ABSTRACT
Osteosarcoma, derived from primitive mesenchymal cells, is the most common primary solid malignant tumor of bone. The cause of osteosarcoma remains unclear. In recent years, the role of non-coding sequences in regulating protein expression in tumors has been paid more and more attention, especially long non-coding RNA (lncRNA). We speculate that SRY-box transcription factor 21 antisense divergent transcript 1 (SOX21-AS1) can regulate the expression of the mechanistic target of rapamycin kinase (mTOR) and Kruppel-like factor 4 (KLF4) through sponging hsa-mir-7-5p and hsa-mir-145-5p. We knocked lncRNA SOX21-AS1 into the genome of 143B cells through CRISPR/Cas9, then screened out a monoclonal cell line. Detect the transcription level and protein expression level of the above-mentioned related genes, and cell proliferation. Then, ginsenoside Rg3 was added to culture the cell line knocked into lncRNA SOX21-AS1, and the expression levels of lncRNA SOX21-AS1, hsa-mir-7-5p, hsa-mir-145-5p, mTOR, and KLF4 were detected by RT-qPCR and Western blot. Cell proliferation method detects cell viability, explores the molecular mechanism of lncRNA SOX21-AS1 in osteosarcoma, and checks whether it can be used as a potential drug target for the treatment of osteosarcoma. Our results demonstrate that the overexpression of lncRNA SOX21-AS1 up-regulates mTOR and KLF4 by sponging hsa-mir-7-5p and hsa-mir-145-5p, and ultimately regulates the proliferation of osteosarcoma. It is proved that ginsenoside Rg3 can inhibit the cell proliferation of osteosarcoma by reducing the expression level of lncRNA SOX21-AS1. It provides an alternative for the treatment of osteosarcoma in the future.
1. Introduction
Osteosarcoma is a malignant tumor originating from mesenchymal stem cells[Citation1]. Its morbidity and mortality rank first in bone tumors. Today, the pathogenesis of osteosarcoma is still unclear. The current treatment of osteosarcoma is still based on surgery combined with adjuvant chemotherapy. Therefore, current clinical treatments cannot effectively cure osteosarcoma[Citation2]. With the continuous development of oncology research, gene-targeted therapy has become possible, bringing hope to overcome osteosarcoma. Gene targeted therapy is to intervene in abnormally expressed cancer-related genes in the development of osteosarcoma through molecular biological means, thereby inhibiting the malignant biological characteristics of the tumor, and achieving the goal of curing osteosarcoma. Therefore, exploring and discovering new molecular targets for osteosarcoma treatment will provide data support and a theoretical basis for gene targeted therapy.
The peak incidence of osteosarcoma is in adolescents who are 10–14 years old in the developmental stage. Juvenile osteosarcoma often appears in the metaphyses (75%) of long bones of the limbs, such as distal femur, proximal tibia, and proximal humerus. This also suggests that the pathogenesis of osteosarcoma is closely related to puberty hormone changes and bone growth[Citation3]. Coincidentally, serine/threonine kinase 3 (AKT3) is a cell signaling regulator that responds to insulin and growth factors. It is involved in a variety of biological processes, including cell proliferation, differentiation, apoptosis, and tumorigenesis[Citation4-6]. The AKT serine/threonine kinase can interact with Kruppel-like factor 4 (KLF4)[Citation7] and mechanistic target of rapamycin kinase (mTOR)[Citation8].
