ABSTRACT
Interleukin (IL)-8 has been shown to play an important role in obstructive sleep apnea syndrome (OSAS). However, its role in OSAS development is still controversial. This meta-analysis was to explore the correlation between interleukin (IL)-8 concentration and OSAS. Database (from the inception to July 2021) searches on PubMed, Web of Science, Medline, EMBASE, and Cochrane Library were conducted for studies analyzing the correlation between IL-8 concentration and OSAS, regardless of the language of publication. Standardized mean difference (SMD) and 95% confidence intervals (CI) were used to analyze any prospective association between IL-8 concentration and OSAS. A total of 25 eligible studies, including 2301 participants and 1123 controls, were included in this meta-analysis. The included studies evaluating the association between serum IL-8 concentration and OSAS indicated that adults and children with OSAS had elevated serum concentrations of IL-8 compared with controls (SMD = 0.997, 95% CI = 0.437–1.517, P < 0.001; SMD = 0.431, 95% CI = 0.104–0.759, P = 0.01). Categorization of the study population into subgroups according to body mass index, apnea–hypopnea index (AHI), ethnicity, and sample size also showed that individuals with OSAS had elevated serum concentrations of IL-8 compared with controls. Additionally, the results demonstrated that the higher the AHI, higher was the IL-8 concentration. Similar results were observed in the literature on the association between plasma IL-8 concentration and OSAS. This meta-analysis verified that compared with controls, children and adults with OSAS have significantly elevated IL-8 concentrations.
1. Introduction
Obstructive sleep apnea syndrome (OSAS) is a common sleep-related breathing disorder characterized by snoring and repetitive pharyngeal collapse. OSAS is associated with an increased risk of cardiovascular and cerebrovascular morbidity and mortality [Citation1]. Additionally, several studies demonstrated that patients with OSAS had a high prevalence of cancer and diabetes [Citation2,Citation3]. Epidemiological studies showed that the prevalence of OSAS varied from 14.7% to 36.5% depending on age, sex, and ethnicity. Furthermore, the incidence of OSAS in people aged >65 years ranges from 20% to 40% [Citation4,Citation5]. Consequently, OSAS is increasingly becoming a serious health problem [Citation6].
Researchers noted that in individuals with OSAS (children and adults), chronic intermittent hypoxia led to a systemic inflammatory response. Furthermore, typical inflammatory cytokines, including interleukin (IL)-8, tumor necrosis factor-α, and IL-6, are upregulated, which may be related to the activation of the nuclear factor pathway [Citation7] OSAS constitutes a low-grade chronic inflammatory respiratory disease because recurrent chronic intermittent hypoxia during sleep causes and triggers an anti-inflammatory cascade. Inflammatory factors, such as IL-8, mediate and aggravate vascular inflammation and damage the homeostasis of the cardiovascular system [Citation8]. As an inflammatory cell chemokine, IL-8 is primarily generated by mononuclear macrophages. Epithelial cells and endothelial cells can also produce IL-8 under suitable stimulation conditions [Citation9]. IL-8 can promote inflammation by attracting and inducing neutrophil degranulation to release protein hydrolase, arachidonic acid, and other substances that cause tissue damage [Citation10,Citation11].
However, the level of IL-8 concentration as a predisposition factor for OSAS remains controversial. The association between serum concentration of IL-8 and OSAS has been previously examined in two meta-analyses conducted in 2013 and 2021 [Citation7,Citation12]. Their findings illustrated that compared to controls, individuals with OSAS had increased IL-8 concentrations. IL-8 concentrations may be used to assist in both the diagnosis and post-treatment follow-up in patients with OSAS. However, the meta-analysis conducted in 2021 included only 10 articles published between 2003 and 2013 and did not include a subgroup analysis according to disease severity or type of sample [Citation12], whereas in the 2013 meta-analysis, only two articles were included [Citation7]. Moreover, meta-regression analysis was not performed in these two meta-analyses.
Therefore, this study aimed to conduct a meta-analysis to explore the correlation between IL-8 concentration and OSAS. Considering the drawbacks of the previous meta-analyses, we included a larger number of studies in our meta-analysis and used standardized mean difference (SMD) to analyze IL-8 concentrations in adults and children with OSAS. Additionally, we performed subgroup analysis and meta-regression analysis according to ethnicity, disease severity, age, sample type, and body mass index (BMI). We tried to investigate whether IL-8 concentration could be a useful clinical biological indicator, which can be used to objectively evaluate the severity of OSAS and the immune function of patients with OSAS.
2. Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis guidelines for reporting systematic reviews and meta-analysis [Citation13]. The meta-analysis was registered at the prospective register of systematic reviews (PROSPERO, the website is https://www.crd.york.ac.uk/PROSPERO/, and the ID is CRD42021262013).
2.1. Retrieval strategy and literature screening
A researcher conducted a comprehensive search of articles published in the PubMed, Medline, EMBASE, Cochrane Library, and Web of Science databases from the respective times of establishment of the databases to 8 July 2021. The following search terms and keywords were applied to identify eligible publications: ‘obstructive sleep apnea OR sleep apnea OR OSA OR obstructive sleep apnea syndrome OR OSAS’, ‘expression’, ‘interleukin-8 OR IL-8ʹ. The reference lists of the eligible studies were also manually screened to determine other relevant articles.
