ABSTRACT
Colorectal cancer is a common cause of cancer-related death worldwide. Thus, there is an urgent need to determine the mechanism of progression of colorectal cancer. In this study, we investigated the function and mechanism of long non-coding RNA LINC00958, providing a new biomarker for colorectal cancer. The expression of LINC00958, miR-3064-5p, and LEM domain containing 1 (LEMD1) in colorectal cancer tissues and cell lines was analyzed using reverse transcription quantitative polymerase chain reaction (RT-qPCR). The interaction between LINC00958, miR-3064-5p, and LEMD1 was assessed using the luciferase assay. The viability, proliferation, migration, invasion, and apoptosis of colorectal cancer cells with silenced LINC00958, miR-3064-5p, and LEMD1 were investigated using the cell counting kit-8 (CCK-8), 5′-Bromo-2′-deoxyuridine (BrdU), flow cytometry, wound healing, and transwell assays. Phosphorylated phosphoinositide 3-kinase (p-PI3K) and phosphorylated protein kinase B (p-AKT) protein levels were measured by western blotting. LINC00958 and LEMD1 were found to have increased, while the expression of miR-3064-5p was decreased in colorectal cancer tissues and cell lines. Silencing of LINC00958 hampered cell viability, proliferation, migration, and invasion, while enhancing the apoptosis in colorectal cancer cells. Notably, LINC00958 inhibited miR-3064-5p and promoted LEMD1; the miR-3064-5p inhibitor abrogated the effect of LINC00958 silencing in colorectal cancer cells. Additionally, LEMD1 knockdown inhibited the activation of PI3K/AKT signaling. Our analyses have shown that LINC00958 could facilitate the progression of colorectal cancer by sponging miR-3064-5p and releasing LEMD1, leading to the activation of the PI3K/AKT pathway. Thus, LINC00958 may be considered as an effective biomarker for the treatment of colorectal cancer.
Introduction
Colorectal cancer is a digestive system malignancy with a high prevalence and death rate worldwide, leading to approximately 600,000 deaths each year [Citation1,Citation2]. In the recent decades, therapies for patients with colorectal cancer, including surgical resection, chemotherapy, and radiotherapy, have improved rapidly. However, many patients show a low survival rate because they are diagnosed at an advanced stage [Citation3,Citation4]. Thus, investigation of effective biomarkers is important for diagnosing and treating patients with colorectal cancer.
Long non-coding RNAs (lncRNAs) are 200 nucleotides long non-coding RNAs, which play important roles in cellular growth and apoptosis to modulate cancer pathogenesis and development [Citation5,Citation6]. Notably, evidences indicate that lncRNAs regulate microRNA (miRNA) expression and affect the occurrence and development of a variety of cancers [Citation7–9]. Recently, lncRNA LINC00958 has been found to be involved in the pathogenesis of different cancers. LINC00958 regulates bladder cancer by inhibiting miR-378a-3p and upregulating insulin-like growth factor 1 receptor (IGF1R) [Citation10]. LINC00958 facilitates cell growth by reducing miRNA-625 and promoting NUAK family SnF1-like kinase-1 (NUAK1) in nasopharyngeal carcinoma [Citation11]. LINC00958 is also known to accelerate cell growth and migration by activating JNK/c-JUN signaling in non-small cell lung cancer [Citation12]. Notably, only one study has indicated that LINC00958 inhibits miR-3619-5p and further enhances cell growth and reduces apoptosis in colorectal cancer [Citation13]. However, whether the LINC00958-miR-3064-5p-LEM Domain Containing 1 (LEMD1)-phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) axis plays any important role in the onset and development of colorectal cancer remains unclear.
Based on the malignant behavior of cancer cells, we investigated the effects of LINC00958/miR-3064-5p/LEMD1 on the viability, proliferation, apoptosis, migration, and invasion of colorectal cancer cells. LINC00958 might promote colorectal cancer progression by repressing the effect of miR-3064-5p on LEMD1 to activate the PI3K/AKT signaling pathway; this may potentially provide therapeutic targets for patients with colorectal cancer.
Materials and methods
Patient samples, cells, and cell transfection
Colorectal cancer and normal tissues were collected from 29 patients with colorectal cancer, and informed consent was obtained from our hospital. This study was approved by the Ethics Committee of Tianyou Hospital Affiliated to the Wuhan University of Science and Technology. shows patient information. Human colorectal cancer cell lines SW620, HCT116, and HCT8 as well as normal colorectal epithelial cells (FHC) were obtained from ATCC (Manassas, VA, USA), and were cultured in DMEM medium (Gibco, USA) with 10% fetal bovine serum (Gibco, USA) at 37°C in 5% CO2. HCT116 and HCT8 cells were transfected with siRNA-LINC00958, miR-3064-5p mimics and inhibitor, SiRNA-LEMD1, and negative control (NC) obtained from GenePharm (Shanghai, China) using Lipofectamine 2000 (Invitrogen, USA) for 48 h.
Table 1. Baseline characteristics of patients with CRC
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Trizol (Cat#: 15596018, Thermo, USA) was used to obtain LINC00958 and LEMD1 mRNA. SuperScript IV One-Step RT-PCR System with ezDNase (12595100, Invitrogen, USA) was used for both RT-qPCR steps, viz. cDNA synthesis and PCR amplification.