MTOR mediates cell responses to stresses, such as DNA damage and nutritional deficiencies. There have been thousands of papers on mTOR research, which have confirmed that it is a proto-oncogene[Citation8-10]. KLF4 is thought to control the transition from G1 to S in the cell cycle after DNA damage by mediating the tumor suppressor gene p53, it is a tumor suppressor gene[Citation11,Citation12]. Over the past 10 years, the discovery and characterization of long non-coding RNA (lncRNA) with a length of more than 200 nucleotides has revealed the diversity of its regulatory role in tumors, and is considered as an important target for the treatment of human cancer. One of the roles of lncRNA is to sponge miRNAs as ceRNA to regulate the expression of related proteins[Citation13]. If the expression levels of genes that are antagonistic to each other in the downstream genes regulated by the same lncRNA increase at the same time, then what will the final result of the regulation of this lncRNA be? Coincidentally, both mTOR and KLF4 are the target genes of hsa-mir-7-5p [Citation14-16] and hsa-mir-145-5p [Citation17,Citation18]. More coincidentally, both hsa-mir-7-5p[Citation19] and hsa-mir-145-5p[Citation20] can be sponged by lncRNA SRY-box transcription factor 21 antisense divergent transcript 1 (SOX21-AS1). Therefore, we speculate that SOX21-AS1 can regulate the expression of mTOR and KLF4 through sponging hsa-mir-7-5p and hsa-mir-145-5p, thereby regulating the proliferation of osteosarcoma.
Therefore, we use CRISPR/Cas9 gene editing technology to knock lncRNA SOX21-AS1 into the “safe harbor” AAVS1 site, and then use RT-qPCR and western blot to detect the expression level of related genes, and use the cell proliferation method to detect the proliferation of osteosarcoma.
Ginseng is a herb native to Northeast Asia and is widely used in traditional medicine in East Asia. In recent years, more and more medicinal values of ginseng have been discovered and have attracted worldwide attention. Ginsenoside is regarded as the most important biologically active substance in ginseng[Citation21]. In vivo and in vitro experiments confirmed that ginsenoside Rg3 can exert anti-tumor effects in a dose- and time-dependent manner[Citation22,Citation23]. In summary, we hypothesize that lncRNA SOX21-AS1 may enhance the proliferative properties of osteosarcoma by upregulating mTOR and KLF4, and verify whether ginsenoside Rg3 can inhibit osteosarcoma proliferation by targeting lncRNA SOX21-AS1, thus hopefully providing an alternative for future clinical treatment of osteosarcoma.
2. Materials and methods
2.1. Bioinformatics analysis
The data were acquired through TCGA database. The tools deepbase v3.0[Citation24], UALCAN[Citation25], RNAInter[Citation26], UCSC Genome Browser[Citation27], GEO DataSets, and GEPIA2[Citation28] were used to analyze Gene Differential Expression, Gene Survival Analysis and gene interactions.
2.2. Plasmid
The PX459 vector (plasmid #62988) expressing the cas9 protein was obtained from Addgene. The gRNA sequence targeting AAVS1 intron 1 is 5ʹ-GGGGCCACTAGGGGACAGGAT-3ʹ[Citation29]. As mentioned above, the SgRNA oligonucleotide was ordered (Sangon Biotech, Shanghai) and annealed into the PX459 vector digested with Bbs I. Then, we got the PX459-AAVS1-sgRNA plasmid. The Donor- SOX21-AS1 vector is kept by our laboratory (NCBI Reference Sequence: NR_046514.1). The vector was prepared by using Qiagen Endofree Plasmid Kit (Qiagen) and quantified by NanoDrop 2000 (Thermo Fisher).
2.3. Cell culture and transfection
The 143B cell line was cultured in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (Sigma) and 1% penicillin–streptomycin (Hyclone, USA). 143B cells that reached 70%–80% confluence were co-transfected with sgRNA/Cas9-expressing PX459-AAVS1-sgRNA vector and Donor-SOX21-AS1 vector by lipofectamine 3000 (Thermo Fisher Scientific). Puromycin (0.5 μg/ml) was used to select transfected cells. The resulting cells are cloned and expanded by separating individual cells with limiting dilution method. Next, single-cell clones are picked and cultured in 96-well plates. The SOX21-AS1 knocked-in AAVS1 in a single isolated colony was detected by PCR, and then the amplicon was analyzed by Sanger sequencing (Sangon Biotech, Shanghai, China). Reporter gene Fluorescent protein is observed under 488 nm light wave. As mentioned in the previous study[Citation30], a monoclonal cell line that successfully knocked into the SOX21-AS1 gene was cultured by adding ginsenoside Rg3.