2.2. Eligibility criteria
The inclusion criteria for the studies in this meta-analysis were as follows: 1) case-control studies that assessed the relationship between IL-8 concentration in plasma, serum, or other tissue samples and OSAS, regardless of age, sex, or BMI; 2) the diagnostic criteria for OSAS were an apnea–hypopnea index (AHI) of >5 events/hour for adults or an AHI of > 1 event/hour for children; 3) OSAS was diagnosed using overnight polysomnography; 4) studies that reported IL-8 concentration before treatment; and 5) studies that provided sufficient data for the calculation of SMD and 95% confidence intervals (CI) in the case and control groups.
The exclusion criteria were as follows: 1) studies without relevant data or with inadequate general or clinical data; 2) meta-analyses, review articles, animal research studies, and conference papers; 3) studies with no control group; 4) studies in which the control group constituted individuals with an AHI of >5 events/h for adults or AHI of >1 event/h for children; and 5) articles with data that overlapped with those in the articles already included in this study.
2.3. Data abstraction
Data from each study were abstracted independently by two researchers. Disagreements, if any, between the two researchers were resolved through a discussion with a third researcher, who helped reach a consensus. The data included the first author, the number of years post-publication, the country of origin of the study, the ethnicity and age of the participants, experimental methods, sample types, and the average and standard deviation of IL-8 concentration.
2.4. Quality evaluation
Two researchers utilized the Newcastle-Ottawa-Scale (NOS) [Citation14] to evaluate the quality of the included literature. Disputes or disagreements were resolved through discussion with a third researcher. The maximum total score of the NOS is 9 points.
2.5. Statistical analysis
The data were analyzed by a researcher. SMD and 95% CI were calculated. Stata 11.0 software (StataCorp, Texas, USA) was used to evaluate the importance of merging SMD and to conduct the Z-test. Cochrane Q and the I2 statistic were used to detect heterogeneity. When heterogeneity was detected (P heterogeneity < 0.1 and I2 > 50%) – which suggests statistically remarkable heterogeneity – random-effects model analysis was used to explore the combined SMD and 95% CI; otherwise, the fixed-effects model was used. Begg’s and Egger’s tests were used to evaluate publication bias in the results. Subgroup assessment was performed according to ethnicity, disease severity, age, sample type, and BMI. Sensitivity analysis, namely ‘cumulative analysis’ and ‘one study removed,’ was employed to explore the consistency and stability of our data. Statistical significance was set at p < 0.05.
Meta-regression analysis was performed to assess the impact of moderator variables on the study effect size. The trim-and-fill approach was adopted to determine articles lost due to publication bias in the funnel chart and to adjust the estimation of the overall effect.
Few articles reported the ‘median and interquartile range’ or ‘median and range’ of IL-8 concentration. In such cases, data were converted to ‘mean and standard deviation’ the methods outlined by Hozo et al. and Wan et al. [Citation15,Citation16].
2.6. Ethics
Approval of the Ethics Committee was not applicable for this study because it does not contain any human or animal subjects and the data used were extracted from previously published papers.
3 Results
A total of 25 eligible studies, including 2301 participants and 1123 controls, was included in this meta-analysis. The included studies evaluating the association between serum IL-8 concentration and OSAS indicated that adults and children with OSAS had elevated serum concentrations of IL-8 compared with controls. Categorization of the study population into subgroups according to body mass index, apnea–hypopnea index (AHI), ethnicity, and sample size also showed that individuals with OSAS had elevated serum concentrations of IL-8 compared with controls. Additionally, the results demonstrated that the higher the AHI, higher was the IL-8 concentration. Similar results were observed in the literature on the association between plasma IL-8 concentration and OSAS. Furthermore, the concentrations of IL-8 in the tonsil tissue, sputum, alveolar lavage fluid, and nasopharyngeal lavage fluid obtained from patients with OSAS were higher than those in the specimens obtained from controls. The specifics of this meta-analysis for each outcome are provided below.
3.1. Study selection
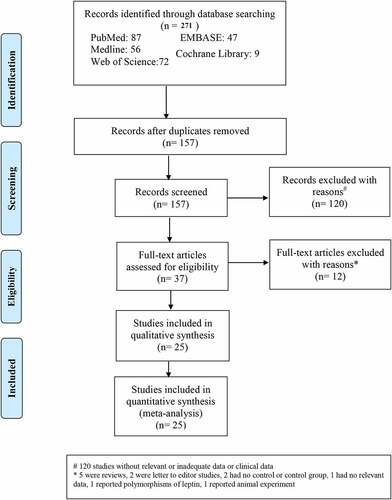
Overall, 271 articles were abstracted from the database. Of these, 157 articles were selected after removing duplicates. Thereafter, an additional 120 articles that did not have relevant data or adequate clinical data were excluded; thus, 37 full-text articles remained. Twelve full-text articles were further excluded because five of them were reviews, two were letters to the editor, two were reports of studies that had no control group, one had no relevant data, one reported polymorphisms of leptin, and one reported animal experiments. illustrated the process of literature screening with a flowchart. Twenty-five studies [Citation8,Citation17–40], having 2301 participants in the OSAS group and 1123 participants in the control group, were included in this meta-analysis (). In these studies, serum (n = 16) [Citation17–22,Citation26–33,Citation35,Citation36], plasma (n = 4) [Citation8,Citation23,Citation37,Citation38], sputum (n = 2) [Citation25,Citation34], nasal and pharyngeal lavage (n = 2) [Citation37,Citation40], bronchoalveolar lavage fluid (n = 1) [Citation39], and tonsil tissue (n = 1) [Citation24] were examined ().