PureLink miRNA Isolation Kit (K157001, Invitrogen, USA) was used to obtain miR-3064-5p. miRcute miRNA First Strand cDNA Synthesis kit (KR211, Tiangen, China) was used for cDNA synthesis and miRcute enhanced miRNA fluorescence quantitative detection kit (FP411, Tiangen, China) was used for RT-qPCR carried out on the ABI PRISM 7500 real-time PCR System (Applied Biosystems, USA).
The level of LINC00958 in the nuclear and cytosolic fractions obtained using the PARIS Kit (AM1921, Life, USA) was separately confirmed by RT-qPCR.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control for LEMD1 and cytoplasm control, and small nuclear RNA 6 (U6) was used as an internal control for miR-3064-5p and nuclear control; the analysis was carried out using the 2−ΔΔCt method [Citation14]. Primer sequences are listed in .
Table 2. The sequences of the primers in this study
Cell counting kit (CCK-8) assay
Cell viability was detected using CCK-8 kit (Cat#: K1018; APExBIO, China). Transfected HCT116 and HCT8 cells (5 × 103) were seeded in 96-well plates. At 0, 24, 48, 72, and 96 h, 10 µL CCK-8 solution was added to the plate at 37°C followed by 2 h incubation. Then, the optical density (OD) at 450 nm was obtained using a multimode plate reader (Biotek, USA) [Citation15].
5-bromo-2′-deoxyuridine (BrdU) assay
Cell proliferation was measured using a BrdU cell proliferation kit (ab142567, Abcam, UK). Approximately 1 × 104 transfected HCT116 and HCT8 cells were cultured in 96-well plates for 48 h. Afterward, the medium was replaced with DMEM medium, and 10 μL BrdU solution was added to the plate. After 4 h of incubation, the cells were washed twice and incubated with secondary antibody for 1 h. OD450 nm was measured using a microplate reader (Biotek, USA) [Citation16].
Apoptosis assay
Cell apoptosis was determined using the Annexin V-fluorescein isothiocyanate (FITC)-propidium iodide (PI) apoptosis detection kit (Cat#: 556547; BD, USA). Transfected HCT116 and HCT8 cells were harvested, washed twice, and suspended in binding buffer. After 20 min incubation with 5 µL FITC and 10 µL PI in the dark at 4°C, cells were washed twice and suspended in binding buffer and subjected to flow cytometry (BD, USA) using the Cell-Quest software (BD) [Citation17].
Cell migration assay
Cell migration was analyzed by wound healing assay as described previously [Citation18]. Transfected HCT116 and HCT8 cells (1 × 106) were cultured in 6-well plates. When cells reached 80% confluence, a 200 μL pipette tip was used to draw a straight line in the middle of each cell monolayer, and cells were washed twice to remove the detached cells. Images of the scratch at 0 and 24 h were captured using a light microscope.
Cell invasion assay
A transwell chamber with 8-µm pores (Cat#: #3244, Coring, USA) was prepared with matrigel (Corning, USA). A 10% FBS cell culture medium was added to the basolateral chambers. Transfected HCT116 and HCT8 cells (8 × 104) were seeded into the apical chamber. After 48 h, the invaded cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Finally, images were captured using a BX43 light microscope (Olympus, Japan), and the number of invasive cells was counted [Citation19].
Dual luciferase reporter assay
The assay was performed as described previously [Citation20]. The LINC00958 mutated (MUT) sequences and LEMD1 3ʹ-UTR mutated sequences were synthesized using QuikChange XL Site-Directed Mutagenesis Kit (Stratagene, USA). Then, LINC00958 wild-type (WT) or MUT sequences and the LEMD1 3ʹ-UTR WT or MUT sequences were cloned in the psiCHECK2 vector (GenePharm, Shanghai, China). The miR-3064-5p mimic or mimic-NC was transfected into HCT116 and HCT8 cells with either of the psiCHECK2 vector for 48 h. Luciferase activities of firefly and Renilla were measured using the Dual-Luciferase Reporter Assay System (Cat#: E1910, Promega, USA). Renilla luciferase activity was used as the control.
RNA immunoprecipitation (RIP) assay
The interaction of LINC00958 and miR-3064-5p in HCT116 and HCT8 cells was determined using the RIP kit (Cat#: P0101, Geneseed, China). Cell lysates from cells treated with miR-3064-5p mimic or NC were incubated with anti-immunoglobulin G (IgG) or anti-Argonaute 2 (AGO2). After 2 h, magnetic beads were added to the solution at 4°C. The next day, LINC00958 was purified and analyzed by RT-qPCR [Citation21].
RNA-pull down detection
The HCT116 and HCT8 cells were tagged with biotin-labeled miR-3064-5p (Bio-miR-3064-5p) or Bio-miR-NC (Thermo, USA) for 48 h. Then, the cell lysates were added to the beads (Cat#: S1420S, NEB, UK) at 4°C on a rotator and incubated overnight. The enriched RNAs were purified using the PureYield RNA Midiprep System (Promega, USA). RT-qPCR was performed to measure LEMD1 enrichment [Citation22].