2.4. qRT-PCR analysis
TRIzol reagent (Thermo Fisher Scientific, USA) was used to extract total RNA from cells. For specific experimental steps, please refer to this article-Quantitative RT-PCR[Citation31]. Each experiment was repeated 3 times. The expression level of the target genes relative to β-actin was determined by the 2−ΔΔCt method. The results are expressed as mean ± standard deviation. We use the two-tailed paired Student’s t-test for comparison. P < 0.05 is considered to be statistically significant.
The primer sequences were listed below:
sgRNA-F:CACCGGGGCCACTAGGGACAGGAT;
sgRNA-R: AAACATCCTGTCCCTAGTGGCCCC.
SOX21-AS1-F: ACGAGTAGGAGAGCCTCTCC;
SOX21-AS1-R: GCATACTCTCAGTCTGGGCTAGC.
mTOR-F: CAAAGCCGCCAAGGAGCTCC;
mTOR-R: ATGGCAAGACGGCCAATGGC.
KLF4-F: GGAGCTCTCCCACATGAAGCG;
KLF4-R: GGATAGGTGAAGCTGCAGGTGG.
KI Detection primer-F: CGAGAGATCTGGCAGCGGAG;
KI Detection primer-R: CGCTACAGGGCGCGTACTATGG.
2.5. Cell proliferation
143B cells were seeded into a 96-well cell culture plate at a density of 5000 cells per well. CCK-8 reagent (10 μl) was added to each well and incubated for 1 h. We use a microplate reader (Bio-Rad, Hercules, CA, USA) to measure the absorbance at 450 nm.
2.6. Western blot
The cell lysed supernatant was mixed with 4x SDS loading buffer and boiled for 5 min. The proteins were separated by SDS-PAGE and transferred to the PVDF membrane. After incubating with the primary antibody and the secondary antibody, we observe the protein bands with a chemiluminescent substrate working solution and image with X-ray film. We use Image J software to quantify the density of protein bands.
3. Results
Osteosarcoma has the highest morbidity and mortality rate among bone tumors. To date, the pathogenesis of osteosarcoma is still unclear, so current clinical treatment cannot effectively cure osteosarcoma. Therefore, it is urgent to explore and discover new molecular targets for osteosarcoma treatment. The discovery and characterization of long non-coding RNAs (lncRNAs) with a length of more than 200 nucleotides have revealed the diversity of their regulatory roles in tumors and are considered to be important targets for the treatment of human cancers. Therefore, in this study, the lncRNA SOX21-AS1, which is aberrantly highly expressed in sarcoma, was found by bioinformatics analysis. Hsa-mir-7-5p and hsa-mir-145-5p were also found to be sponged by lncRNA SOX21-AS1. Coincidentally, both mTOR and KLF4 are target genes of hsa-mir-7-5p and hsa-mir-145-5p, and both are also aberrantly highly expressed in sarcoma. Therefore, we speculate that SOX21-AS1 can regulate the expression of mTOR and KLF4 through sponging hsa-mir-7-5p and hsa-mir-145-5p, thereby regulating the proliferation of osteosarcoma. We use CRISPR/Cas9 gene editing technology to knock lncRNA SOX21-AS1 into the “safe harbor” AAVS1 site, and then use RT-qPCR and WB to detect the expression level of related genes, and use the CCK-8 method to detect the proliferation of osteosarcoma. Finally, we explored whether ginsenoside Rg3 could inhibit osteosarcoma by targeting lncRNA SOX21-AS1 to inhibit the proliferative properties of osteosarcoma cells.