Table 1. Characteristics of studies included in the meta-analysis
3.2. Serum IL-8 concentration
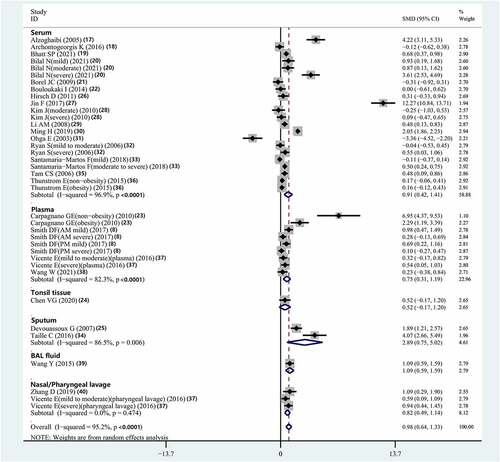
The association between serum IL-8 concentration and OSAS was analyzed in 16 studies [Citation17–22,Citation26–33,Citation35,Citation36]. The results indicated that compared with controls, patients with OSAS had significantly elevated serum IL-8 concentrations (SMD = 0.914, 95% CI = 0.418–1.411, P < 0.001, I2 = 96.9%, Ph<0.001) ().
3.3. Plasma IL-8 concentration
The relationship between plasma IL-8 concentration and OSAS was evaluated in four studies [Citation8,Citation23,Citation37,Citation38]. In these studies, compared with controls, individuals with OSAS had significantly elevated serum IL-8 concentrations (SMD = 0.748, 95% CI = 0.308–1.189, P = 0.001, I2 = 82.3%, Ph<0.001) ().
3.4. Sputum IL-8 concentration
The association between sputum IL-8 concentration and OSAS was assessed in two studies [Citation25,Citation34]. The results of these studies indicated that compared to controls, individuals with OSAS had significantly elevated sputum IL-8 concentrations (SMD = 2.889, 95% CI = 0.754–5.024, P = 0.008, I2 = 86.5%, Ph = 0.006) ().
3.5. Bronchoalveolar fluid IL-8 concentration
The relationship between the IL-8 concentration in bronchoalveolar lavage fluid and OSAS was evaluated in only one study [Citation39]. In this study, compared with controls, individuals with OSAS had significantly elevated bronchoalveolar lavage IL-8 concentration (P < 0.05).
3.6. Nasal and pharyngeal lavage IL-8 concentration
The association between nasal and pharyngeal lavage concentrations of IL-8 and OSAS was examined in two studies [Citation37,Citation40]. The results showed that compared with controls, individuals with OSAS had significantly increased nasal and pharyngeal lavage concentrations of IL-8 (SMD = 0.819, 95% CI = 0.494–1.143, P < 0.001, I2 = 0%, Ph = 0.474) ().
3.7. Tonsil tissue IL-8 concentration
The relationship between IL-8 concentration in the tonsils of children and OSAS was investigated in only one study [Citation24]. In this study, compared with controls, children with OSAS had significantly elevated IL-8 concentrations in tonsil tissue (P = 0.04).
3.8. Subgroup analysis of serum IL-8 concentration
3.8.1. Age
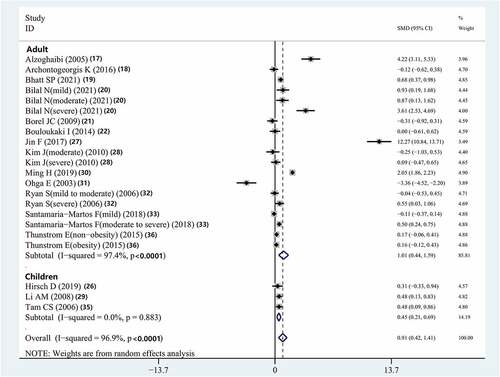
The result indicated that the serum IL-8 concentrations in adults (SMD = 1.013, 95% CI = 0.437–1.587, P < 0.001) and children (SMD = 0.445, 95% CI = 0.214–0.689, P = 0.01) with OSAS in the included studies were higher than those of controls (, ).
Table 2. Subgroup analysis for serum and plasma levels of interleukin-8
3.8.2. Mean body mass index
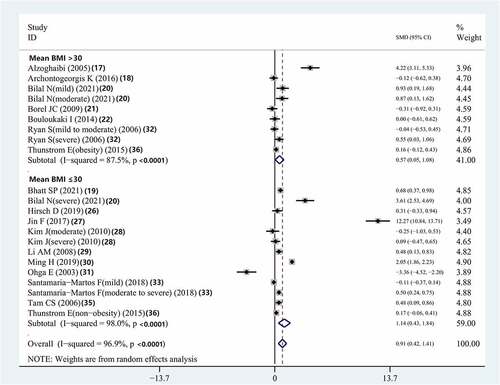
The serum IL-8 concentrations of patients with OSAS with an average BMI >30 kg/m2 and that of those with an average BMI ≤30 kg/m2 were higher than those of controls (SMD = 0.568, 95% CI = 0.052–1.084, P = 0.031; SMD = 1.137, 95% CI = 0.429–1.844, P < 0.001, respectively) (, ).