Western blot analysis
Lysates of transfected HCT116 and HCT8 cells were obtained using RIPA buffer (Cat#: #20-188, Sigma, USA). Protein samples were separated using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene (PVDF) membranes; the membranes were blocked using 5% nonfat milk for 1 h. After washing twice, the membranes were incubated with anti-LEMD1 (1:1000, Cat#: ab201206, Abcam, UK), PI3K (1:1000, Cat#: ab86714, Abcam), p-PI3K (1:750, Cat#: ab182651. Abcam), AKT (1:1000, Cat#: 4691, Cell Signaling Technology, USA), p-AKT (1:2000, Cat#: 4060; 1:2000, Cell Signaling Technology), and anti-GAPDH (1:2000, Cat#: 5174, CST, USA) overnight at 4°C. The membranes were then washed twice and were incubated with horse radish peroxidase (HRP)-linked anti-rabbit antibody (1:5000, Cat#: ab6721, Abcam, UK) for 1 h. Finally, protein bands were developed using the EasyBlot ECL kit (#C506668; Sangon, China), and band densities were analyzed using Image J software (National Institute of Health, USA). Relative LEMD1 protein level was normalized to that of GAPDH [Citation23].
Statistical analysis
Data were analyzed using GraphPad Prism 8.0 (GraphPad Prism, USA) and presented as the mean ± standard deviation obtained from triplicate experiments. Comparisons between two and more groups were evaluated using Student’s t-test or analysis of variance (ANOVA), respectively. Pearson correlation analysis was performed for analyzing the associations between LINC00958 and miR-3064-5p expression, and LEMD1 and miR-3064-5p expression. Differences were considered significant at P < 0.05.
Results
To begin with, we identified miR-3064-5p and LEMD1 as genes of interest through bioinformatics analysis. In addition, the expression level of LINC00958 was found to have increased in colorectal cancer. Biological function analysis showed that knockdown of LINC00958 inhibited the viability, proliferation, migration, and invasion of cancer cells and promoted cell apoptosis. Targeting analysis showed that LINC00958 affected tumor progression by negatively regulating miR-3064-5p. Finally, we demonstrated that miR-3064-5p controls the phosphorylation of PI3K and AKT by binding to the classical transcription factor LEMD1. Thus, the LINC00958/miR-3064-5p/LEMD1/PI3K/AKT axis was confirmed to influence the progression of colorectal cancer.
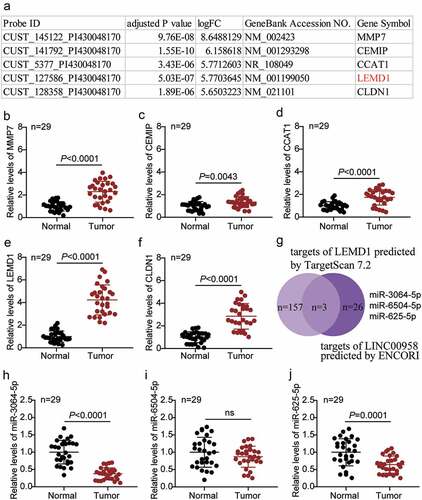
LEMD1 was significantly upregulated and miR-3064-5p was downregulated in colorectal cancer
The top five most upregulated genes in colorectal cancer in the GSE126095 data series are listed in . The expression of five genes, namely MMP7, CEMIP, CCAT1, LEMD1, and CLDN1, was also detected in the tissue samples collected in this study. We found that LEMD1 was the most significantly upregulated gene in our samples (). The expression of LEMD1 in cancer tissues was almost fourfold higher than that in the adjacent healthy tissues (). We then analyzed the possible target miRNAs of LEMD1 using the TargetScan Human 7.2 algorithm and the potential target miRNAs of LINC00958 using the ENCORI algorithm. miR-3064-5p, miR-6504-5p, and miR-625-5p were identified as common targets of LINC00958 and LEMD1 mRNA (). Among the three miRNAs, miR-3064-5p was the most downregulated in the cancer tissue samples collected in our study ().
Figure 1. LEMD1 was found significantly upregulated and miR-3064-5p was significant downregulated in colorectal cancer
LINC00958 facilitated colorectal cancer cells progression
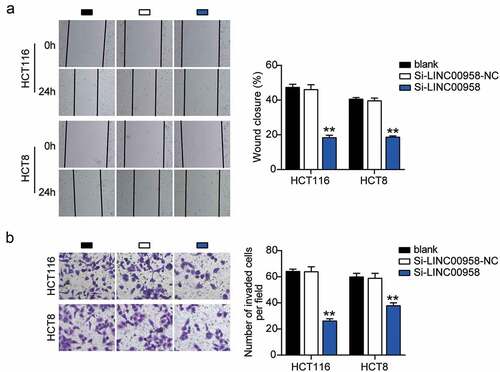
First, we determined the levels of LINC00958 in colorectal cancer cells and tissues. As the data show, colorectal cancer cells showed dramatically higher LINC00958 expression than that observed in normal FHC cells; this was especially true for HCT116 and HCT8 cells, which were chosen for subsequent studies. At the same time, colorectal cancer tissues also displayed enhanced LINC00958 expression compared to the normal tissues (). Next, we confirmed that LINC00958 mainly existed in the cytoplasm of both HCT116 and HCT8 cells (). After transfecting siRNA-LINC00958 and negative control (NC) into HCT116 and HCT8 cells, the si-LINC00958 was downregulated by approximately 70% when compared with control cells, in both cell lines (). CCK-8 assay showed that si-LINC00958 groups significantly inhibited cell viability in both cell lines when compared to the control cells (). The si-LINC00958 groups also showed approximately 30% decrease in cell proliferation compared with control cells in both cell lines (). Furthermore, the si-LINC00958 groups showed nearly twofold cell apoptosis compared with control cells in both cell lines (). On the other hand, si-LINC00958 groups showed approximately half of the cell migration and invasion compared to that observed for the control cells, in both cell types (). These results indicated that LINC00958 facilitated the progression of colorectal cancer.
