ABSTRACT
β-galactosidase catalyzes lactose hydrolysis and transfers reactions to produce prebiotics such as galacto-oligosaccharides (GOS) with potential applications in the food industry and pharmaceuticals. However, there is still a need for improved transgalactosylation activity of β-galactosidases and reaction conditions of GOS production in order to maximize GOS output and reduce production costs. In this study, a β-galactosidase gene, galA, from Bacillus circulans was expressed in Pichia pastoris, which not only hydrolyzed lactose but also had strong transgalactosylation activity to produce GOS. Response surface methodology was adopted to investigate the effects of temperature, enzyme concentration, pH, initial lactose concentration, and reaction time on the production of GOS and optimize the reaction conditions for GOS. The optimal pH for the enzyme was 6.0 and remained stable under neutral and basic conditions. Meanwhile, GALA showed most activity at 50°C and retained considerable activity at a lower temperature 30–40°C, indicating this enzyme could work under mild conditions. The enzyme concentration and temperature were found to be the critical parameters affecting the transgalactosylation activity. Response surface methodology showed that the optimal enzyme concentration, initial lactose concentration, temperature, pH, and reaction time were 3.03 U/mL, 500 g/L, 30°C, 5.08, and 4 h, respectively. Under such conditions, the maximum yield of GOS was 252.8 g/L, accounting for approximately 50.56% of the total sugar. This yield can be considered relatively high compared to those obtained from other sources of β-galactosidases, implying a great potential for GALA in the industrial production and application of GOS.
1. Introduction
β-galactosidase (EC 3.2.1.23), also known as lactase, catalyzes two different types of reactions, namely hydrolysis and transgalactosylation [Citation1]. It hydrolyzes lactose into glucose and galactose, which has been broadly exploited in the food industries, with alleviating lactose maldigestion and enhancing sweetness, flavor, and solubility of lactose [Citation2]. On the other hand, transgalactosylation reaction is commonly applied to produce lactose-based prebiotics such as galacto-oligosaccharides (GOS) which have beneficial effects on human health [Citation3]. GOS is a low-caloric oligosaccharide composed of 2 ~ 10 galactose units with one terminal glucose residue. When using lactose as the substrate, β-galactosidase cuts the β-1,4 glycosidic bond and connects the free galactosyl group with other glycosidic receptors by glycosidic bond such as β-1,3, β-1,4, or β-1,6 [Citation4], and ultimately results in the formation of GOS. Although GOS cannot be absorbed by the body, they can modulate colonic flora by stimulating beneficial bacteria such as Bifidobacterium and Lactobacillus, and inhibiting less desirable bacteria, providing favorable benefits on immunological stimulation, mineral absorption, and the prevention of colon cancer [Citation5,Citation6]. Therefore, GOS has bright prospects in the field of functional foods and pharmaceuticals.
There are various GOS production methods, including extraction from natural raw materials, chemical synthesis, acid hydrolysis, direct fermentation synthesis, and enzymatic synthesis [Citation1,Citation50]. As an environment-friendly biocatalyst, β-galactosidase has been widely studied for its recognized advantages. The ratio between lactose hydrolysis and GOS synthesis crucially depends on the concentration of lactose, temperature of the reactor, and intrinsic properties of enzymes De [Citation7, Citation8]. Therefore, it is of importance to optimize the conditions for GOS synthesis and to discover β-galactosidases with high transgalactosylation capacity.
β-galactosidases are widely distributed in microorganisms, plants, and animal tissues [Citation9]. Among them, β-galactosidase from microbial sources has the greatest potential for industrial applications mainly due to their easy handling, greater catalytic activity, and high production yield [Citation10]. And so far, most β-galactosidases of commercial interest have been isolated from Kluyveromyces lactis, Kluyveromyces fragilis, Candida kefyr, Thermotoga maritima, Sulfolobus solfataricus, Aspergillus niger, Aspergillus oryzae, and Bacillus circulans [Citation11,Citation12]. In particular, the Bacillus circulans β-galactosidase synthesizes GOS with a major presence of β (1→4) bonds [Citation13,Citation14] and gives rise to a notable GOS yield (approximately 49% w/v, starting with 400 g/L lactose) [Citation15,Citation16].
Many studies have been conducted in pursuit of a higher production of GOS. When the amino acids of the thermostable recombinant β-galactosidase from Halothermothrix orenii were altered from Phe417 to Tyr417 and from Tyr296 to Phe296, the GOS yield from lactose increased by approximately 46% and 33.6%, respectively, where the reactions were performed at 70°C with an initial lactose concentration of 300 g/L in sodium phosphate buffer (pH 6.0) [Citation17,Citation51] performed multiple sequence alignment to identify the potential mutation sites associated with increased GOS formation. They found that Phe426, Phe401, and Phe441 were conserved in β-glycosidase of Pyrococcus furiosus, Thermus thermophilus, and Sulfolobus solfataricus, respectively, and therefore mutant F441Y was constructed to investigate GOS production. Under the optimal conditions, yields of GOS for wild-type enzyme and mutant F441Y could reach 50.9% and 61.7%, respectively. Additionally, β-galactosidase was immobilized on chitosan-coated magnetic nanoparticles to produce GOS from lactulose. The stability of β-galactosidase was significantly improved, and the maximum yield of GOS was 17% at the initial lactulose concentration of 2.34 M for 36 h of reaction [Citation18]. Nonetheless, it is still imperative to find new β-galactosidases with higher transgalactosylation activity and to optimize reaction conditions of GOS production in order to maximize GOS output and reduce production costs.