3.8.3. Ethnicity
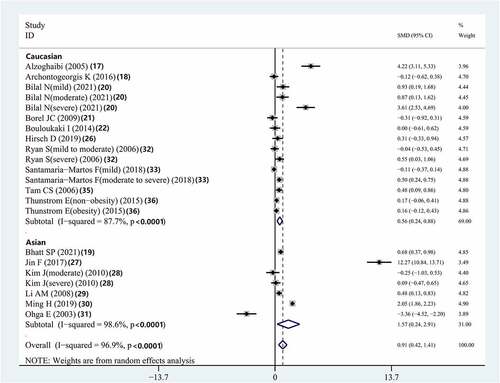
The results showed that Caucasian (SMD = 0.557, 95% CI = 0.237–0.877, P < 0.001) and Asian (SMD = 1.573, 95% CI = 0.240–2.905, P < 0.001) patients with OSAS had higher serum IL-8 concentrations than did the controls. (, ).
3.8.4. Total number of subjects
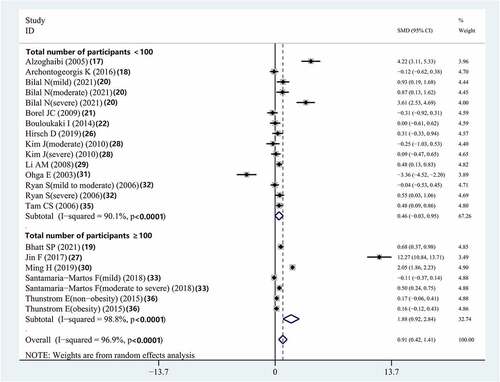
Combined assessment of studies with a sample size of more than 100 patients showed that compared to controls, individuals with OSAS had significantly elevated serum IL-8 concentrations (SMD = 1.883, 95% CI = 0.925–2.841, P < 0.001). However, there was no significant difference between the serum IL-8 concentrations of patients with OSAS and that of the controls in studies with a sample size of fewer than 100 patients (SMD = 0.461, 95% CI = −0.027–0.950, P = 0.064) (, ).
3.8.5. Mean apnea-hypopnea index
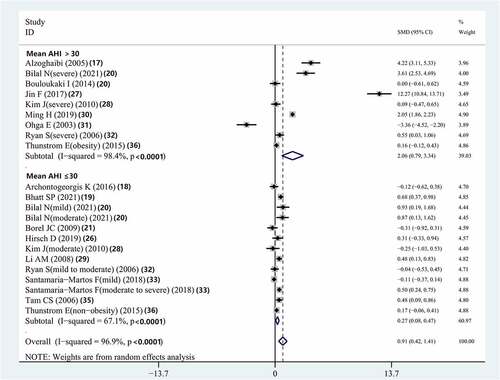
Subgroup analysis according to disease severity demonstrated that compared to controls, patients with OSAS with a mean AHI >30 had significantly elevated serum IL-8 concentrations (SMD = 2.064, 95% CI = 0.791–3.337, P = 0.001). Compared with controls, patients with OSAS who had a mean AHI ≤30 also had significantly elevated serum IL-8 concentrations (SMD = 0.275, 95% CI = 0.077–0.473; P = 0.007) (, ).
Figure 7. Meta-analysis for the association between serum IL-8 levels and OSAS: subgroup analysis by mean AHI
3.8.6 Meta-regression analysis of the serum IL-8 concentrations of patients with obstructive sleep apnea syndrome
The meta-regression data of serum IL-8 concentrations are outlined in . Age, mean BMI, number of patients, mean AHI, and ethnicity had no independent significant impact on serum IL-8 concentration.
Table 3. Meta-regression analysis of variables predicting serum and plasma levels of IL-8
3.9. Subgroup analysis of plasma IL-8 concentration
3.9.1. Age
The combined analysis showed that compared with controls and children with OSAS, adult patients with OSAS had significantly higher plasma IL-8 concentrations (SMD = 1.832, 95% CI = 0.521–3.144, P = 0.006) (SMD = 0.440, 95% CI = 0.120–0.760, P = 0.007) (Supplementary Figure 1, ).
3.9.2. Ethnicity
The results showed that compared to controls, Caucasian patients with OSAS had significantly higher plasma IL-8 concentrations (SMD = 0.837, 95% CI = 0.345–1.328, p = 0.001) (Supplementary Figure 2, ).
3.9.3. Mean body mass index
Compared with controls, patients with OSAS with BMI ≤30 kg/m2 had significantly elevated plasma IL-8 concentrations (SMD = 0.589, 95% CI = 0.177–1.001, P = 0.005) (Supplementary Figure 3, ).
3.9.4. Mean apnea-hypopnea index
Subgroup analysis according to disease severity indicated that compared with controls, individuals with OSAS with a mean AHI >30 had significantly elevated plasma IL-8 (SMD = 0.936, 95%CI = 0.240–1.631, P = 0.008). Plasma IL-8 concentrations were also significantly elevated in individuals with OSAS with a mean AHI ≤30 compared with that in controls (SMD = 0.660, 95% CI = 0.297–1.022, P < 0.001) (Supplementary Figure 4, ).