Figure 2. LINC00958 promoted cell growth, but inhibited cell apoptosis of colorectal cancer cells
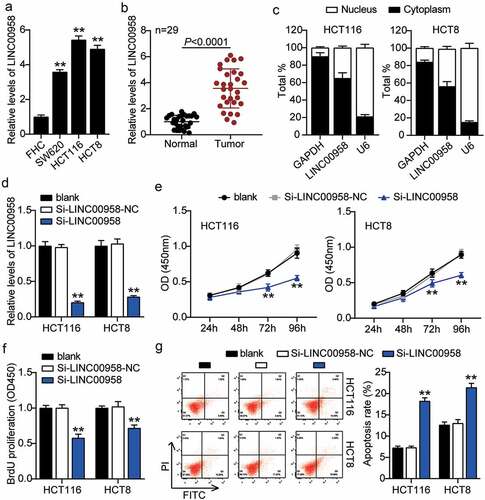
Figure 3. LINC00958 promoted cell migration and invasion of colorectal cancer cells
LINC00958 sponged miR-3064-5p in colorectal cancer cells
The binding sequence between LINC00958 and miR-3064-5p is shown in . miR-3064-5p mimics or NC and psiCHECK2 LINC00958-wide type (WT) vectors or psiCHECK2 LINC00958-Mut vectors were co-transfected into HCT116 and HCT8 cells. The cells treated with miR-3064-5p mimics and psiCHECK2 LINC00958-Mut showed 50% reduction in luciferase activity compared with the NC group (). The interaction between LINC00958 and miR-3064-5p was confirmed by RNA immunoprecipitation (RIP) in both HCT116 and HCT8 cells (). miR-3064-5p levels were negatively correlated with LINC00958 levels in colorectal cancer tissues (). HCT116 and HCT8 cells also showed more than 50% decrease in miR-3064-5p levels compared with normal cells (). Next, we transfected Si-LINC00958, miR-3064-5p inhibitor, and NC into HCT116 and HCT8 cells to determine the role of the LINC00958-miR-3064-5p axis. The si-LINC00958 groups showed a 1.5-fold increase in miR-3064-5p levels, but a 70% reduction in LINC00958 levels compared with control cells. The miR-3064-5p inhibitor groups showed 70% reduction in miR-3064-5p levels but the LINC00958 levels were similar to those observed in the control cells. In addition, the si-LINC00958+ miR-3064-5p inhibitor groups showed a 70% decrease in LINC00958 levels, but the same miR-3064-5p levels compared with control cells (). Collectively, LINC00958 sponged miR-3064-5p and inhibited its expression in colorectal cancer cells.
Figure 4. LINC00958 interacted with miR-3064-5p in colorectal cancer cells
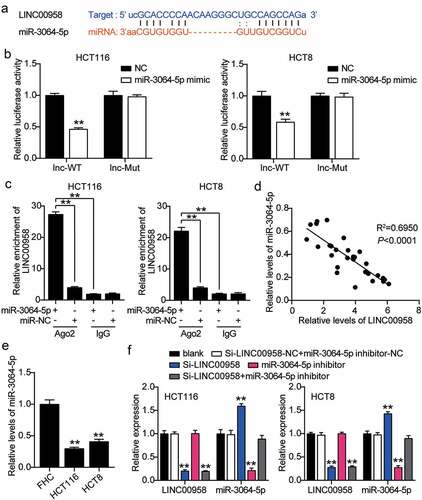
Sponging miR-3064-5p by LINC00958 facilitated the development of colorectal cancer cells
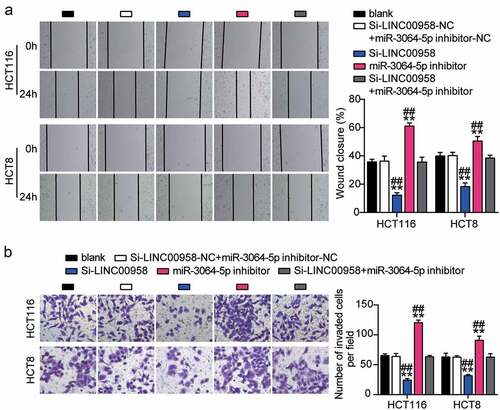
Next, we assessed the function of the LINC00958-miR-3064-5p axis in colorectal cancer and found that miR-3064-5p inhibitor groups showed significantly enhanced cell viability compared with the control cells (). Consistently, miR-3064-5p inhibitor groups were observed to promote the cell proliferation 1.5-fold higher than that observed for the control cells (). However, miR-3064-5p inhibitor groups showed decreased cell apoptosis compared with the control cells (). On the other hand, these groups showed approximately twofold enhanced cell migration and 1.3-fold enhanced cell invasion when compared with the control cells in HCT116 and HCT8 cells, respectively (). It was further found that si-LINC00958 significantly inhibited the effect of miR-3064-5p inhibitor in both HCT116 and HCT8 cells.