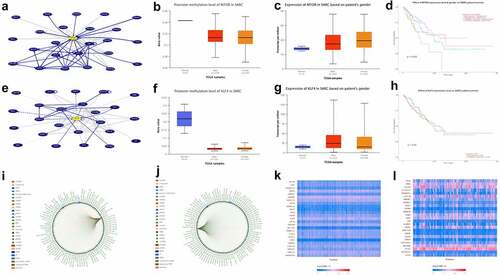
3.1. KLF4 and mTOR are overexpressed in sarcoma(SARC)
The incidence of osteosarcoma is closely related to puberty hormone changes and bone growth. AKT3 is a cell signal regulator that responds to hormones, such as insulin and growth factors, and participates in a variety of biological processes, including cell proliferation, differentiation, apoptosis, and tumorigenesis [Citation4-6]. Then, we use UCSC Genome Browser (http://genome.ucsc.edu/index.html) to analyze the network interacting with AKT3, and we find that both mTOR and KLF4 can interact with AKT3 (). There have been thousands of papers on mTOR research, which have confirmed that it is a proto-oncogene [Citation8-10]. KLF4 is thought to control the transition from G1 to S in the cell cycle after DNA damage by mediating the tumor suppressor gene p53, it is a tumor suppressor gene [Citation11,Citation12]. Through TCGA database, we found that the promoter methylation level of mTOR and KLF4 decreased in sarcoma (). So, we speculate that their expression level in sarcoma should be elevated. Therefore, we further analyzed the expression of mTOR and KLF4 in patients with sarcoma of different genders, and the results did indeed increase in their expression (). KLF4 is a tumor suppressor gene, and mTOR is a proto-oncogene, so we speculate that overexpressed mTOR is not conducive to the prognosis of patients with sarcoma; the effect of KLF4 should be just the opposite. Then, we analyze the relationship between these two genes and the survival rate of sarcoma patients. Kaplan–Meier survival analysis shows that in sarcoma, the overall survival rate of patients with high mTOR expression is lower than that of patients with low mTOR expression, and the effect of KLF4 is really just the opposite (). Coincidentally, both mTOR and KLF4 are the target genes of hsa-mir-7-5p[Citation14-16] and hsa-mir-145-5p[Citation17,Citation18] (). Genes positively related to mTOR and KLF4 in SARC indicate that these genes contain both proto-oncogene and tumor suppressor gene (), indicating that their regulation of tumors is an extremely complex network, which is the result of promoting tumor development and reducing patient survival by its combined effect.
Figure 1. KLF4 and mTOR are overexpressed in SARC
3.2. Highly expressed SOX21-AS1 significantly reduces the survival rate of SARC patients
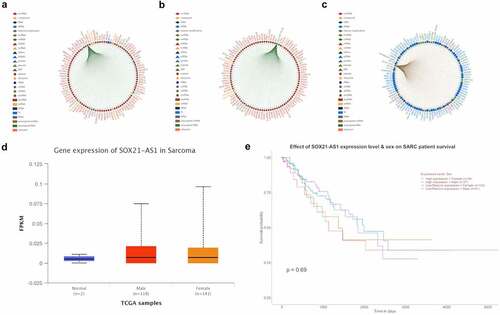
More coincidentally, both hsa-mir-7-5p[Citation19] and hsa-mir-145-5p[Citation20] can be sponged by lncRNA SOX21-AS1), SOX21-AS1 can promote tumorigenesis[Citation20], in the figure, the genes that interact with SOX21-AS1, hsa-mir-7-5p and hsa-mir-145-5p are all confirmed by previous studies. Therefore, we speculate that SOX21-AS1 can regulate the expression of mTOR and KLF4 through sponging hsa-mir-7-5p and hsa-mir-145-5p, thereby regulating the proliferation of osteosarcoma. It is worth noting that lncRNA SOX21-AS1 can sponge a lot of miRNAs. Among these miRNAs, hsa-mir-7-5p and hsa-mir-145-5p can also regulate many mRNAs and lncRNAs, so this is a very large and complex network. So if the expression of mTOR and KLF4 are regulated and increased by SOX21-AS1 at the same time, should the tumorigenesis be promoted or suppressed? The expression of SOX21-AS1 in sarcoma is slightly increased ()), but Kaplan–Meier survival analysis shows that in sarcoma, the overall survival rate of patients with high SOX21-AS1 expression is lower than that of patients with low SOX21-AS1 expression ()).
Figure 2. Highly expressed SOX21-AS1 significantly reduces the survival rate of SARC patients.