3.9.5. Meta-regression analysis of plasma interleukin-8 concentrations of patients with obstructive sleep apnea syndrome
The meta-regression data of plasma IL-8 concentration are shown in . Age, mean AHI, mean BMI, number of participants, and ethnicity had no independent significant effect on plasma IL-8 concentration.
3.10. Quality evaluation
The quality scores of the included articles were shown in .
3.11. Sensitivity analysis
The two sensitivity analyses, including ‘cumulative analysis’ and ‘one study removed,’ demonstrated the stability of the data for adults and children.
3.12 Publication bias of the included studies
The funnel chart of the analysis of serum IL-8 concentration was shown in Supplementary Figure 5. For serum concentration of IL-8, Begg’s test (P = 0.152) and Egger’s test (P = 0.324) showed that there was no bias in the included studies.
For the analysis of the serum concentration of IL-8 without imputed articles, the point estimate and 95% CI for the combined studies was 0.633 (0.558, 0.707) in the random-effects model. Analysis using the trim-and-fill approach showed the same imputed point estimate as aforementioned. This finding illustrated that the overall effect sizes for IL-8 concentration reported in the forest plot were valid.
4. Discussion
In this systematic review and meta-analysis, we analyzed the existing literature for the relationship between OSAS and IL-8 concentrations. The results illustrated that the plasma and serum IL-8 concentrations of adults and children with OSAS were significantly higher than those of healthy controls. Subgroup analysis of IL-8 concentrations in other samples, such as tonsil tissue, sputum, bronchoalveolar lavage fluid, and nasopharyngeal lavage fluid was not conducted owing to the small number of studies on IL-8 concentrations in these samples. However, the results of the present meta-analysis indicated that compared to controls, patients with OSAS showed higher concentrations of IL-8 in the above mentioned samples. This deduction carries clinical significance because an elevated IL-8 concentration may be an independent predisposing factor of cardiovascular disease in adults with OSAS [Citation27,Citation36]. Consequently, the findings of this meta-analysis suggest that in addition to routine tests for the assessment of sleep and sleep-disordered breathing, the immune systems of both children and adults with OSAS need to be monitored in clinical settings.
A previous study showed that OSAS could alter the levels of several hormones in the body [Citation41]. Additionally, vascular inflammation and systemic inflammation were the main mechanisms behind cardiac metabolic disorders in patients with OSAS. Qing Cui et al. [Citation12] concluded that serum IL-8 concentration was associated with OSAS in adults, whereas elevated serum IL-8 level might promote OSAS progression. Although the precise mechanism behind the influence of OSAS on IL-8 concentration is unclear, sleep deprivation and intermittent hypoxemia are considered to be important pathogenic factors [Citation42].
IL-8 is one of the strongest known inflammatory cell chemokines that play an indispensable role in systemic inflammation in OSAS and related cardiovascular diseases [Citation43]. IL-8 can induce neutrophils to release myeloperoxidase and recruit inflammatory cells to help sustain inflammation. Moreover, exposure to IL-8 can cause rapid mobilization of macrophage antigen 1 to the neutrophil surface in OSAS; thus, overexpression of IL-8 in human bronchial epithelial cells has been seen in response to a vibration stimulus generated by snoring [Citation7]. Akyol et al. [Citation44] reported that IL-8 bonded to specific receptors on the surface of neutrophils, which led to cell deformation, degranulation, and increased production of reactive oxygen species. This process might trigger the secretion of lysosomes to activate arachidonic acid, resulting in elevated vascular permeability and plasma protein exudation, leading to tissue damage, atherosclerosis, and other diseases [Citation45]. In healthy people, inflammatory cytokines, such as IL-8, participate in the modulation of physiological sleep and are related to physiological secretory patterns [Citation29]. Good sleep at night and a good physical state the next day are related to decreased secretion of IL-8, whereas increased secretion of IL-8 may be associated with excessive drowsiness and fatigue during the day [Citation46]. Daytime sleepiness is not the only main clinical manifestation of OSAS, but also one of the critical physiological consequences of OSAS [Citation47]. Strawbridge et al. measured the levels of inflammatory factors in individuals with depression and established that patients with depression also had generally high serum IL-8, which gradually increased with the progression of the condition [Citation48].
Previous studies demonstrated that serum IL-8 concentration was linked to obesity and that visceral adipose tissue may be involved in the secretion of IL-8 [Citation49]. Carpagnano et al. [Citation23] assessed serum IL-8 concentration in obese patients with OSAS and healthy controls. They established that the obese individuals with OSAS had a serum IL-8 concentration three times higher than that of healthy controls, whereas that of non-obese patients with OSAS was twice as high as that of healthy controls. Additionally, the serum IL-8 concentration of obese persons without OSAS in that study was also higher than that of healthy controls. The reason for this phenomenon may be that oxidative stress and sympathetic activation and inflammation, which are known to be involved in the pathophysiological mechanism of OSAS, are also observed in obese patients [Citation19,Citation50]. In the present meta-analysis, the serum IL-8 concentrations of both obese and non-obese persons with OSAS were higher than those of healthy controls. We also found that the serum and plasma IL-8 concentrations of obese patients with OSAS were lower than those of non-obese patients with OSAS. Some previous studies indicated that there was no significant correlation between BMI and IL-8 concentrations in patients with OSAS [Citation19,Citation29]. The IL-8 concentrations of non-obese patients with OSAS in this study were higher than those of obese patients with OSAS, suggesting that BMI itself does not influence IL-8 concentration in individuals with OSAS. However, other confounding factors may affect serum and plasma IL-8 concentration.