Figure 5. LINC00958 sponging miR-3064-5p facilitated cell proliferation and repressed cell apoptosis of colorectal cancer cells
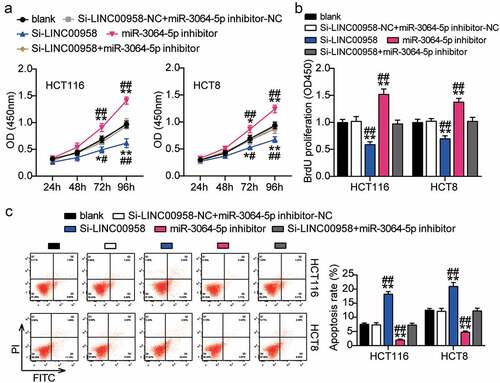
Figure 6. LINC00958 sponging miR-3064-5p enhanced cell migration and invasion of colorectal cancer cells
LEMD1 was targeted by miR-3064-5p in colorectal cancer cells
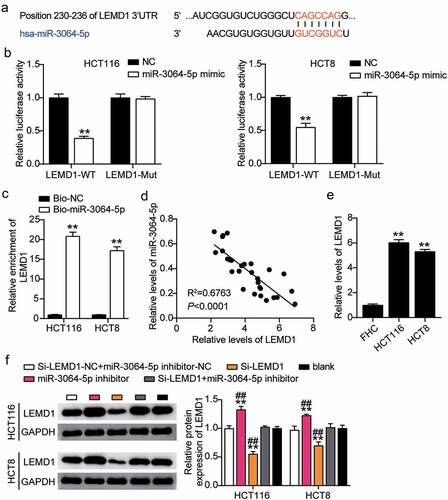
The binding site between LEMD1 and miR-3064-5p was obtained from starBase (). HCT116 and HCT8 cells co-transfected with miR-3064-5p mimics and psiCHECK2 LEMD1 3′-UTR WT vectors showed nearly 50% decreased luciferase activity; however, this was not observed for the psiCHECK2 LEMD1 3′-UTR Mut vectors, suggesting an interaction between miR-3064-5p and LEMD1 (). The interaction between miR-3064-5p and LEMD1 was verified by an RNA pull-down assay in both the cell lines (). A negative correlation between miR-3064-5p and LEMD1 was found in colorectal cancer tissues (). The expression of LEMD1 in HCT116 and HCT8 cells was found to have been significantly elevated by approximately sixfold compared to that in the normal cells (). To determine the effect of miR-3064-5p-LEMD1 axis in colorectal cancer, we transfected siRNA-LEMD1, miR-3064-5p inhibitor, and NC into HCT116 and HCT8 cells. The si-LEMD1 groups showed approximately 50% downregulation in LEMD1 protein levels, whereas those in the miR-3064-5p inhibitor groups were promoted by 1.3-fold when compared with the control cells ().
Figure 7. MiR-3064-5p targeted to LEMD1 and inhibited the expression of LEMD1 in colorectal cancer cells
miR-3064-5p attenuated colorectal cancer cell progression by inhibiting LEMD1
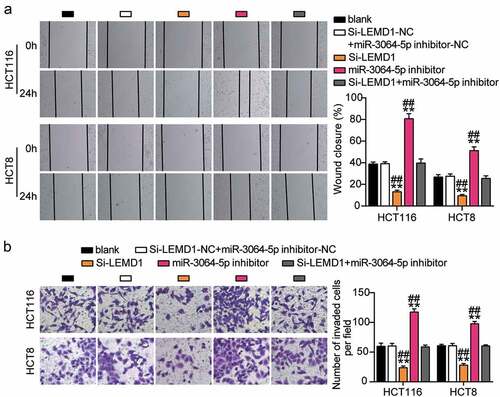
The si-LEMD1 groups showed significant inhibition of cell viability when compared with the control cells (). The si-LEMD1 groups showed nearly 50% repression of cell proliferation when compared with the control cells (). However, the si-LEMD1 groups showed an approximately twofold increase in cell apoptosis compared with the control cells (). Additionally, we found that the si-LEMD1 groups showed nearly 50% reduction in cell migration and invasion compared with the control cells (). However, these effects were abrogated by si-LEMD1+ miR-3064-5p inhibitor treatment in both HCT116 and HCT8 cells. Thus, miR-3064-5p was observed to attenuate colorectal cancer cell progression by inhibiting LEMD1 expression.