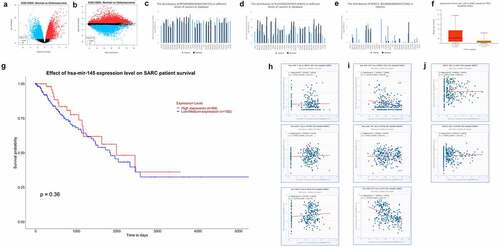
3.3. Expression of SOX21-AS1, mTOR and KLF4 in pan cancers and co-expression with hsa-mir-7-5p and hsa-mir-145-5p in sarcomas
We analyzed the differentially expressed genes in normal tissues and osteosarcoma through GEO DataSets database (GSE12865), mapped the volcanoes () and found that mTOR and KLF4 were indeed significantly higher expressed in osteosarcoma. These are consistent with the results of the TCGA analysis above. Then, by collecting transcriptome sequencing results from previous investigators in different cancers, histograms of SOX21-AS1, mTOR and KLF4 expression in different cancers were plotted, which showed elevated expression of these three genes in most cancer tissues, especially in sarcoma tissues (). Then, we analyzed the expression of hsa-mir-145-5p in patients with osteosarcoma with TP53 mutation and its effect on patient survival (). TP53 is an oncogene and its mutation leads to increased proliferation of tumor cells. We found that hsa-mir-145-5p was increased in sarcoma with TP53 mutation. It should be that it has a synergistic effect with TP53, both have oncogenic properties, so when TP53 mutation cells enhance other oncogenic signaling pathways, the expression of hsa-mir-145-5p is increased, and high expression of hsa-mir-145-5p significantly enhances the survival rate of patients (high expression is 64 samples, low to medium expression is 192 samples), it was further demonstrated that hsa-mir-145-5p has cancer inhibitory effect. We then analyzed the relationship between hsa-mir-7-5p, hsa-mir-145-5p, SOX21-AS1, mTOR and KLF4 co-expression (more than 260 sample sizes) by transcriptome sequencing results of osteosarcoma from the database, and further prove the above conclusion ().
Figure 3. Expression of SOX21-AS1, mTOR and KLF4 in pan cancers and co-expression with hsa-mir-7-5p and hsa-mir-145-5p in sarcomas
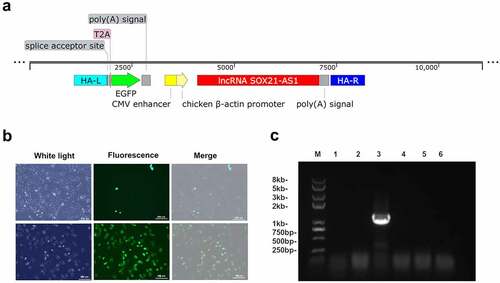
3.4. Knock lncRNA SOX21-AS1 into 143B through CRISPR/cas9 gene editing technology
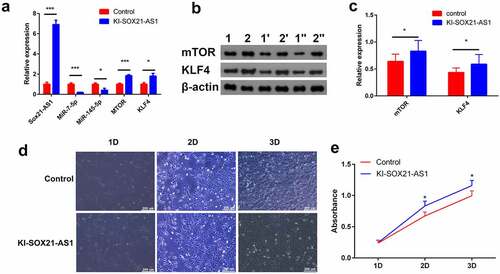
In order to verify our hypothesis, we used CRISPR/Cas9 to knock SOX21-AS1 into the genome of 143B cells[Citation29]. After co-transfection of Donor-SOX21-AS1 and PX459-AAVS1-sgRNA plasmids, we observed a small amount of 143B cells expressing green fluorescent protein through fluorescence microscope ()). Then, the monoclonal cell line was obtained by the limiting dilution method, and the KI-SOX21-AS1 143B monoclonal cell line was detected by PCR ()). The screened KI-SOX21-AS1 143B cell line continued to be expanded and cultured ()), and then sequenced to further confirm the successful SOX21-AS1 gene knock-in.
Figure 4. The lncRNA SOX21-AS1 was successfully knocked into 143B cells.