We hypothesized that β-galactosidase expressed in P. pastoris would have higher transgalactosylation activity and GOS yields. Therefore, the aim of this study was to characterize the β-galactosidase from B. circulans which featured quite high transgalactosylation properties and to optimize the main reaction parameters (pH, temperature, time, initial lactose concentration, and the amount of β-galactosidase) of transgalactosylation activity to produce more GOS.
2. Materials and methods
2.1. Strains, plasmids, and medium
Escherichia coli (E. coli) Trans10 (TransGen, Beijing, China) and P. pastoris GS115 (Invitrogen, Carlsbad, CA, USA) were used as the gene cloning and expression hosts, respectively. The plasmid pUC57-galAm harboring the gene of β-galactosidase from B. circulans and His-tag was optimized and synthesized by the GenScript Corporation (Nanjing, China). The GenBank accession number of the β-galactosidase gene is MN443117. The P. pastoris-E. coli shuttle expression vector pPICZαA (Invitrogen, Carlsbad, CA, USA) was used for gene cloning and expression in those two hosts. All medium, including Luria-Bertani (LB) medium, yeast peptone dextrose (YPD) medium, buffered glycerol-complex (BMGY) medium, buffered methanol-complex (BMMY) medium, and minimal methanol (MM) medium, were prepared according to the instructions in the Pichia expression kit (Invitrogen, Carlsbad, CA, USA).
2.2 Chemicals
5-Bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal), trichloroacetic acid (TCA), D-β-galactose, D-glucose, O-nitrophenyl-β-D-galactopyranoside (oNPG), Zeocin, and bovine serum albumin (BSA) were purchased from Sigma Aldrich (St. Louis, USA). Amino acid-free yeast nitrogen source (YNB) was derived from Difco (Detroit, MI). Enzymes and protein ladders were purchased from Thermo Scientific (Rockford, IL, USA).
2.3 Construction of recombinant plasmid pPICZαA-galAm
The strains with plasmids pUC57-galAm and pPICZαA were cultivated at 37°C overnight in LB medium containing 50 mg/L ampicillin and 25 mg/L zeocin, respectively. The plasmids were extracted using the DNA Extraction Kit (TIANGEN Cor., Beijing, China) according to the manufacturer’s guide and then treated with endonucleases FastDigest EcoRI and NotI at 37°C for 3 h. The digested products were purified using the DNA Gel Extraction Kit (TIANGEN Cor., Beijing, China). The resultant two fragments were ligated in the presence of T4 DNA ligase at 25°C for 1 h, and the recombinant plasmids were transformed into E. coli Trans10 for gene cloning. The strain with expression plasmid pPICZαA-galAm was cultivated at 37°C and the plasmid was extracted. Finally, the plasmids were linearized with SacI and transformed into P. pastoris GS115 competent cells by electroporation.
2.4 Positive transformant screening
Transformants were cultivated on YPD plates containing 300 mg/L zeocin at 28°C for 48 h, and then individual positive transformants were placed in 48-well microtiter plates containing 500 µL of BMGY medium, and then grown at 28°C for 48 h at 200 rpm in a shaker incubator. BMGY medium was discarded by centrifugation and the transformants were further cultivated in BMMY at 28°C for 72 h where 1% methanol was added every 24 h. The enzyme solutions were harvested by centrifugation, and the enzyme activity was measured. A transformant with the highest enzyme activity was finally selected for the target strain.
2.5 Protein expression, purification, and analysis
The target strain was cultured in 2 ml of YPD containing 300 mg/L of zeocin for 48 h and then inoculated and induced in 50 ml of BMGY at 28°C for 48 h in shaking flasks. The cells were harvested by centrifugation (5000 rpm for 5 min) and then grown in 25 ml of BMMY with methanol induction (1.0% v/v) at 28°C for 72 h. The supernatant was collected by centrifugation (5000 rpm for 5 min) and concentrated using a 10 kDa molecular weight cutoff membrane, and dialyzed in 50 mL of buffer A (20 mM phosphate–citrate buffer, pH 6.0). The retentate was applied on a Histrap HP column (1 mL) that was pre-equilibrated with buffer on an automated fast protein liquid chromatography (FPLC) system (Äkta Purifier, GE Healthcare, USA). The recombinant β-galactosidase was eluted using a linear gradient of imidazole from 0 to 0.5 M. The fractions showing β-galactosidase were further purified via molecular sieve chromatography (GE Healthcare) with buffer A as the mobile phase. Protein concentration was measured with the BCA Protein Assay Kit (CWBIO, Beijing, China). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Native-PAGE analysis were performed using a 12% running gel. Protein bands were stained using either Coomassie Blue or X-gal as a substrate for active staining. In order to obtain more protein, the high-cell-density fermentation was performed according to the Pichia fermentation guidelines (Invitrogen, Carlsbad, CA, USA) in a 2 L bioreactor, followed by protein purification using the same method as described above.
2.6 Enzyme activity assay
β-galactosidase activity was determined using the oNPG method [Citation19]. The reaction was initiated by adding 200 μL of dissolved enzyme solution to 800 μL of 0.25% oNPG in buffer A, which was incubated at 45°C for 15 min. The reaction was stopped by the incorporation of 1 mL of 10% TCA. Then, 2 mL of 10% Na2CO3 was added to stabilize the color of the reaction. The released o-nitrophenol was determined by measuring the absorbance at 420 nm using a Benchmark Plus Microplate Spectrophotometer (Bio-Rad, California, USA). One unit (U) of enzyme was defined as the amount of the enzyme that liberates 1 μmol of o-nitrophenol from oNPG per minute under the assay conditions.