Several studies showed that the serum IL-8 concentrations of individuals with severe OSAS were higher than those of individuals with mild OSAS or controls [Citation20,Citation28,Citation32,Citation37]. Additionally, Devouassoux et al. [Citation25] reported that there was a significant correlation between serum IL-8 concentration and AHI in patients with OSAS (r = 0.81, P < 0.01). The subgroup analysis in the present study also showed that the serum and plasma IL-8 concentrations of individuals with AHI >30/h were higher than those of individuals with AHI ≤30/h. The serum and plasma IL-8 concentrations of patients with OSAS increased with the severity of the disease, suggesting that the concentration of serum and plasma IL-8 may reflect the severity of OSAS. Chronic intermittent hypoxia can lead to an increase in the pro-inflammatory cytokine IL-8. The higher the number of hypoxia events per unit time, the more serious is the degree of hypoxia and more obvious is the increase in IL-8 [Citation51,Citation52]. The pathophysiological basis of OSAS is that chronic intermittent hypoxia/reoxygenation leads to sleep oxygen deficiency, which is similar to hypoxia/reperfusion injury, causing damage to target organs. Thus, serum and plasma IL-8 concentrations increase with an increase in the severity of OSAS [Citation53]. Furthermore, subgroup analyses were conducted according to age and number of samples, and the serum and plasma IL-8 concentrations were still higher in the OSAS patients. The increase in the serum/plasma IL-8 level was more obvious in adults than in children. This could be related to immature and reduced immune functions in children. It has been reported that chronic rhinitis in children is related to adenoid hypertrophy, thereby causing upper airway obstruction leading to OSAS, especially at night [Citation54]. Inflammation has been suggested as a potential mechanism for children with OSAS. Hirsch et al. [Citation26] also indicated that the impaired cardiac responses correlated with higher serum IL-8 levels in children with OSAS. Moreover, it should be noted that Asians have significantly higher serum IL-8 concentrations than Caucasians. This suggests that ethnicity may affect the concentration of serum IL-8 in individuals with OSAS. Different ethnic groups have different genetic backgrounds, lifestyles, and living environments, which may lead to differences in the level of IL-8 secretion. Nevertheless, the data included in our meta-analysis is not sufficient to clarify the potential physiological mechanism by which ethnicity affects IL-8 concentration. Therefore, future studies are needed to explore the extent to which genetic background influences the susceptibility to OSAS.
Although the findings of the present meta-analysis are novel, the following limitations should be considered. First, the results of the included studies do not suggest that adjustments were made for prospective confounding factors, such as obesity, smoking, staying up late, sex, or drinking. Second, the results of the included studies with a small sample size (<100) were not reliable as low statistical power leads to a higher variability; thus, the relationship between IL-8 concentration and OSAS could not be considered to be adequately justified. In addition, only a few studies involved the analysis of samples other than serum or plasma. Third, there was a high degree of heterogeneity between studies in some analyses. Meta-regression analysis showed that ethnicity, age, BMI, AHI, and sample size had little effect on heterogeneity. We could not identify the source of the obvious heterogeneity, which may be related to experimental conditions, test reagents, physical differences in participants, and other confounding factors, all of which may affect the reliability of the results. Fourth, IL-8 concentrations were considered secondary results in some studies; thus, the evidence of support the effect of IL-8 in OSAS might be low. Some of the studies suffer from significant sources of bias and this should be also taken into consideration.
Despite these limitations, this meta-analysis has several advantages. First, the overall results of the meta-analysis suggest that IL-8 concentration may be a useful clinical biological indicator, which can be used to objectively evaluate the severity of OSAS and the immune function of patients with OSAS and to better understand the immune mechanism behind OSAS. Second, to the best of our knowledge, this is the largest meta-analysis of relevant literature on the relationship between IL-8 concentration and OSAS. Furthermore, we performed a subgroup analysis to provide more reliable results. Third, unlike previous meta-analyses, we included data on children with OSAS.
5. Conclusion
This meta-analysis shows that individuals with OSAS have elevated IL-8 concentrations, which is linked to the severity of the disease. Additionally, age and ethnicity have an influence on the association between OSAS and IL-8 concentration. Future studies should be focused on OSAS interventions (for example, use of continuous positive airway pressure devices) for the investigation of IL-8 concentrations and immune function.
Author contributions
XL, RH, XR, and JH had full access to all study data and take responsibility for the analytical integrity and accuracy of the data. RH, XR, and JH conceived and designed the study. XL conducted the data acquisition and extraction. RH conducted the assessment of bias. XL conducted the data analysis and interpretation. XL drafted the manuscript. XL, RH, XR, and JH conducted a critical review of the manuscript for important intellectual content. XR and XL conducted the statistical analyses. JH supervised the study. All authors contributed to the article and approved the submitted version.