Figure 8. MiR-3064-5p attenuated cell proliferation and elevated cell apoptosis by inhibiting LEMD1
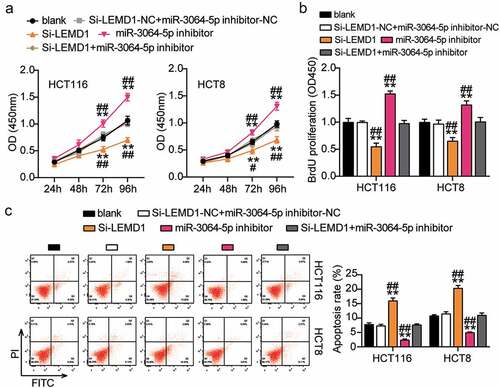
Figure 9. MiR-3064-5p repressed cell migration and invasion by inhibiting LEMD1
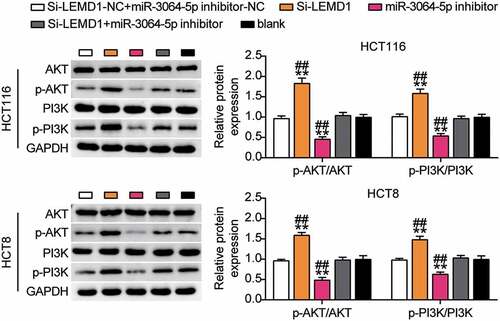
miR-3064-5p was found to regulate the downstream PI3K-AKT signaling pathway through LEMD1
Furthermore, we studied the expression levels of PI3K-AKT signaling pathway-related proteins. Western blotting showed that p-PI3K and p-AKT protein levels in the si-LEMD1 group were significantly lower than those in the control group, while p-PI3K and p-AKT protein levels were elevated in the miR-3064-5p inhibitor group. In addition, silencing LEMD1 reversed the effects of miR-3064-5p interference on the levels of p-PI3K and p-AKT (). Considering the targeted regulation of LEMD1 by miR-3064-5p, we hypothesized that interference with miR-3064-5p could activate the downstream PI3K-AKT signaling pathway via LEMD1.
Discussion
The current study showed that LINC00958 inhibited miR-3064-5p expression and promoted the expression of LEMD1, which effectively enhanced cell growth and arrested apoptosis of colorectal cancer cells.
Accumulating evidence suggests that LINC00958 plays an important role in cancer progression [Citation24–26]. Wang et al. reported that LINC00958 accelerated cell growth and metastasis by reducing miR-625-5p to elevate LRRC8E expression in cervical cancer [Citation24]. Chen et al. have revealed that LINC00958 inhibited miR-627-5p and upregulated YBX2 to enhance oral squamous cell carcinoma cell growth [Citation25]. Another study indicated that LINC00958 accelerated tumor initiation in pancreatic cancer by downregulating miRNA-330-5p but upregulating PAX8 [Citation26]. Regarding colorectal cancer, only one study has demonstrated that LINC00958 significantly promoted cell growth by inhibiting miR-3619-5p expression. Downregulation of LINC00958 reversed tumorigenesis by increasing miR-3619-5p expression [Citation13]. Our study showed that LINC00958 levels in colorectal cancer tissues and cells were evidently increased. LINC00958 silencing attenuated cell growth and boosted apoptosis in HCT116 and HCT8 cells. Additionally, LINC00958 sponged miR-3064-5p, which suppressed the role of miR-3064-5p in preventing colorectal cancer progression.
miR-3064-5p has been previously reported to participate in the pathogenesis of cancers [Citation27–29]. For instance, CircPRRX1 was shown to promote doxorubicin resistance in gastric cancer by inhibiting miR-3064-5p and enhancing protein tyrosine phosphatase, non-receptor type 14 (PTPN14) signaling [Citation27]. In addition, lncRNA PXN-AS1 repressed cell growth by reducing miR-3064-5p and upregulating phosphatidylinositol-5-phosphate 4-kinase type 2 beta (PIP4K2B) expression in pancreatic cancer [Citation28]. Furthermore, miRNA-3064-5p dramatically hampered the cell growth and invasion by suppressing telomerase reverse transcriptase in ovarian cancer [Citation29]. Notably, the role of miR-3064-5p in colorectal cancer has remained unknown. We demonstrated that miR-3064-5p expression in colorectal cancer tissues and cells was reduced. Notably, miR-3064-5p was negatively correlated with LINC00958 or LEMD1 expression in colorectal cancer tissues. miR-3064-5p inhibitor accelerated cell growth but hampered cell apoptosis; however, LINC00958 reduced miR-3064-5p expression to elevate the progression of colorectal cancer.
Previous studies have identified LEMD1 as an oncogene in various cancers [Citation30–32]. For instance, LEMD1 promotes cell growth, invasion, and endothelial transmigration in oral squamous cell carcinoma (OSCC) [Citation30]. Additionally, they found that the sushi repeat-containing protein X-linked 2 (SRPX2) is a downstream signal of LEMD1, which acts as a tumor genesis gene in OSCC [Citation31]. Overexpression of LEMD1 was also observed during the initiation of colorectal cancer, and downregulation of LEMD1 significantly destroyed the maintenance of colorectal cancer [Citation33,Citation34]. In our study, LEMD1 expression in colorectal cancer tissues and cells was found to have been significantly increased. The silencing of LEMD1 dramatically hampered cell growth and enhanced apoptosis in colorectal cancer cells. Notably, we observed that LEMD1 expression was attenuated by the LINC00958-miR-3064-5p axis in colorectal cancer.