2.7 Effects of temperature and pH on enzyme activity
The optimum temperature of the β-galactosidase was determined at temperatures ranging from 20°C to 65°C for 0.25% oNPG in buffer A. The enzyme activity at optimum temperature was defined as 100%. For thermal stability measurements, each enzyme dissolved in buffer A at a final concentration of 0.3 mg/mL was pre-incubated for 1 h at temperatures ranging from 20°C to 65°C, and the remaining activity was assayed using oNPG as a substrate, as described above. The ideal pH for the β-galactosidase was measured using dissolved oNPG as substrates in 50 mM phosphate–citrate buffer at pH values ranging from 4.5 to 9.5 at 45°C. For pH stability measurements, the enzyme was incubated at 30°C in 50 mM phosphate–citrate buffer at pH 4.5–9.5 for 120 min in the absence of the substrate at the final concentration of 0.3 mg/mL. The remaining activity was then assayed using dissolved oNPG in buffer A as a substrate by the method described above. The enzyme activity at optimum pH was defined as 100%.
2.8 Transgalactosylation reaction analysis
Transgalactosylation efficiency of the β-galactosidase was analyzed by high performance liquid chromatography (HPLC) [Citation20,Citation21]. Briefly, all reactions were performed at 30–50°C in conical flasks on an orbital shaker set at 200 rpm. The reaction mixture was composed of 440 μL of 200–500 g/L lactose in 0.1 M phosphate–citrate buffer (pH 5–7) and 60 μL of 3–12 U/mL enzyme (measured in the presence of oNPG). Samples were taken at different times and then heated at 100°C for 10 min to stop the reaction by the inactivation of the enzyme. In order to collect the supernatant from the reaction mixture, samples were centrifuged at 10,000 rpm for 10 min and filtered through 0.2 μm syringe filters. Seven hundred μL of the reaction mixture diluted with deionized water was analyzed by HPLC. Control samples without enzymes were prepared simultaneously by exposure to the same temperature treatment; no galacto-oligosaccharide reaction products were detected in these control samples. All experiments were carried out in duplicates and the average concentration values for the products obtained are presented in the figures; all standard deviations were less than 5%. The amounts of extra lactose and reaction products galactose and glucose were determined by HPLC with a 6.5 × 300 mm Sugar-Pak I column (Waters) and 50 mg/ml CaNa2-EDTA buffer as the mobile phase (Choi, et al. 2020). The concentration of GOS was calculated from the concentrations of lactose, galactose, and glucose using the equation:
GOS = Ci-Cf-galactose-glucose
The lactose conversion and GOS yield were calculated using the following equations.
Lactose conversion = (Ci-Cf)/Ci×100
GOS yield = Cp/Ci×100
where Ci and Cf are the initial and final concentrations of lactose, respectively, and Cp is the concentration of GOS [Citation1].
In addition, thin-layer chromatography (TLC) was used to determine the composition of GOS mixture, following previously described methods [Citation22,Citation23]. The analysis of GOS and other products was accomplished on a precoated 10 × 10 cm high-performance lamina chromatography (HPTLC) silica gel 60 plate (Merck KGaA, Germany). 5 µL of 5% lactose (m/v), 5% galactose (m/v), 5% glucose (m/v), 20% GOS sample, and 20% commercial GOS product (m/v) were pipetted on the plate. The linear ascending development of the plate was accomplished in a pre-saturated glass chamber (21× 9× 21 cm) with solvent consisting of n-butyl alcohol:isopropanol: water (3:12:4, v/v/v) for 1 h at room temperature. The plate was air-dried at room temperature for 10 min. The spots were visualized by fully submerging the plate into sulfuric acid ethanol (30%, v/v) for 10 s. The plate was dried in an oven set at 100°C for 10 min.
2.9 Optimization of the synthesis of high GOS
Rational optimization of the synthesis of GOS catalyzed by the β-galactosidase was performed by response surface methodology (RSM) [Citation24,Citation25,Citation26,Citation52]. Firstly, a factorial experimental design was carried out to assess the simultaneous and combined effect of the main reaction parameters affecting GOS production using Design Expert version 8 software (Stat-Ease Inc., Minneapolis, MN, USA) [Citation27]. Such parameters include temperature, enzyme concentration, pH, initial lactose concentration and reaction time [Citation28]. A 53 factorial experimental design (five factors at three levels) was carried out using a Plackett–Burman design (PBD), to determine the significant influencing factor and the optimum reaction conditions for GOS production. The central point was repeated three times to determine the variability of the results and to assess the experimental error. The selected response for the analysis was GOS yield. The selection of the levels for each factor was based on either experimental results or previously reported data.
3. Result and discussion
The aim of this study was to find higher transgalactosylation activity β-galactosidases and increase GOS yields by optimizing reaction conditions. The β-galactosidase GALA from B. circulans was expressed in P. pastoris and purified for further characterization. The optimal pH and temperature of GALA were examined based on oNPG converting activity. Moreover, the response surface methodology was used to optimize the main parameters including pH, temperature, time, enzyme concentration, and initial lactose concentration of transgalactosylation reaction to produce more GOS.
3.1 Protein expression and purification of GALA
Ten transformants were chosen to be induced with 1% methanol and the supernatants were used in the measurement of enzyme activity. Clone #2 demonstrated the highest activity and was chosen for further analyses (Figure S1).