3.5. SOX21-AS1 regulates the proliferation of osteosarcoma cells
The expression of SOX21-AS1 mRNA in the KI-SOX21-AS1 143B cell line was significantly increased (6.8 times). SOX21-AS1 as a sponge reduced the expression of miR-7-5p (5.18 times) and miR-145-5p (2.47 times), and finally indirectly increased the expression of mTOR (1.84 times) and KLF4 (1.79 times) ()). WB results showed that the expression of mTOR (1.3 times) and KLF4 (1.35 times) was also significantly up-regulated at the protein level (). mTOR is a proto-oncogene and KLF4 is a tumor suppressor gene. Overexpression of SOX21-AS1 up-regulates the expression of both, so should 143B cell proliferation be promoted or inhibited? The results of cell proliferation experiments showed that SOX21-AS1 promoted the proliferation of 143B cells(). But KLF4 is a tumor suppressor gene, and its expression has also increased. Why do not KLF4 inhibit the proliferation of 143B cells? Therefore, we speculate that overexpressed SOX21-AS1 regulates many proto-oncogenes and tumor suppressor genes, mTOR and KLF4 are just two of these regulatory genes. The enhancement of cell proliferation is not only the sole effect of mTOR, but the result of combined action of many genes regulated by SOX21-AS1.
Figure 5. SOX21-AS1 up-regulates mTOR and KLF4 by sponging miR-7-5p and miR-145-5p, and ultimately regulates the proliferation of osteosarcoma cells.
3.6. Ginsenoside Rg3 inhibits the proliferation of osteosarcoma cells by inhibiting the expression of SOX21-AS1
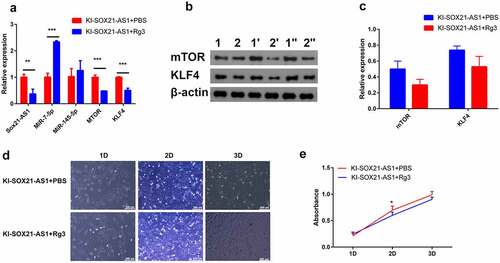
To determine whether ginsenoside Rg3 affects the growth of 143B cells in culture, cell proliferation experiment, WB and RT-PCR were performed on 143B cells treated with 80 µM Rg3 for 48 hours[Citation32]. Compared with control cells, the expression of SOX21-AS1 in 143B cells treated with ginsenoside Rg3 was significantly reduced ()). Rg3 also significantly reduced the expression of mTOR and KLF4 (). Cell proliferation experiments have shown that Rg3 can inhibit the proliferation of 143B cells (). It provides an alternative for the treatment of osteosarcoma in the future. In conclusion, our results show that SOX21-AS1 can simultaneously up-regulate the expression of proto-oncogene mTOR and tumor suppressor gene KLF4, but its combined effect is to enhance the proliferation of 143B cells.
Figure 6. Ginsenoside Rg3 inhibits the proliferation of osteosarcoma cells by inhibiting the expression of SOX21-AS1.
Therefore, we conclude that the regulation of lncRNA is not achieved through a single lncRNA/miRNA/mRNA axis, but through the comprehensive effect of a complex network. Perhaps, it can provide some reference value for studying the function of lncRNA in the future. Therefore, SOX21-AS1 may be a new indicator and potential therapeutic target for the progression and prognosis of osteosarcoma.
4. Discussion
Osteosarcoma is a malignant tumor originating from mesenchymal stem cells, its morbidity and mortality rank first among bone tumors. To this day, the pathogenesis of osteosarcoma is still unclear. Long non-coding RNA (lncRNA) is involved in the development of cancer. The lncRNA/miRNA/mRNA axis is very popular in the research of lncRNA. But metabolic regulation is a very complex network, some regulation has synergistic effects, some regulation has antagonistic effects. Therefore, if the expression levels of genes that are antagonistic to each other in the downstream genes regulated by the same lncRNA increase at the same time, then what will the final result of the regulation of this lncRNA be?