Supplemental Material
Download Zip (14.2 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All relevant data in the study are included in the article/supplementary material. Further inquiries could be directed to the corresponding author.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Tan LL, Ting J, Balakrishnan I, et al. Sleep apnea evolution and left ventricular recovery after percutaneous coronary intervention for myocardial infarction. J Clin Sleep Med. 2018;14:1773–1781.
- Hunyor I, Cook KM. Models of intermittent hypoxia and obstructive sleep apnea: molecular pathways and their contribution to cancer. Am J Physiol Regul Integr Comp Physiol. 2018;315:R669–R87.
- Morsy NE, Farrag NS, Zaki NFW, et al. Obstructive sleep apnea: personal, societal, public health, and legal implications. Rev Environ Health. 2019;34:153–169.
- Yamagishi K, Ohira T, Nakano H, et al. Cross-cultural comparison of the sleep-disordered breathing prevalence among Americans and Japanese. Eur Respir J. 2010;36:379–384.
- Arnardottir ES, Bjornsdottir E, Olafsdottir KA, et al. Obstructive sleep apnoea in the general population: highly prevalent but minimal symptoms. Eur Respir J. 2016;47:194–202.
- Torelli F, Moscufo N, Garreffa G, et al. Cognitive profile and brain morphological changes in obstructive sleep apnea. Neuroimage. 2011;54:787–793.
- Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med. 2013;9:1003–1012.
- Smith DF, Hossain MM, Hura A, et al. Inflammatory milieu and cardiovascular homeostasis in children with obstructive sleep apnea. Sleep. 2017;40:zsx022.
- Jiang WG, Sanders AJ, Ruge F, et al. Influence of interleukin-8 (IL-8) and IL-8 receptors on the migration of human keratinocytes, the role of PLC-γ and potential clinical implications. Exp Ther Med. 2012;3:231–236.
- Matlawska-Wasowska K, Finn R, Mustel A, et al. The vibrio parahaemolyticus type III secretion systems manipulate host cell MAPK for critical steps in pathogenesis. BMC Microbiol. 2010;10:329.
- Ozretić P, Da Silva Filho MI, Catalano C, et al. Association of NLRP1 coding polymorphism with lung function and serum IL-1β concentration in patients diagnosed with Chronic Obstructive Pulmonary Disease (COPD). Genes (Basel). 2019;10:783.
- QingCui Z, Qin S, Min Z, et al. Relation between IL-8 level and obstructive sleep apnea syndrome. Open Med. 2021;16:683–691.
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
- Moskalewicz A, Oremus M. No clear choice between Newcastle-Ottawa scale and appraisal tool for cross-sectional studies to assess methodological quality in cross-sectional studies of health-related quality of life and breast cancer. J Clin Epidemiol. 2020;120:94–103.
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
- Alzoghaibi MA, Bahammam AS. Lipid peroxides, superoxide dismutase and circulating IL-8 and GCP-2 in patients with severe obstructive sleep apnea: a pilot study. Sleep Breath. 2005;9:119–126.
- Archontogeorgis K, Nena E, Tsigalou C, et al. Cystatin C levels in middle-aged patients with obstructive sleep apnea syndrome. Pulm Med. 2016;2016:8081723.
- Bhatt SP, Guleria R, Kabra SK. Metabolic alterations and systemic inflammation in overweight/obese children with obstructive sleep apnea. PLoS One. 2021;16:e0252353.
- Bilal N, Kurutas EB, Orhan I, et al. Evaluation of preoperative and postoperative serum interleukin-6, interleukin-8, tumor necrosis factor α and raftlin levels in patients with obstructive sleep apnea. Sleep Breath. 2021;25:819–826.
- Borel JC, Roux-Lombard P, Tamisier R, et al. Endothelial dysfunction and specific inflammation in obesity hypoventilation syndrome. PLoS One. 2009;4:e6733.
- Bouloukaki I, Papadimitriou V, Sofras F, et al. Abnormal cytokine profile in patients with obstructive sleep apnea-hypopnea syndrome and erectile dysfunction. Mediators Inflamm. 2014;2014:568951.
- Carpagnano GE, Spanevello A, Sabato R, et al. Systemic and airway inflammation in sleep apnea and obesity: the role of ICAM-1 and IL-8. Transl Res. 2010;155:35–43.
- Chen VG, Fonseca V, Amaral JB, et al. Inflammatory markers in palatine tonsils of children with obstructive sleep apnea syndrome. Braz J Otorhinolaryngol. 2020;86:23–29.
- Devouassoux G, Lévy P, Rossini E, et al. Sleep apnea is associated with bronchial inflammation and continuous positive airway pressure-induced airway hyperresponsiveness. J Allergy Clin Immunol. 2007;119:597–603.
- Hirsch D, Evans CA, Wong M, et al. Biochemical markers of cardiac dysfunction in children with obstructive sleep apnoea (OSA). Sleep Breath. 2019;23:95–101.
- Jin F, Liu J, Zhang X, et al. Effect of continuous positive airway pressure therapy on inflammatory cytokines and atherosclerosis in patients with obstructive sleep apnea syndrome. Mol Med Rep. 2017;16:6334–6339.