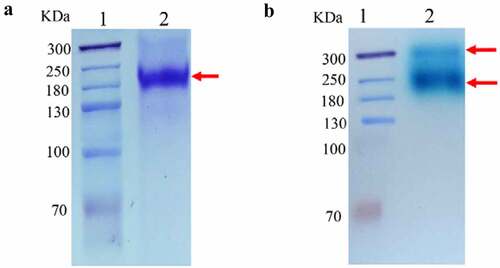
The target strain Clone #2 was cultured and induced in shaking flasks, and the hydrolysis activity of the supernatant measured with oNPG was 0.5 U/mL, which was increased to 1.4 U/mL following the high-cell-density fermentation. The obtained GALA protein was predicted to encode 1703 residues with a molecular weight of 190 kDa (analyzed by Vector NTI 11.5.1). As shown in , SDS-PAGE analysis of the purified GALA showed that the band size was consistent with the theoretical molecular weight of 190 kDa. Active staining on Native-PAGE with X-gal yielded two bands showed β-galactosidase activity (), indicating that glycosylation occurred during post-translational modification in P. pastoris.
Figure 1. Protein analysis of GalA: (a) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis(SDS-PAGE) of purified β-galactosidase. Lane 1, molecular weight marker; lane 2, purified enzyme sample treated in SDS buffer at 100°C for 10 min, with Coomassie brilliant blue R250 staining. (b) Native polyacrylamide gel electrophoresis(Native-PAGE) of the culture supernatant from Pichia pastoris. Lane 1, molecular weight marker; lane 2, crude enzyme sample, with X-gal staining
P. pastoris, an established protein expression host, is mainly applied for the production of biopharmaceuticals and industrial enzymes. This methylotrophic yeast is a distinguished production system for its growth to very high cell densities, the available strong and tightly regulated promoters, and the options to produce gram amounts of recombinant protein per liter of culture both intracellularly and in secretory fashion [Citation29]. In this study, a β-glucosidase from B. circulans was expressed in P. pastoris. However, the expression level of the enzyme was still not ideal, and strategies are needed to further improve the expression. In addition to the yeast expression system, the recombinant β-galactosidase gene has been successfully expressed in Escherichia coli, Lactobacillus plantarum, Lactococcus lactis, and Saccharomyces cerevisiae [Citation23]. However, the recombinant expression of β-galactosidases in E. coli, Lactobacillus, and Lactococcus are usually cytoplasmic, making the purification tedious and costly [Citation12]. Besides heterogeneous expression, many of β-galactosidases were produced by native fermentation such as Alicyclobacillus vulcanalis [Citation30], Kluyveromyces lactis De [Citation31] and Aspergillus terreus [Citation2]. However, some typical problems exist during their production, e.g., the fermentation may contain other endogenous proteins leading to complex downstream processing and high economic costs.
3.2 Effect of temperature and pH on enzymatic hydrolysis activity and stability
Results showed that the optimal temperature of the recombinant β-galactosidase was 50°C (). However, the enzyme still retained considerable activity at lower temperatures 20–30°C, suggesting that the enzyme could work under milder conditions, which can reduce energy consumption and environmental pollution. shows that the recombinant β-galactosidase was quite thermally stable. The residual hydrolysis activity was more than 80% at 20°C–40°C, and about 50% after incubation at 55°C for 60 min.
Figure 2. The effect of temperature and pH on purified β-galactosidase. (a) The optimal temperature for the purified β-galactosidase. The enzyme activity assay was carried out at different temperatures ranging from 20 to 65°C in 5-unit increments. (b) The heat tolerance of the purified β-galactosidase. The enzyme activity assay was carried out at temperatures ranging from 20 to 60°C. (c) The optimal pH for the purified β-galactosidase. (d) The pH tolerance of the purified β-galactosidase. The enzyme activity assay was carried out in buffers with different pH values ranging from 4.5 to 9.5 in 0.5-unit increments. The experiments were carried out three times using ONPG as the substrate, and the data are presented as Mean ± SD
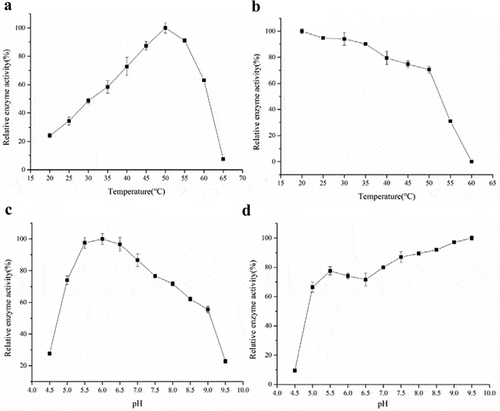
As shown in , the recombinant enzyme demonstrated significant oNPG hydrolysis in the pH range of 5.5–6.5 (optimal at 6.0), but the enzymatic activity decreased dramatically at pH less than 5.0 and higher than 9.0, which aligns well with the natural pH of milk. The recombinant enzyme worked predominantly in basic conditions, as reflected by the results that the residual hydrolysis activity was found to be more than 80% in alkaline region (pH 8.0–9.5) (). These results were similar to those reported in the literature for other β-galactosidases, in response to temperature and pH (). For example, the recombinant β-galactosidase from the Antarctic bacterium Alteromonas sp. ANT48 cloned and expressed in E. coli showed most activity at 50°C and retained more than 80% of its initial activity below 40°C [Citation23Citation24–26]. A cold active β-galactosidase from Planococcus sp-L4 was optimized for expression in P. pastoris [Citation9]. The recombinant enzyme displayed significant activity at pH 6.5 but the activity decreased dramatically at pH below 5.5 and above 8. Moreover, the β-galactosidase from B. subtilis was expressed in E. coli, and displayed an optimum activity at pH 6.5 and 40°C [Citation32,Citation33]. The optimum temperature was 10°C lower than in the present study, which may be caused by the lack of post-translational modifications in the E. coli expression system.
