13:67.
- Pula G, Mundell SJ, Roberts PJ, et al. Agonist-independent internalization of metabotropic glutamate receptor 1a is arrestin- and clathrin-dependent and is suppressed by receptor inverse agonists. J Neurochem. 2004 May;89(4):1009–1020.
- Gasparini F, Lingenhöhl K, Stoehr N, et al. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999 Oct;38(10):1493–1503.
- White AM, Kylänpää RA, Christie LA, et al. Presynaptic group I metabotropic glutamate receptors modulate synaptic transmission in the rat superior colliculus via 4-AP sensitive K(+) channels. Br J Pharmacol. 2003 Dec;140(8):1421–1433.
- Chen HH, Liao PF, Chan MH. mGluR5 positive modulators both potentiate activation and restore inhibition in NMDA receptors by PKC dependent pathway. J Biomed Sci. 2011 Feb 22;18(1):19.
- O’Leary DM, Movsesyan V, Vicini S, et al. Selective mGluR5 antagonists MPEP and SIB-1893 decrease NMDA or glutamate-mediated neuronal toxicity through actions that reflect NMDA receptor antagonism. Br J Pharmacol. 2000 Dec;131(7):1429–1437.
- Pisani A, Gubellini P, Bonsi P, et al. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106(3):579–587.
- Sheppard A, Hayes SH, Chen GD, et al. Review of salicylate-induced hearing loss, neurotoxicity, tinnitus and neuropathophysiology. Acta Otorhinolaryngol Ital. 2014 Apr;34(2):79–93.