We have proved through bioinformatics that the expression of the proto-oncogene mTOR in sarcoma cells is increased, and its high expression leads to a low survival rate of patients. The expression of the tumor suppressor gene KLF4 in sarcoma cells is also increased, and its high expression leads to a high survival rate of patients. Coincidentally, both mTOR and KLF4 are the target genes of hsa-mir-7-5p[Citation14-16] and hsa-mir-145-5p [Citation17,Citation18]. More coincidentally, both hsa-mir-7-5p[Citation19] and hsa-mir-145-5p[Citation20] can be sponged by lncRNA SOX21-AS1. Therefore, we speculate that SOX21-AS1 can regulate the expression of mTOR and KLF4 through sponging hsa-mir-7-5p and hsa-mir-145-5p, thereby regulating the proliferation of osteosarcoma.
Then, we successfully obtained the 143B cell line knocked into SOX21-AS1 through CRISPR/Cas9 gene editing technology. Our results prove that overexpression of lncRNA SOX21-AS1 up-regulates mTOR and KLF4 through sponging miR-7-5p and miR-145-5p, and ultimately promotes the proliferation of osteosarcoma. It is proved that Rg3 can inhibit the cell proliferation of osteosarcoma by reducing the expression level of SOX21-AS1. It provides an alternative for the treatment of osteosarcoma in the future.
In addition, the tumor suppressor gene KLF4 is overexpressed in sarcoma, which should be a negative feedback regulation of cell proliferation. Although the cell proliferation of sarcoma is still increased, this should be the result of negative feedback regulation, otherwise cell proliferation must be much higher than this result.
5. Conclusion
In this study, we demonstrated that the overexpression of lncRNA SOX21-AS1 upregulated mTOR and KLF4 through sponging hsa-mir-7-5p and hsa-mir-145-5p, and ultimately promoted osteosarcoma proliferation. And it was demonstrated that ginsenoside Rg3 could inhibit cell proliferation of osteosarcoma by reducing the expression level of lncRNA SOX21-AS1. It provides an option for the future treatment of osteosarcoma.
Acknowledgements
The authors are thankful for the support of equipments provided by Shanghai Engineering Research Center of Nano-Biomaterials and Regenerative Medicine, College of Chemistry, Chemical Engineering and Biotechnology, Donghua University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Jo VY, Fletcher CD. Who classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46:95–104.
- Zhang YL, Pu YC, Wang J, et al. Research progress regarding the role of long non-coding RNAs in osteosarcoma. Oncol Lett. 2020;20:2606–2612.
- Miller RW. Contrasting epidemiology of childhood osteosarcoma, ewing’s tumor, and rhabdomyosarcoma. Natl Cancer Inst Monogr. 1981;56:9–15.
- Takahashi H, Rokudai S, Kawabata-Iwakawa R, et al. AKT3 is a key regulator of head and neck squamous cell carcinoma. Cancer Sci. 2021;112:2325–2334.
- Suyama K, Yao JH, Liang HZ, et al. An AKT3 splice variant lacking the serine 472 phosphorylation site promotes apoptosis and suppresses mammary tumorigenesis. Cancer Res. 2018;78:103–114.
- Turner KM, Sun YT, Ji P, et al. Genomically amplified AKT3 activates DNA repair pathway and promotes glioma progression. Proc Natl Acad Sci U S A. 2015;112:3421–3426.
- Sousa L, Pankonien I, Clarke LA, et al. Klf4 acts as a wt-CFTR suppressor through an akt-mediated pathway. Cells-Basel. 2020;9:1607.
- Liu J, Shangguan Y, Sun J, et al. Baiap2l2 promotes the progression of gastric cancer via akt/mtor and Wnt3a/beta-catenin signaling pathways. Biomed Pharmacother. 2020;129:110414.
- Xu GY, Yang Hl, Liu MC, et al. lncrna tincr facilities bladder cancer progression via regulating mir-7 and mtor. Mol Med Rep. 2020;22:4243–4253.
- Mossmann D, Park S, Hall MN. Mtor signalling and cellular metabolism are mutual determinants in cancer. Nat Rev Cancer. 2018;18:744–757.
- Yang WT, Zheng PS. Kruppel-like factor 4 functions as a tumor suppressor in cervical carcinoma. Cancer-Am Cancer Soc. 2012;118:3691–3702.