Table 1. Literature review on GOS production from lactose by microbial β-galactosidases
3.3 Enzymatic synthesis of galacto-oligosaccharides
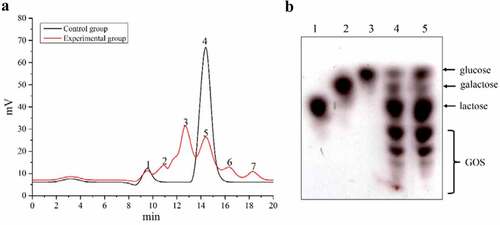
The reaction mixture was analyzed using HPLC and TLC to detect the presence of different sugars. As shown in , the formation of GOS could be significantly seen under standard conditions. During hydrolysis, aside from lactose (peaks 4 and 5), galactose (peak 7), glucose (peak 6), and GOS (peaks 2 and 3) were also formed as a result of transgalactosylation catalyzed by the enzyme, compared with control group without the enzyme, which was consistent with TLC result. In addition, the relative proportions of all kinds of oligosaccharides in the GOS generated in this study were close to commercial GOS products (), which further demonstrated that GALA is a potential candidate for catalyzing the production of GOS. The results in clearly indicate that the GOS produced by β-galactosidase was a mixture of transgalactosylated oligosaccharides, unreacted lactose, glucose, and galactose. Extensive research has been carried out on the components of GOS products despite inconsistent results. Simultaneous synthesis and purification of GOS from lactose was conducted using a combi-catalyst, which was composed by β-galactosidase and S. cerevisiae cell aggregates to improve the purity of GOS [Citation13] [Citation34], showed that GOS mixture contained distinct oligosaccharides varying in glycosidic linkages with a polymerization degree of 2–8 [Citation34]. The glycosidic bond structures of GOS synthesized by β-galactosidase from L. plantarum were β-D-Galp-(1→6)-D-Lac, β-D-Galp-(1→3)-D-Lac, and β-D-Galp-(1→6)-DGlc [Citation35]. The majority of the produced GOS by a novel thermophile β-galactosidase was trisaccharides, while low concentrations of disaccharides and tetrasaccharides were also detected [Citation36]. Taken together with these studies, more work is still needed to identify and purify GOS in the future.
Figure 3. Analysis of GOS production achieved by enzymatic transgalactosylation activity of the β-galactosidase, using lactose as the substrate. (a) The presence of the different sugars in the reaction mixture was analyzed by HPLC: (1) solvent; (2, 3) GOS product; (4, 5) lactose; (6) glucose; (7) galactose. (b) High performance thin layer chromatogram of 20% galacto-oligosaccharides sample on precoated silica gel. 5 µL of each sample was spotted on the plate using micropipettes: (1) 5% lactose; (2) 5% galactose; (3) 5% glucose; (4) GOS product obtained in this study, (5) commercial GOS product
3.4 Optimization of the synthesis of GOS by response surface methodology
Five parameters were chosen to optimize the synthesis of GOS using a response surface methodology designed by Design Expert software (version 8). The three selected levels for each factor in the design were as follows: pH 5, pH 6, and pH 7, 300, 400, and 500 g/L for initial lactose concentration, 4, 10, and 20 h for reaction time, 30, 40, and 50°C for temperature, and 3, 6, and 12 U/mL for enzyme concentration. The rationale for such selection includes the optimal temperature and pH of enzymatic hydrolysis activity, from the above results, was 50°C and pH 6, respectively; the initial lactose concentration is limited by the maximum lactose solubility in water and high lactose concentrations are often accompanied by high GOS productivity [Citation34,Citation37]; 5 U/mL was considered as the optimum enzyme concentration to provide maximum GOS yields; the previous study showed that GOS reached maximum after 6 h of reaction time [Citation38]. Results on the effect of these five factors are shown in . Moreover, two linear models were postulated to fit the experimental results and two equations for the response model were obtained as illustrated below.
Table 2. Experimental results for the production of GOS, GOS yield, and lactose conversion by the β-galactosidase
Y1=36.14–4.18 × A-1.37 × B-4.06 × C + 2.03 × D-2.91 × E
Y2 = 63.86 + 4.18 × A + 4.06 × B-0.41 × C − 2.03 × D + 2.91 × E
where Y1 was the response of GOS yield and Y2 was the response of lactose conversion, A, B, C, D, and E represented enzyme concentration, pH, temperature, initial lactose concentration, and reaction time, respectively. As shown in and , the experimental results are well in agreement with their respective predicted values obtained using the mathematical models. The correlation coefficients for the two equations were 0.93 and 0.95, respectively, and the model was statistically significant (p = 0.0303), indicating that the vast majority of the variables could be explained by this model.
Figure 4. Effect of temperature and enzyme concentration on GOS synthesis: (a) Correlation between model-predicted values and experimental values of YGOS (%). (b)Three-dimensional surface plots for YGOS (%) in response to temperature and enzyme concentration. (c) Correlation between model-predicted values and experimental values of CGOS(%). (B)Three-dimensional surface plots for CGOS(%) in response to temperature and enzyme concentration
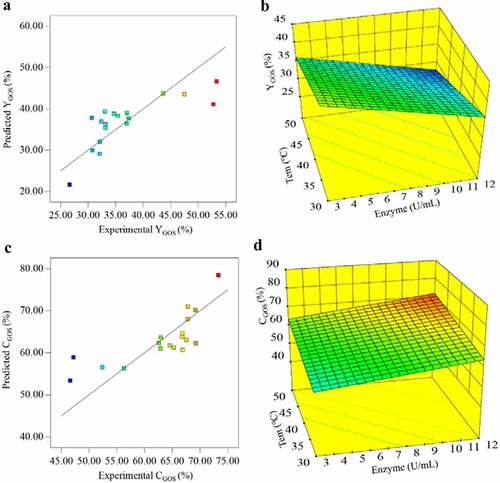
Among the parameters analyzed, the response surface was significantly impacted by enzyme concentration (p = 0.0234) and temperature (p = 0.0290). Some studies have shown that the enzyme concentration had an important influence on the transgalactosylation reaction [Citation37, Citation39]. The effect of temperature and enzyme concentration on the GOS synthesis can be better understood by observing the three-dimensional surface plots shown in .