- Kim J, Lee CH, Park CS, et al. Plasma levels of MCP-1 and adiponectin in obstructive sleep apnea syndrome. Arch Otolaryngol Head Neck Surg. 2010;136:896–899.
- Li AM, Lam HS, Chan MH, et al. Inflammatory cytokines and childhood obstructive sleep apnoea. Ann Acad Med Singap. 2008;37:649–654.
- Ming H, Tian A, Liu B, et al. Inflammatory cytokines tumor necrosis factor-α, interleukin-8 and sleep monitoring in patients with obstructive sleep apnea syndrome. Exp Ther Med. 2019;17:1766–1770.
- Ohga E, Tomita T, Wada H, et al. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J Appl Physiol (1985). 2003;94:179–184.
- Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2006;174:824–830.
- Santamaria-Martos F, Benítez I, Girón C, et al. Biomarkers of carcinogenesis and tumour growth in patients with cutaneous melanoma and obstructive sleep apnoea. Eur Respir J. 2018;51:1701885.
- Taillé C, Rouvel-Tallec A, Stoica M, et al. Obstructive sleep apnoea modulates airway inflammation and remodelling in severe asthma. PLoS One. 2016;11:e0150042.
- Tam CS, Wong M, McBain R, et al. Inflammatory measures in children with obstructive sleep apnoea. J Paediatr Child Health. 2006;42:277–282.
- Thunström E, Glantz H, Fu M, et al. Increased inflammatory activity in nonobese patients with coronary artery disease and obstructive sleep apnea. Sleep. 2015;38:463–471.
- Vicente E, Marin JM, Carrizo SJ, et al. Upper airway and systemic inflammation in obstructive sleep apnoea. Eur Respir J. 2016;48:1108–1117.
- Wang W, Xu Z, Zhang J, et al. Tim-3 is a potential regulator that inhibits monocyte inflammation in response to intermittent hypoxia in children with obstructive sleep apnea syndrome. Clin Immunol. 2021;222:108641.
- Wang Y, Hu K, Liu K, et al. Obstructive sleep apnea exacerbates airway inflammation in patients with chronic obstructive pulmonary disease. Sleep Med. 2015;16:1123–1130.
- Zhang D, Xiao Y, Luo J, et al. Measurement of fractional exhaled nitric oxide and nasal nitric oxide in male patients with obstructive sleep apnea. Sleep Breath. 2019;23:785–793.
- Chopra S, Rathore A, Younas H, et al. Obstructive sleep apnea dynamically increases nocturnal plasma free fatty acids, glucose, and cortisol during sleep. J Clin Endocrinol Metab. 2017;102:3172–3181.
- Vakil M, Park S, Broder A. The complex associations between obstructive sleep apnea and auto-immune disorders: a review. Med Hypotheses. 2018;110:138–143.
- Kim YA, Kim DH, Park CB, et al. Anti-inflammatory and skin-moisturizing effects of a flavonoid glycoside extracted from the aquatic plant nymphoides indica in human keratinocytes. Molecules. 2018;23:2342.
- Akyol S, Çörtük M, Baykan AO, et al. Mean platelet volume is associated with disease severity in patients with obstructive sleep apnea syndrome. Clinics (Sao Paulo). 2015;70:481–485.
- Azagra-Calero E, Espinar-Escalona E, Barrera-Mora JM, et al. Obstructive sleep apnea syndrome (OSAS). Review of the literature. Med Oral Patol Oral Cir Bucal. 2012;17:e925–9.
- Yang H, Engeland CG, King TS, et al. The relationship between diurnal variation of cytokines and symptom expression in mild obstructive sleep apnea. J Clin Sleep Med. 2020;16:715–723.
- Casale M, Pappacena M, Rinaldi V, et al. Obstructive sleep apnea syndrome: from phenotype to genetic basis. Curr Genomics. 2009;10:119–126.
- Strawbridge R, Marwood L, King S, et al. Inflammatory proteins and clinical response to psychological therapy in patients with depression: an exploratory study. J Clin Med. 2020;9:3918.
- López-Pulido EI, Ramírez-De Los Santos S, González-Sánchez GD, et al. Association of obesity with depressive symptomatology, eating habits, interleukin-8 and cortisol in a young population. Ecol Food Nutr. 2021;60:324–333.
- Alam I, Lewis K, Stephens JW, et al. Obesity, metabolic syndrome and sleep apnoea: all pro-inflammatory states. Obes Rev. 2007;8:119–127.
- Wang Z, Zeng Y, Lin H. The effect of chronic intermittent hypoxia on atherosclerosis in rat offspring. Int J Cardiol. 2021;335:98–103.
- Ke D, Kitamura Y, Lejtenyi D, et al. Enhanced interleukin-8 production in mononuclear cells in severe pediatric obstructive sleep apnea. Allergy Asthma Clin Immunol. 2019;15:23.
- Belaidi E, Morand J, Gras E, et al. Targeting the ROS-HIF-1-endothelin axis as a therapeutic approach for the treatment of obstructive sleep apnea-related cardiovascular complications. Pharmacol Ther. 2016;168:1–11.
- Gao YY, Wang HJ, Wu Y. Superficial punctate keratopathy in a pediatric patient was related to adenoid hypertrophy and obstructive sleep apnea syndrome: a case report. BMC Ophthalmol. 2018;18:55.