Abnormal activation of PI3K, a member of the 3ʹ-hydroxy phosphorylated conserved lipokinase family of phosphatidylinositol, is commonly observed in human cancers [Citation35]. This protein phosphorylates PIP2 to produce PIP3 by regulating the extracellular protein kinase levels [Citation36]. PIP3 is an important second messenger involved in AKT recruitment and activation of the cell survival signal response [Citation37]. A previous study found that LEMD1 regulates the proliferation and apoptosis of gastric cancer cells by activating the PI3K/AKT signaling pathway [Citation32]. Furthermore, phosphorylation of PI3K and AKT has been shown to support the tumor environment in non-small cell lung carcinoma and colorectal cancer [Citation38,Citation39]. Similar to previous studies, this study found that knocking down LEMD1 inhibited the phosphorylation of PI3K and AKT. In addition, rescue experiments showed that low expression of LEMD1 reversed the inhibitory effect of miR-3064-5p interference on the PI3K/AKT signaling pathway.
Nevertheless, this study has several limitations. The absence of animal studies has resulted in insufficient evidence to support the effect of LINC00958 on the growth of colorectal cancer cells in vivo. In addition, the correlation between LINC00958/miR-3064-5p/LEMD1, patient prognosis, and histology needs to be further investigated. We have only examined the effects of LINC00958, miR-3064-5p, and LEMD1 on the activity, proliferation, migration, invasion, and apoptosis of HCT116 and HCT8 cells based on the colorectal cancer cell level. However, the therapeutic effect of LINC00958/miR-3064-5p/LEMD1, such as exploring the correlation with prognosis, has not been studied at the clinical level, which will be explored in the future studies to provide an effective biomarker for the treatment of colorectal cancer.
Conclusion
Our study showed that LINC00958 and LEMD1 were upregulated and miR-3064-5p was downregulated in colorectal cancer. In summary, we uncovered for the first time that LINC00958 sponging miR-3064-5p promotes the viability, proliferation, migration, and invasion of colorectal cancer cells and inhibits apoptosis by elevating LEMD1 expression and activating the PI3K/AKT signaling pathway. This may provide effective biomarkers based on the LINC00958-miR-3064-5p-LEMD1 axis for the treatment of colorectal cancer.
Consent for publication
Consent for publication was obtained from the participants.
Ethics approval and informed consent
The present study was approved by the Ethics Committee of Tianyou Hospital Affiliated to Wuhan University of Science and Technology (Wuhan, China). The processing of clinical tissue samples is in strict compliance with the ethical standards of the Declaration of Helsinki. All patients signed written informed consent.
Supplemental Material
Download ()Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author ([email protected]) on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Zheng Y, Nie P, Xu S. Long noncoding RNA CASC21 exerts an oncogenic role in colorectal cancer through regulating miR-7-5p/YAP1 axis. Biomed Pharmacothe. 2020;121:109628.
- Meng S, Jian Z, Yan X, et al. LncRNA SNHG6 inhibits cell proliferation and metastasis by targeting ETS1 via the PI3K/AKT/mTOR pathway in colorectal cancer. Mol Med Rep. 2019;20(3):2541–2548.
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193.
- van der Werf A, Arthey K, Hiesmayr M, et al. The determinants of reduced dietary intake in hospitalised colorectal cancer patients. Support Care Cancer. 2018;26(6):2039–2047.
- Bao Y, Tang J, Qian Y, et al. Long noncoding RNA BFAL1 mediates enterotoxigenic bacteroides fragilis-related carcinogenesis in colorectal cancer via the RHEB/mTOR pathway. Cell Death Dis. 2019;10(9):675.
- Shi X, Zhang W, Nian X, et al. The previously uncharacterized lncRNA APP promotes prostate cancer progression by acting as a competing endogenous RNA. Int J Cancer. 2020;146(2):475–486.
- Chen G, Gu Y, Han P, et al. Long noncoding RNA SBF2-AS1 promotes colorectal cancer proliferation and invasion by inhibiting miR-619-5p activity and facilitating HDAC3 expression. J Cell Physiol. 2019;234(10):18688–18696.
- Liu T, Liu Y, Wei C, et al. LncRNA HULC promotes the progression of gastric cancer by regulating miR-9-5p/MYH9 axis. Biomed Pharmacothe. 2020;121:109607.
- Niu X, Yang B, Liu F, et al. LncRNA HOXA11-AS promotes OSCC progression by sponging miR-98-5p to upregulate YBX2 expression. Biomed Pharmacothe. 2020;121:109623.
- Cui Y, Xie M, Zhang Z. LINC00958 involves in bladder cancer through sponging miR-378a-3p to elevate IGF1R. Cancer Biother Radiopharm. 2020;35(10):776–788.
- Guo XB, Zhang XC, Chen P, et al. miR‑378a‑3p inhibits cellular proliferation and migration in glioblastoma multiforme by targeting tetraspanin 17. Oncol Rep. 2019;42(5):1957–1971.
- Luo Z, Han Z, Shou F, et al. LINC00958 accelerates cell proliferation and migration in non-small cell lung cancer through JNK/c-JUN signaling. Hum Gene Ther Methods. 2019;30(6):226–234.
- Sun Y, Liu Y, Cai Y, et al. Downregulation of LINC00958 inhibits proliferation, invasion and migration, and promotes apoptosis of colorectal cancer cells by targeting miR‑3619‑5p. Oncol Rep. 2020;44(4):1574–1582.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408.
- Sun C, Li M, Feng Y, et al. MDM2-P53 signaling pathway-mediated upregulation of CDC20 promotes progression of human diffuse large B-cell lymphoma. Onco Targets Ther. 2020;13:10475–10487.