By limiting these five factors (temperature, enzyme concentration, pH, initial lactose concentration, and reaction time), the maximum GOS yield was predicted by the Design Expert software (Table S1 and Table S2). As shown in Table S2, the number 1 represented the maximum production of GOS was 252.8 g/L (50.56% of the total sugar) at 30°C, pH 5.08, 500 g/L of initial lactose concentration, 3.03 U/mL of enzyme concentration, and 4 h reaction time. This yield can be considered relatively high compared to the reported yields obtained with other β-galactosidases without protein engineering as shown in .
In particular, the GOS yield obtained in this study was higher than that of Palai et al. (39% of the total sugar) who also used purified β-galactosidases derived from B. circulans [Citation40]. Although the GOS yield obtained in this work was lower than that of Hung et al. (63% of the total sugar) in which recombinant β-galactosidases were derived from Bifidobacterium infantis, the concentration of GOS in our work was up to 252.8 g/L, which was 33% higher than their results (190 g/L) [Citation41]. In addition, the reaction temperature was 30°C lower than the temperature used in their work, leading to more energy saving. In recent years, certain strategies have been used to improve the GOS yield, [Citation42] predicted the key residues to transglycoside interactions of β-galactosidase from Aspergillus oryzae; then, a double mutant N140C/W806F was constructed and the resultant GOS yield was 59.8%, which represented substantial improvements over that of the wild type (35.7%) [Citation42]. In another study, an intelligent double-hydrophobic amino acid scanning strategy was employed to predict key residues forming the glycan-binding site (−1 subsite) of β-galactosidase; two mutants C510V and H512I significantly improved GOS synthesis efficiency, with 59.1% for C510V and 51.5% for H512I under optimal conditions [Citation43].
4. Conclusions
In this study, the characteristics of a novel β-galactosidase GALA from B. circulans were examined and demonstrated that the enzyme could effectively hydrolyze β-glucosides with higher transgalactosylation capacity at a lower reaction temperature and higher GOS yield than some previous studies. The optimal pH and temperature for the enzyme was 6.0°C and 50°C, which allows this enzyme to hydrolyze lactose in milk products, thereby overcoming the complications of lactase deficiency. Given the fact that the synthesis of GOS is affected by various parameters including temperature, pH, enzyme concentration, reaction time and lactose concentration, response surface methodology was employed to simultaneously evaluate the influence of these parameters and optimize the conditions for the maximum yield of GOS. The yield of GOS was approximately 50.56% of the total sugar at 30°C, pH 5.08, 3.03 U/mL of enzyme concentration, 4 h reaction time, and 500 g/L of lactose concentration, indicating potential applications of this novel enzyme in the food industry. Nonetheless, the expression level of the enzyme in this work is still not ideal, and more work is warranted to improve its expression or obtain higher transgalactosylation activity through protein engineering.
Supplemental Material
Download ()Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant number 31972601) and The Agricultural Science and Technology Innovation Program.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Cardoso BB, Silvério SC, Abrunhosa L, et al. β-galactosidase from Aspergillus lacticoffeatus: a promising biocatalyst for the synthesis of novel prebiotics. Int J Food Microbiol. 2017;257:67–74.
- Vidya B, Palaniswamy M, Angayarkanni J, et al. Purification and characterization of β-galactosidase from newly isolated Aspergillus terreus (KUBCF1306) and evaluating its efficacy on breast cancer cell line (MCF-7). Bioorg Chem. 2019;94:103442.
- Silvério SC, Macedo EA, Teixeira JA, et al. Perspectives on the biotechnological production and potential applications of lactosucrose: a review. J Funct Foods. 2015;19:74–90.
- Warmerdam A, Zisopoulos FK, Boom RM, et al. Kinetic characterization of galacto-oligosaccharide (GOS) synthesis by three commercially important β-galactosidases. Biotechnol Prog. 2014;30(1):38–47.
- Geiger B, Nguyen HM, Wenig S, et al. From by-product to valuable components: efficient enzymatic conversion of lactose in whey using β-galactosidase from streptococcus thermophilus. Biochem Eng J. 2016;116:45–53.
- González-Delgado I, López-Muñoz M-J, Morales G, et al. Optimisation of the synthesis of high galacto-oligosaccharides (GOS) from lactose with β-galactosidase from Kluyveromyces lactis. Int Dairy J. 2016;61:211–219.
- Andrade B, Timmers L, Renard G, et al. Microbial β-Galactosidases of industrial importance: computational studies on the effects of point mutations on the lactose hydrolysis reaction. Biotechnol Prog. 2020;36(4):e2982.
- Rodriguez-Colinas B, Fernandez-Arrojo L, Santos-Moriano P, et al. Continuous packed bed reactor with immobilized β-galactosidase for production of galactooligosaccharides (GOS). Catalysts. 2016;6(12):12.
- Mahdian SM, Karimi E, Tanipour MH, et al. Expression of a functional cold active β-galactosidase from planococcus sp-L4 in Pichia pastoris. Protein Expr Purif. 2016;125:19–25.
- Vera C, Guerrero C, Aburto C, et al. Conventional and non-conventional applications of β-galactosidases. Biochim Biophys Acta Proteins Proteom. 2020;1868(1):140271.