- Guan HF, Xie LK, Leithauser F, et al. Klf4 is a tumor suppressor in b-cell non-Hodgkin lymphoma and in classic Hodgkin lymphoma. Blood. 2010;116:1469–1478.
- Luan T, Zhang XM, Wang SY, et al. Long non-coding RNA miat promotes breast cancer progression and functions as cerna to regulate dusp7 expression by sponging mir-155-5p. Oncotarget. 2017;8:76153–76164.
- Cui Y, Xiao Z, Chen T, et al. The mir-7 identified from collagen biomaterial-based three-dimensional cultured cells regulates neural stem cell differentiation. Stem Cells Dev. 2014;23:393–405.
- Li YZ, Wen L, Wei X, et al. Inhibition of mir-7 promotes angiogenesis in human umbilical vein endothelial cells by upregulating vegf via klf4. Oncol Rep. 2016;36:1569–1575.
- Glover AR, Zhao JT, Gill AJ, et al. microRNA-7 as a tumor suppressor and novel therapeutic for adrenocortical carcinoma. Oncotarget. 2015;6:36675–36688.
- Xu N, Papagiannakopoulos T, Pan Gj, et al. microRNA-145 regulates oct4, sox2, and klf4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–658.
- Liu Z, Yan Y, Cao SZ, et al. long non-coding RNA snhg14 contributes to gastric cancer development through targeting mir-145/sox9 axis. J Cell Biochem. 2018;119:6905–6913.
- Xie Y, Zhang SJ, Lv ZY, et al. Sox21-as1 modulates neuronal injury of MMP+-treated sh-sy5y cells via targeting mir-7-5p and inhibiting irs2. Neurosci Lett. 2021;746:135602.
- Wei AW, Li LF. long non-coding RNA sox21-as1 sponges mir-145 to promote the tumorigenesis of colorectal cancer by targeting myo6. Biomed Pharmacother. 2017;96:953–959.
- Kim YJ, Zhang DB, Yang DC. Biosynthesis and biotechnological production of ginsenosides. Biotechnol Adv. 2015;33:717–735.
- Joo EJ, Chun J, Ha YW, et al. Novel roles of ginsenoside rg3 in apoptosis through downregulation of epidermal growth factor receptor. Chem Biol Interact. 2015;233:25–34.
- kim bm, kim dh, park jh, et al. Ginsenoside Rg3 induces apoptosis of human breast cancer (mda-mb-231) cells. J Cancer Prev. 2013;18:177–185.
- Xie F, Liu S, Wang J, et al. Deepbase v3.0: expression atlas and interactive analysis of ncrnas from thousands of deep-sequencing data. Nucleic Acids Res. 2021;49:d877–d83.
- Chandrashekar DS, Bashel B, Balasubramanya Sah, et al. Ualcan: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–658.
- Lin Y, Liu T, Cui T, et al. Rnainter in 2020: RNA interactome repository with increased coverage and annotation. Nucleic Acids Res. 2020;48:d189–d97.
- Kent WJ, Sugnet CW, Furey TS, et al. The human genome browser at ucsc. Genome Res. 2002;12:996–1006.
- Tang ZF, Kang BX, Li CW, et al. Gepia2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47(w1):w556–w60.
- Castano J, Bueno C, Jimenez-Delgado S, et al. Generation and characterization of a human ipsc cell line expressing inducible Cas9 in the “safe harbor” aavs1 locus. Stem Cell Res. 2017;21:137–140.
- He BC, Gao Jl, Luo X, et al. Ginsenoside rg3 inhibits colorectal tumor growth through the down-regulation of wnt/ß-catenin signaling. Int J Oncol. 2011;38:437–445.
- Lambolez B, Rossier J. Quantitative rt-pcr. Nat Biotechnol. 2000;18:5.
- Luo Y, Zhang P, Zeng HQ, et al. Ginsenoside Rg3 induces apoptosis in human multiple myeloma cells via the activation of Bcl-2-associated x protein. Mol Med Rep. 2015;12:3557–3562.