- Mukai T, Tojo A, Nagamura-Inoue T. Umbilical cord-derived mesenchymal stromal cells contribute to neuroprotection in neonatal cortical neurons damaged by oxygen-glucose deprivation. Front Neurol. 2018;9:466.
- Lin H, Yang L, Tian F, et al. Up-regulated LncRNA-ATB regulates the growth and metastasis of cholangiocarcinoma via miR-200c signals. Onco Targets Ther. 2019;12:7561–7571.
- Zhao Z, Wang L, Song W, et al. Reduced miR-29a-3p expression is linked to the cell proliferation and cell migration in gastric cancer. World J Surg Oncol. 2015;13:101.
- Kong Y, Geng C, Dong Q. LncRNA PAPAS may promote triple-negative breast cancer by downregulating miR-34a. J Int Med Res. 2019;47(8):3709–3718.
- Liu Y, Li M, Yu H, et al. lncRNA CYTOR promotes tamoxifen resistance in breast cancer cells via sponging miR‑125a‑5p. Int J Mol Med. 2020;45(2):497–509.
- Zhao CC, Jiao Y, Zhang YY, et al. Lnc SMAD5-AS1 as ceRNA inhibit proliferation of diffuse large B cell lymphoma via Wnt/β-catenin pathway by sponging miR-135b-5p to elevate expression of APC. Cell Death Dis. 2019;10(4):252.
- Awan HM, Shah A, Rashid F, et al. Comparing two approaches of miR-34a target identification, biotinylated-miRNA pulldown vs miRNA overexpression. RNA Biol. 2018;15(1):55–61.
- Wu W, Guo L, Liang Z, et al. Lnc-SNHG16/miR-128 axis modulates malignant phenotype through WNT/β-catenin pathway in cervical cancer cells. J Cancer. 2020;11(8):2201–2212.
- Wang L, Zhong Y, Yang B, et al. LINC00958 facilitates cervical cancer cell proliferation and metastasis by sponging miR-625-5p to upregulate LRRC8E expression. J Cell Biochem. 2020;121(3):2500–2509.
- Chen F, Liu M, Yu Y, et al. LINC00958 regulated miR-627-5p/YBX2 axis to facilitate cell proliferation and migration in oral squamous cell carcinoma. Cancer Biol Ther. 2019;20(9):1270–1280.
- Chen S, Chen JZ, Zhang JQ, et al. Silencing of long noncoding RNA LINC00958 prevents tumor initiation of pancreatic cancer by acting as a sponge of microRNA-330-5p to down-regulate PAX8. Cancer Lett. 2019;446:49–61.
- Wang S, Ping M, Song B, et al. Exosomal CircPRRX1 enhances doxorubicin resistance in gastric cancer by regulating MiR-3064-5p/PTPN14 signaling. Yonsei Med J. 2020;61(9):750–761.
- Yan J, Jia Y, Chen H, et al. Long non-coding RNA PXN-AS1 suppresses pancreatic cancer progression by acting as a competing endogenous RNA of miR-3064 to upregulate PIP4K2B expression. J Exp Clin Cancer Res. 2019;38(1):390.
- Bai L, Wang H, Wang AH, et al. MicroRNA-532 and microRNA-3064 inhibit cell proliferation and invasion by acting as direct regulators of human telomerase reverse transcriptase in ovarian cancer. PloS One. 2017;12(3):e0173912.
- Sasahira T, Kurihara M, Nakashima C, et al. LEM domain containing 1 promotes oral squamous cell carcinoma invasion and endothelial transmigration. Br J Cancer. 2016;115(1):52–58.
- Sasahira T, Kurihara-Shimomura M, Nishiguchi Y, et al. Sushi repeat containing protein X-linked 2 is a downstream signal of LEM domain containing 1 and acts as a tumor-promoting factor in oral squamous cell carcinoma. Int J Mol Sci. 2020;21:10.
- Li Q, Ge Y, Chen X, et al. LEM domain containing 1 promotes proliferation via activating the PI3K/Akt signaling pathway in gastric cancer. J Cell Biochem. 2019;120(9):15190–15201.
- Martinez-Romero J, Bueno-Fortes S, Martín-Merino M, et al. Survival marker genes of colorectal cancer derived from consistent transcriptomic profiling. BMC Genomics. 2018;19(Suppl 8):857.
- Takeda R, Hirohashi Y, Shen M, et al. Identification and functional analysis of variants of a cancer/testis antigen LEMD1 in colorectal cancer stem-like cells. Biochem Biophys Res Commun. 2017;485(3):651–657.
- Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27(41):5497–5510.
- Czech MP. PIP2 and PIP3: complex roles at the cell surface. Cell. 2000;100(6):603–606.
- Lien EC, Dibble CC, Toker A. PI3K signaling in cancer: beyond AKT. Curr Opin Cell Biol. 2017;45:62–71.
- Sun B, Hu N, Cong D, et al. MicroRNA-25-3p promotes cisplatin resistance in non-small-cell lung carcinoma (NSCLC) through adjusting PTEN/PI3K/AKT route. Bioengineered. 2021;12(1):3219–3228.
- Lian G, Chen S, Ouyang M, et al. Colon cancer cell secretes EGF to promote M2 polarization of TAM through EGFR/PI3K/AKT/mTOR pathway. Technol Cancer Res Treat. 2019;18:1533033819849068.