- Martínez-Villaluenga C, Cardelle-Cobas A, Corzo N, et al. Optimization of conditions for galactooligosaccharide synthesis during lactose hydrolysis by β-galactosidase from kluyveromyces lactis (Lactozym 3000 L HP G). Food Chem. 2008;107(1):258–264.
- Movahedpour A, Ahmadi N, Ghalamfarsa F, et al. β-Galactosidase: from its source and applications to its recombinant form. Biotechnol Appl Biochem. 2021. DOI:10.1002/bab.2137
- Aburto C, Guerrero C, Vera C; Illanes. Improvement in the yield and selectivity of lactulose synthesis with Bacillus circulans β-galactosidase. LWT-Food Sci Technol. 2020;118:108746–110874.
- Rodriguez-Colinas B, Fernandez-Arrojo L, Abreu MD, et al., On the enzyme specificity for the synthesis of prebiotic galactooligosaccharides. Adv Enzym Biotechnol. Springer, New York.2013;pp:23-39.
- Beneová UR,Z, Těínsk M, Spiwok V, et al. Transglycosylation abilities of β-d-galactosidases from GH family 2. 3 Biotech. 2021;11(4):168.
- Rodriguez-Colinas B, Poveda A, Jimenez-Barbero J, et al. Galacto-oligosaccharide synthesis from lactose solution or skim milk using the β-galactosidase from Bacillus circulans. J Agric Food Chem. 2012;60(25):6391–6398.
- Hassan N, Geiger B, Gandini R, et al. Engineering a thermostable halothermothrix orenii β-glucosidase for improved galacto-oligosaccharide synthesis. Appl Microbiol Biotechnol. 2016;100(8):3533–3543.
- Nguyen VD, Styevko G, Madaras E, et al. Immobilization of β-galactosidase on chitosan-coated magnetic nanoparticles and its application for synthesis of lactulose-based galactooligosaccharides. Process Biochem. 2019;84:30–38.
- Nagy Z, Kiss T, Szentirmai A, et al. β-galactosidase of Penicillium chrysogenum: production, purification, and characterization of the enzyme. Protein Expr Purif. 2001;21(1):24–29.
- Schultz G, Alexander R, Lima FV, et al. Kinetic modeling of the enzymatic synthesis of galacto-oligosaccharides: describing galactobiose formation. Food Bioprod Process. 2021;127:1–13.
- Thuy DTB, Nguyen AT, Khoo KS, et al. Optimization of culture conditions for gamma-aminobutyric acid production by newly identified Pediococcus pentosaceus MN12 isolated from ‘mam nem’, a fermented fish sauce. Bioengineered. 2021;12(1):54–62.
- Li D, Li S, Wu Y, et al. Cloning and characterization of a new β-galactosidase from Alteromonas sp. QD01 and Its potential in synthesis of galacto-oligosaccharides. Mar Drugs. 2020;18(6):6.
- Li Z, Xing. A New β-Galactosidase from the Antarctic Bacterium Alteromonas sp. ANT48 and its potential in formation of prebiotic galacto-oligosaccharides. Mar Drugs. 2019;17(11):11.
- Barbosa MS, Freire C, Almeida LC, et al. Optimization of the enzymatic hydrolysis of Moringa oleifera Lam oil using molecular docking analysis for fatty acid specificity. Biotechnol Appl Biochem. 2019;66(5):823–832.
- Box GEP, Wilson KB. Introduction to box and Wilson on the experimental attainment of optimum conditions. J R Stat Soc B (Methodological). 1951;13(1):1–38.
- Marvdashti LM, Ayatollahi SA, Salehi B, et al. Optimization of edible Alyssum homolocarpum seed gum-chitosan coating formulation to improve the postharvest storage potential and quality of apricot (Prunus armeniaca L.). J Food Saf. 2020;40:4.
- Trang NTH, Tang DYY, Chew KW, et al. Discovery of α-glucosidase inhibitors from marine microorganisms: optimization of culture conditions and medium composition. Mol Biotechnol. 2021;6(11):1004-1015.
- Ober PB. Introduction to linear regression analysis. J Appl Stat. 2013;40(12):2775–2776.
- Raschmanová WA, Knejzlík Z, Melzoch K, et al. Engineering of the unfolded protein response pathway in Pichia pastoris: enhancing production of secreted recombinant proteinsy. Appl Microbiol Biotechnol. 2021;105(11):4397–4414.
- Murphy J, Ryan MP, Walsh G. Purification and characterization of a novel β-galactosidase from the thermoacidophile Alicyclobacillus vulcanalis. Appl Biochem Biotechnol. 2020;191(3):1190–1206.
- Freitas LCH, Albuquerque T, Rocha M, et al. Simultaneous hydrolysis of cheese whey and lactulose production catalyzed by β-galactosidase from Kluyveromyces lactis NRRL Y1564. Bioprocess Biosyst Eng. 2020;43(4):711–722.
- Carneiro L, Yu L, Paul D, et al. Characterization of a β-galactosidase from Bacillus subtilis with transgalactosylation activity. Int J Biol Macromol. 2018;120:279–287.
- Carla A, Cecilia G, Carlos, et al. Co-immobilized β-galactosidase and Saccharomyces cerevisiae cells for the simultaneous synthesis and purification of galacto-oligosaccharides. Enzyme Microb Technol. 2018;118:102–108.
- Lili L, Guo L, Wang K, et al. β-galactosidases: a great tool for synthesizing galactose-containing carbohydrates. Biotechnol Adv. 2020;39:107465.
- Zhang X, Yao C, Wang T, et al. Production of high-purity galacto-oligosaccharides (GOS) by Lactobacillus-derived β-galactosidase. European Food Res and Tech. 2021;247(6):1501–1510.
- Zerva A, Limn A Ios A, Kritikou AS, et al. A novel thermophile β-galactosidase from Thermothielavioides terrestris producing galactooligosaccharides from acid whey. N Biotechnol. 2021;63:45–53.
- González-Delgado I, López-Muñoz M-J, Morales G, et al. Covalent immobilization of Enterococcus faecalis Esawy dextransucrase and dextran synthesis. Int J Biol Macromol. 2016;82:905–912.
- Torres DPM, Gonçalves MDPF, Teixeira JA, et al. Galacto-oligosaccharides: production, properties, applications, and significance as prebiotics. Compr Rev Food Sci Food Saf. 2010;9(5):438–454.
- Liu Y, Chen Z, Jiang Z, et al. Biochemical characterization of a novel β-galactosidase from Paenibacillus barengoltzii suitable for lactose hydrolysis and galactooligosaccharides synthesis. Int J Biol Macromol. 2017;104:1055–1063.
- Palai T, Mitra S, Bhattacharya PK. Kinetics and design relation for enzymatic conversion of lactose into galacto-oligosaccharides using commercial grade β-galactosidase. J Biosci Bioeng. 2012;114(4):418–423.
- Hung MN, Lee B. Purification and characterization of a recombinant β-galactosidase with transgalactosylation activity from Bifidobacterium infantis HL96. Appl Microbiol Biotechnol. 2002;58(4):439–445.
- Gao X, Wu J, Wu D. Rational design of the β-galactosidase from Aspergillus oryzae to improve galactooligosaccharide production. Food Chem. 2019;286:362–367.
- Qin Z, Li S, Huang X, et al. Improving galactooligosaccharide synthesis efficiency of β-galactosidase Bgal1-3 by reshaping the active site with an intelligent hydrophobic amino acid scanning. J Agric Food Chem. 2019;67(40):11158–11166.
- Oh SY, Youn SY, Park MS, et al. Synthesis of β-galactooligosaccharide using Bifidobacterial β-galactosidase purified from recombinant Escherichia coli. J Microbiol Biotechnol. 2017;27(8):1392–1400.
- Iqbal S, Nguyen T-H, Nguyen HA, et al. Characterization of a heterodimeric GH2 β-galactosidase from Lactobacillus sakei Lb790 and formation of prebiotic galacto-oligosaccharides. J Agric Food Chem. 2011;59(8):3803–3811.
- Iqbal S, Nguyen T-H, Nguyen TT, et al. β-galactosidase from Lactobacillus plantarum WCFS1: biochemical characterization and formation of prebiotic galacto-oligosaccharides. Carbohydr Res. 2010;345(10):1408–1416.
- Nguyen -T-T, Nguyen HA, Arreola SL, et al. Homodimeric β-Galactosidase from Lactobacillus delbrueckii subsp. bulgaricus DSM 20081: expression in Lactobacillus plantarum and Biochemical Characterization. J Agric Food Chem. 2012;60(7):1713–1721.
- Maischberger T, Leitner E, Nitisinprasert S, et al. β-galactosidase from Lactobacillus pentosus: purification, characterization and formation of galacto-oligosaccharides. Biotechnol J. 2010;5(8):838–847.
- Tien-Thanh N, Hoang Anh N, Sheryl Lozel A, et al. Homodimeric β-galactosidase from Lactobacillus delbrueckii subsp. bulgaricus DSM 20081: expression in Lactobacillus plantarum and biochemical characterization. J Agric Food Chem. 2012;60(7):1713–1721.
- Hashem AM, Gamal AA, Hassan ME, et al. Covalent immobilization of Enterococcus faecalis Esawy dextransucrase and dextran synthesis. Int J Biol Macromol. 2016;82:905–912.
- Wu Y, Yuan S, Chen S, et al. Enhancing the production of galacto-oligosaccharides by mutagenesis of Sulfolobus solfataricus β-galactosidase. Food Chem. 2013;138(2):1588–1595.
- Lim HR, Choo CM, Chong CH, et al. Optimization studies for water defluoridation with two-stage coagulation processes using new industrial-based chemical coagulants. J Water Process Eng. 2021;42:102179.
- Urrutia P, Rodriguez-Colinas B, Fernandez-Arrojo L, et al. Detailed analysis of galactooligosaccharides synthesis with β-galactosidase from Aspergillus oryzae J Agric Food Chem. 2013;61(5):1081–1087.
- Ambrogi V, Bottacini F, Callaghan J, et al. Infant-associated Bifidobacterial β-galactosidases and their ability to synthesize galacto-oligosaccharides. Front Microbiol. 2021;12(949):662959.
- Choi JY, Hong H, Seo H, et al. High galacto-oligosaccharide production and a structural model for transgalactosylation of β-galactosidase II from Bacillus circulans. J Agric Food Chem. 2020;68(47):13806–13814.
- Sun H, You S, Wang M, et al. Recyclable strategy for the production of high-purity galacto-oligosaccharides by Kluyveromyces lactis. J Agric Food Chem. 2016;64(28):5679–5685.
- Xin Y, Guo T, Zhang Y, et al. A new β-galactosidase extracted from the infant feces with high hydrolytic and transgalactosylation activity. Appl Microbiol Biotechnol. 2019;103(20):8439–8448.
- Volford B, Varga M, Szekeres A, et al. β-galactosidase-producing isolates in mucoromycota: screening, enzyme production, and applications for functional oligosaccharide synthesis. Journal of Fungi. 2021;7(3):229.
